Abstract
Isolates of the Burkholderia cepacia complex (BCC) are known as plant and human pathogens. We describe herein BCC infections as the cause of subcutaneous abscesses and purulent cellulitis in 5 cats. All cats were presented with an open wound, and 4 received standard wound care and empiric antibiotic therapy. Despite treatment, clinical signs worsened in 4 cats. Isolates of the BCC were obtained from all 5 cases. Two cats were submitted for postmortem examination. Subcutaneous abscesses with draining fistulas were observed. Histopathology revealed severe, pyogranulomatous cellulitis with intralesional gram-negative bacilli. Based on susceptibility results, the other 3 cats were administered effective antibiotics and recovered without complications. The BCC was cultured from the 2% chlorhexidine surgical scrub solution used in the clinic, suggesting the source of infection for 4 of 5 cats. Given the ability to grow in antiseptic solutions, the extra steps required to culture from antiseptics, and innate multidrug resistance, the BCC poses a challenge to both detect and treat. Although the BCC causes disease almost exclusively in humans with cystic fibrosis or immunodeficiency, the bacteria should also be a differential for nosocomial infections in veterinary patients.
Keywords: Burkholderia cepacia complex, cats
Subcutaneous abscesses are common skin and soft tissue conditions in domestic cats. Actinomyces spp., Clostridium spp., Fusobacterium spp., Pasteurella multocida, Peptostreptococcus spp., Porphyromonas spp., Staphylococcus spp., Streptococcus spp., and members of family Enterobacteriaceae are the most commonly cultured pathogens from such wounds.9 Treatment typically involves debridement, drainage, and administration of antimicrobials. Although, ideally, antimicrobial treatment should be based on results of culture and susceptibility testing, cephalosporins, clindamycin, metronidazole, and penicillins are recommended until results become available.9 Our retrospective case series highlights the importance of microbial culture and susceptibility testing for the diagnosis and treatment of subcutaneous abscesses and infected wounds in cats. It also suggests a potential role of antiseptic solutions as a nosocomial risk in veterinary clinics.
The Burkholderia cepacia complex (BCC; formerly Pseudomonas cepacia) is a group of gram-negative, non–spore-forming, aerobic bacilli composed of several genetically closely related bacteria that are ubiquitous in soil and water and on plant surfaces.8 These bacteria are not considered commensal flora of the canine or feline skin.13 Although all Burkholderia species have been isolated from human clinical samples, Burkholderia cenocepacia is one of the most studied and medically relevant species within the BCC associated with infection.15 Members of the BCC are widely known in the human medical world to cause opportunistic, hospital-associated infections in patients with cystic fibrosis (CF), patients with chronic granulomatous disease, and patients undergoing intensive care.8 Infection can lead to life-threatening, necrotizing pneumonia and septicemia, termed “cepacia syndrome.”8
BCC infections pose a challenge to diagnose, treat, and prevent. Culture-based surveillance studies for isolates of the BCC from presumably contaminated areas, such as food stores, restaurant salad bars, and the homes of uninfected individuals and infected patients, have shown low detection rates of only 1–18% using both MacConkey agar and Pseudomonas cepacia agar,16 despite the latter agar being recommended for selective isolation of the BCC.16 This suggests that, although considered ubiquitous, these bacteria are not commonly isolated from the environment. The organism also has intrinsic resistance to most clinically relevant antibiotics such as aminoglycosides, β-lactams, chloramphenicol, fluoroquinolones, and tetracyclines through a combination of decreased outer membrane permeability to prevent entry of antibiotics, expression of antibiotic-modifying proteins, and expression of multiple efflux pump transporters that remove antibiotics from cells.10
BCC members are also resistant to many antiseptics. There are several reports of human patients acquiring BCC infections from contaminated hospital antiseptics, including alcohol,18 benzalkonium chloride,12 chlorhexidine,20 and povidone–iodine.4 In some cases, these antiseptics may have been contaminated during pharmaceutical production, given that BCC members are one of the commonly reported contaminants in sterile and non-sterile, pharmaceutical product recalls by the U.S. Food and Drug Administration (FDA).11 Furthermore, BCC bacteria have been reported to form biofilms within water pipes, and contaminated water used for antiseptic dilution has been associated with hospital infections in humans.18 Given these challenges in detection, the occurrence of innate antibiotic resistance, and the possibility of unexpected contamination of antiseptic solutions, infections with BCC pathogens can be difficult to diagnose and treat.
There are few reports of BCC infection in veterinary medicine: pyoderma in dogs administered cyclosporin,2 mixed bacterial cultures in a horse with pneumonia,7 diarrhea in specific pathogen–free piglets,19 myocarditis, hepatitis, nephritis, and pneumonia in a pet African gray parrot,1 the sole agent of vegetative endocarditis in 2 horses,21 and subclinical mastitis in a flock of dairy sheep.5 Additionally, there are reports of BCC contamination of veterinary commercial ear cleaners3 and veterinary clinic surgical cold sterilization solutions17 without reported infection in animals. None of these reports identified the BCC to the species level. To date, there is no literature on animals acquiring BCC infection in the hospital setting, as well as no reports of B. cenocepacia or other members of the BCC causing infections in cats, to our knowledge. We describe herein BCC infections in 5 cats presented to a veterinary clinic in the United States, and highlight the importance of microbial culture and susceptibility testing for effective treatment of cats with abscess wounds. In addition, we discuss the possibility of antiseptic solutions as a point source of infection.
The medical records of the cats at a Georgia veterinary clinic for 2012–2014 were reviewed. The criterion for inclusion was a positive aerobic culture for BCC. If available, additional information retrieved from the medical records included the following: signalment, known history, clinical signs, relevant physical examination findings, clinicopathologic test results (hematologic evaluation, serum biochemical analysis, urinalysis, and endocrinology), antibiotic treatments, duration of disease, and outcomes. From November 2012 to May 2014, 5 cats with positive BCC cultures were identified and included in our study (Table 1). All cases were presented because of an open wound. In each case, the cat was pre-medicated with butorphanol (0.2 mg/kg IM; Dolorex, Merck Animal Health, Summit, NJ), sedated with dexmedetomidine (40 µg/kg IM; Dexdomitor, Orion Pharma, Turku, Finland), and maintained on isoflurane gas (<2%; Fluriso, VetOne, Boise, ID). Bacterial culture and susceptibility was initially declined by the submitter or owner in cases 1–4 and approved in case 5. In the first 4 cases, the referring veterinarian routinely clipped the fur and cleaned the wound area 3 times with alternating scrubs of a pre-made solution of 2% chlorhexidine scrub diluted with tap water followed by an alcohol scrub. Each abscess was then lanced with a sterile #10 blade, a drain (Argyle Penrose tubing, Covidien, Mansfield, MA) placed under the skin, and the animal administered cefovecin (7.9 mg/kg SQ; Convenia, Zoetis, Kalamazoo, MI).
Table 1.
Signalment and history for cats in the case series.
| Cat | Age | Sex | Breed | Indoor/Outdoor status | FeLV/FIV status | Vaccine status | Presenting complaint |
|---|---|---|---|---|---|---|---|
| 1 | 4 mo | FI | DSH | Outdoor stray | NK | NK | Hit by car, amputated rear limb |
| 2 | 4 y | FS | DSH | Indoor/outdoor | Negative | FVRCP, RV | History of cat fight, wound on back |
| 3 | 4.5 y | FS | DSH | Indoor/outdoor | Negative | FVRCP, RV, FeLV | Wound on dorsolumbar back |
| 4 | 7 y | MN | DLH | Primarily outdoor | NK | Expired FVRCP, RV | Wound on right hip |
| 5 | 2 y | MN | DSH | Indoor/outdoor | NK | FVRCP, RV | Wound on right ventrolateral abdomen |
DLH = domestic longhair; DSH = domestic shorthair; FI = female intact; FS = female spayed; FVRCP = feline viral rhinotracheitis, calicivirus, and panleukopenia vaccine; MN = male neutered; NK = not known; RV = rabies vaccine.
Within 7 d of presentation, the cats in cases 1–4 were reexamined for lethargy, inappetence, and non-healing wounds with purulent discharge. Three were febrile, and one was hypothermic (35°C). Wounds were reopened, flushed, and devitalized tissue debrided. Supportive care included subcutaneous fluids for 2 cats. Case 3 developed leukocytosis, azotemia, increased pancreatic enzyme levels, hyperbilirubinemia, increased pancreas-specific lipase, and elevated creatine kinase. Because of hypothermia, dehydration, and suspected necrotizing pancreatitis and septicemia, this cat was maintained on warming pads and blankets, sodium chloride intravenous fluid therapy, and feeding by nasoesophageal tube every 4 h, as well as treated with maropitant (1 mg/kg SQ q24h; Cerenia, Zoetis), famotidine (0.5 mg/kg intravenous q12h; West-Ward Pharmaceutical, Eatontown, NJ), and buprenorphine (0.01 mg/kg per os; Buprenex, compounded by RoadRunner Pharmacy, Phoenix, AZ). Animal health did not improve, and skin lesions worsened for each of these 4 cats despite a combination of inpatient and outpatient care for cases 1 and 3, outpatient care for cases 2 and 4, and empirical antibiotic therapy (Table 2) with amoxicillin–clavulanic acid (Clavamox, Zoetis), ampicillin (GC Hanford Manufacturing, Syracuse, NY), enrofloxacin (Baytril, Bayer HealthCare, Animal Health Division, Shawnee Mission, KS), pradofloxacin (Verflox, Bayer HealthCare), and doxycycline (PAR Pharmaceutical, Spring Valley, NY).
Table 2.
Bacteriology results, antibiotic treatment, and outcomes for each cat in the case series.
| Cat | Results of aerobic culture of abscess | Antibiotic treatment | Outcome |
|---|---|---|---|
| 1 | Burkholderia cepacia complex 2+; Stenotrophomonas maltophilia 2+; Pseudomonas putida 1+ | Amoxicillin–clavulanic acid, cefovecin, enrofloxacin, trimethoprim–sulfamethoxazole | Recheck with full recovery after 31 d |
| 2 | B. cepacia complex 1+ | Amoxicillin–clavulanic acid, cefovecin, doxycycline, ciprofloxacin | Recheck with full recovery after 63 d |
| 3 | B. cepacia complex 3+ | Amoxicillin–clavulanic acid, ampicillin | Found deceased after 14 d, submitted for autopsy |
| 4 | B. cepacia complex 1+ | Cefovecin, enrofloxacin, pradofloxacin | Euthanasia after 12 d, submitted for autopsy |
| 5 | B. cepacia complex 1+; Corynebacterium sp. 4+ | Cefovecin, trimethoprim–sulfamethoxazole | Recheck with full recovery after 11 d |
1+ = light growth; 2+ = light-to-medium growth; 3+ = medium-to-heavy growth; 4+ = heavy growth.
At 12 d following initial presentation in case 1, 26 d for case 2, 9 d for case 3, and 12 d for case 4, the cat was pre-medicated and sedated, as previously described. The wound was cleaned with 3 alternating scrubs of pre-made solution of 2% chlorhexidine scrub diluted with tap water followed by an alcohol scrub. Then, the surgical incision was reopened with a sterile #10 blade, and a direct swab of the affected subcutis obtained. All abscess swab samples were transported in culture medium tubes for aerobic culture and susceptibility testing (IDEXX Laboratories, North Grafton, MA). Each swab was rolled on MacConkey agar and trypticase soy agar (TSA) with 5% sheep blood and incubated overnight at 37°C. All bacterial isolates were identified by matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF MS; Bruker Daltronics, Billerica, MA; Table 2). Minimum inhibitory concentration was determined (VITEK 2 automated microbiology analyzer system, bioMérieux, Durham, NC) and interpreted based on the standards established by the Clinical and Laboratory Standards Institute (CLSI).6 If interpretive criteria were not available from CLSI, susceptibility results were determined by Kirby–Bauer disk diffusion susceptibility testing. In all 4 cases, aerobic culture yielded BCC. Based on susceptibility testing (Table 3), the cats in case 1 and case 2 were switched to antimicrobials to which the relevant BCC isolate was susceptible, ciprofloxacin (Pack Pharmaceuticals, Buffalo Grove, IL) and trimethoprim–sulfamethoxazole (RoadRunner Pharmacy), and recovered without further complications 31 and 63 d following initial presentation, respectively.
Table 3.
Antimicrobial susceptibility of Burkholderia cepacia complex isolates from a direct swab of a subcutaneous abscess in each of 5 cats and from swabs of chlorhexidine solution as reported by IDEXX Laboratories (North Grafton, MA).
| Antimicrobial agent | Results for isolate from indicated source | |||||||
|---|---|---|---|---|---|---|---|---|
| Cat 1 | Cat 2 | Cat 3 | Cat 4 | Cat 5 | 2% chlorhexidine scrub | 2% chlorhexidine scrub diluted with tap water stock bottle | 2% chlorhexidine scrub diluted with tap water in surgery suite | |
| Ticarcillin–clavulanic acid | S† | S† | NA | S† | S† | S† | S† | NA |
| Piperacillin | S (8) | S (⩽4) | I (32) | S (16) | S (8) | S (16) | S (16) | I (32) |
| Imipenem | R† | R (⩾16) | R (⩾16) | R (⩾16) | R (⩾16) | R† | R (⩾16) | R (⩾16) |
| Ceftazidime | S† | S† | S† | S† | S† | S† | S† | NA |
| Tobramycin | R (⩾16) | R (⩾16) | R† | R† | R† | R (⩾16) | R (⩾16) | R† |
| Ciprofloxacin | S† | S† | S† | S† | S† | I† | S† | NA |
| Marbofloxacin | I (2) | I (2) | I (2) | I (2) | I (2) | R (⩾4) | I (2) | I (2) |
| Trimethoprim–sulfamethoxazole | S (⩽20) | S (⩽20) | S (⩽20) | S (⩽20) | S (⩽20) | S (⩽20) | S (⩽20) | S (⩽20) |
All antimicrobial concentrations are expressed as µg/mL. All isolates were resistant to amoxicillin (>32 µg/mL), amoxicillin–clavulanic acid (>32 µg/mL), cephalexin (⩾64 µg/mL), cefovecin (⩾8 µg/mL), cefpodoxime (⩾8 µg/mL), ceftiofur (⩾8 µ/mL), amikacin (⩾64 µg/mL), gentamicin (⩾16 µg/mL), enrofloxacin†, tetracycline (⩾16 µg/mL), and chloramphenicol (32 µ/mL); I = intermediate; NA = not available; R = resistant; S = susceptible.
Kirby–Bauer disk diffusion susceptibility test performed; minimum inhibitory concentration data not available.
Before culture results returned, however, the cat in case 3 died, and the owner elected euthanasia in case 4 given the development of new draining tracts and financial constraints. These cases were submitted to the Athens Veterinary Diagnostic Laboratory (AVDL; Athens, GA) for postmortem examination, including autopsy and histopathology. In both cats, autopsy revealed subcutaneous abscesses over the caudodorsal regions with fistulas draining purulent fluid (Fig. 1). Histologic lesions in the skin ranged from subacute, necrohemorrhagic to pyogranulomatous cellulitis, with fibrin thromboses and rare, gram-negative, intrahistiocytic, and extracellular bacilli (Fig. 2). Additionally, case 3 had pyogranulomatous lymphadenitis with intrahistiocytic bacilli, diaphragmatic abscesses, and fibrin thromboses in the kidneys. Case 4 also had fibrin thromboses with necrosis in the kidneys, as well as few fibrin thromboses in the pancreas and cerebrum. These findings were consistent with septicemia in both cases.
Figure 1.
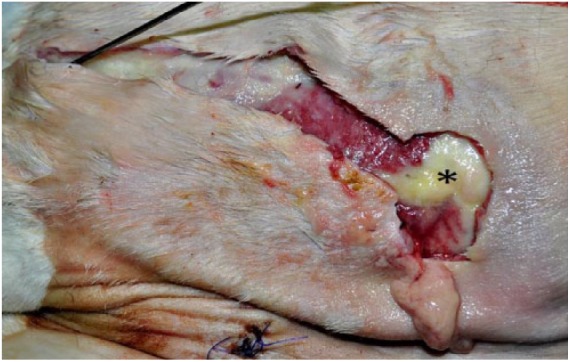
Photograph of the right flank of a 7-y-old, domestic shorthair cat that had been euthanized because of non-healing skin wounds. Subcutaneous abscess (*) with stylet passing through fistula draining purulent exudate. The subcutis was also diffusely reddened and expanded by large amounts of serous fluid that drained during incision.
Figure 2.
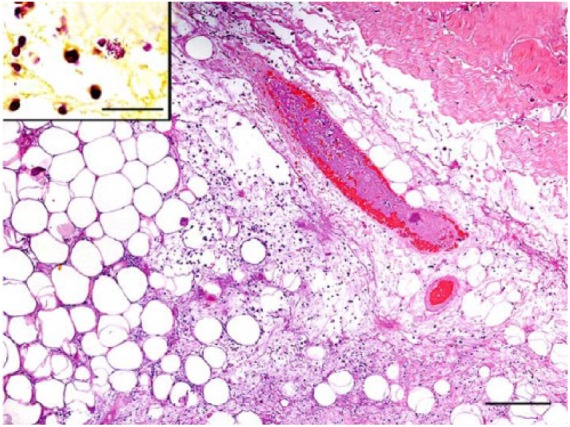
Photomicrograph of sections of the skin around the abscess on the right flank of the cat in Figure 1. Within the subcutis, there are multifocal areas of necrosis and large numbers of predominantly neutrophils and macrophages. Rare, gram-negative, intrahistiocytic, and extracellular bacilli are noted in these areas (inset, Gram stain; bar = 20 μm). A blood vessel within this section contains a fibrin thrombus. H&E. Bar = 200 μm.
A section of liver from case 3 and a direct swab of the subcutaneous tissue affected by the abscess from case 4 were submitted for aerobic culture. The liver sample was aerobically cultured directly on TSA with 5% sheep blood agar and MacConkey agar overnight at 37°C. The swab was diluted in sterile water, filtered using 0.45-µm filters, and cultured on the same media under the same conditions. Bacterial isolates were identified by conventional phenotypical methods and MALDI-TOF MS. In case 3, the liver sample yielded light-to-moderate growth of BCC and light growth of Serratia marcescens. The swab of the abscess in case 4 yielded a pure culture of moderate BCC growth. Sequencing the 1,500 bp of the 16S rRNA gene for the isolates from these 2 cases revealed 99% and 100% similarity to B. cenocepacia GenBank accession CP000458.1, respectively. No susceptibility testing was performed for these samples, and no other isolate was subjected to sequencing.
For case 5, the referring veterinarian was aware of the culture results from the first 4 cases, and as a result, persuaded the owner to agree to bacterial culture at the time of presentation. The referring veterinarian pre-medicated and sedated the cat as previously described, and then plucked fur from the wound site, incised the skin with a sterile #10 scalpel blade, obtained a direct swab sample of the subcutaneous abscess, and submitted the sample in culture medium tubes for aerobic culture (IDEXX Laboratories). This cat was treated with cefovecin (Convenia) and trimethoprim–sulfamethoxazole (RoadRunner Pharmacy) on the day of presentation based on the susceptibility testing for the previous cases while the culture and susceptibility results were pending (Tables 2, 3).
To investigate a point of infection that all 5 cases had in common, swabs of supplies at the veterinary clinic were submitted (IDEXX Laboratories) for aerobic culture and subsequent identification of any bacteria by MALDI-TOF MS as described previously. The supplies sampled included a swab of the clipper blades, an opened container of the 2% chlorhexidine solution (VetOne, Boise, ID) in the manufacturer’s 3.7-L bottle, an opened container of the 2% chlorhexidine scrub (VetOne) in the manufacturer’s 3.7-L bottle, and a pre-made solution of the 2% chlorhexidine scrub diluted with tap water that was periodically poured into smaller containers in the surgery room, treatment area, and examination rooms. Additionally, 3 mL of the veterinary clinic tap water was submitted to the AVDL for aerobic culture. This sample was filtered and cultured as described above.
The 3.7-L manufacturer’s bottle of 2% chlorhexidine scrub, the container of pre-mixed 2% chlorhexidine scrub from the manufacturer’s bottle diluted with tap water, and the container of 2% chlorhexidine scrub diluted with tap water in the surgery room all tested positive for BCC (Table 3). The 2% chlorhexidine scrub yielded BCC in pure culture with light-to-medium growth. The pre-mixed 2% chlorhexidine scrub diluted with tap water that served as a stock solution and the dilution in the surgery suite yielded light-to-medium growth of both Achromobacter xylosoxidans ssp. denitrificans and BCC. The clipper blades, 2% chlorhexidine solution, small containers in the treatment area and examination rooms of the pre-mixed chlorhexidine 2% scrub diluted with tap water, and the veterinary clinic tap water did not yield isolates of BCC.
Our report of BCC infections in 5 cats has several important implications for veterinary medicine. BCC is rarely reported in the veterinary literature, and our investigation provides evidence of BCC as a feline pathogen. BCC is not considered a commensal of the canine or feline skin13 and, thus, we did not consider it an opportunistic pathogen in our study. Furthermore, the identification of B. cenocepacia by PCR sequencing in cases 3 and 4 is consistent with reports of this species as one of the most commonly isolated members of the BCC in human clinical samples, as opposed to the other Burkholderia species, which are more commonly found in environmental samples.15 Although the pathogenesis of BCC infection in animals has not been explored, the dermal lesions in cases 3 and 4 had pyogranulomatous inflammation similar to reports of deep pyoderma in dogs,2 and the multisystemic fibrin thromboses suggested septicemia, which is compatible with “cepacia syndrome” reported in humans.8
In the antemortem abscess swabs from cases 2–4 and the postmortem abscess swab in case 4, BCC was grown in pure culture, implicating this organism as the causative agent of the clinical signs. The other cases yielded mixed cultures of BCC with other pathogens (Table 2). In case 1, BCC and Stenotrophomonas maltophilia were reported to have similar light-to-medium growth from antemortem abscess swabs, and, in addition to light growth of P. putida, all likely contributed to the clinical disease in this animal. In case 3, the postmortem liver sample grew light-to-moderate growth of BCC and very light growth of S. marcescens. However, given that the antemortem abscess swab yielded pure culture of medium-to-heavy growth of BCC organisms, the clinical significance of the S. marcescens isolation is unknown. In cases 1–4, samples for culture were obtained following antibiotic administration, which may have selected for BCC, which is intrinsically multidrug resistant.10 In case 5, the swab of the subcutaneous abscess was obtained prior to antibiotic administration and may explain the heavy growth of Corynebacterium spp. Additionally, the premixed 2% chlorhexidine diluted with tap water and the dilution in the surgery suite yielded mixed culture of BCC with A. xylosoxidans ssp. denitrificans. Interestingly, S. maltophilia and A. xylosoxidans are also emerging gram-negative, nosocomial pathogens in human medicine associated with multidrug resistance, CF patients, and septicemia.14
The report of B. cepacia skin infections in dogs attributed the disease to administration of immunosuppressive cyclosporin.2 In our report, given the open wounds in all cats at the time of presentation, direct inoculation of contaminated solutions into these wounds is the likely route of transmission. It is also possible that the patients were infected from the environment, given that members of the BCC are considered ubiquitous, and all cats had outdoor access. However, the lack of reports of environmental infections in domestic animals and the occurrence of multiple cases presented at a single veterinary hospital suggests that there was a common point source of contamination for all 5 cats. Unfortunately, clinical isolates of the IDEXX-cultured chlorhexidine samples were unavailable for PCR sequencing to compare strains. Nevertheless, the similar antimicrobial susceptibility pattern for each of the BCC isolates (Table 3) indirectly suggests that these isolates are related.
Because of multidrug-resistant capabilities,10 BCC infections can be difficult to treat. In our study, the pathogen was resistant to antibiotics commonly used in cats (Table 3). All isolates in our study exhibited similar antibiotic susceptibility testing results, including susceptibility to trimethoprim–sulfamethoxazole, a feature previously reported in the case series of pyoderma in dogs.2 It is important to note that the antimicrobial susceptibility tests were interpreted using the standards established by CLSI based on treating human infections,6 because there are no established veterinary laboratory standards for this organism. Furthermore, BCC is likely difficult to eradicate from a hospital given resistance to many antiseptics.4,12,18,20 The cat in case 5 was not surgically prepared with 2% chlorhexidine scrub before the wound was swabbed for microbial culture, yet the BCC bacteria were still detected. The clinic tap water and clippers did not yield BCC, and the microbes are not typical commensals of feline skin.13 Thus, these are unlikely sources of infection, and the source of infection for this cat remains unknown. However, it should be noted that members of the BCC can be difficult to culture, with detection rates typically <18% from sources suspected to have the bacteria, including the homes of human patients with confirmed B. cepacia infections.16 Hence, it is possible that the pathogen was present but not isolated from one of the sources we examined. It is also possible that an alternative or even additional source of contamination, such as the alcohol scrub or unopened supplies that were not tested, existed.
Our study suggests the source of infection to have been the contaminated antiseptic solution in the veterinary clinic. Isolates of the BCC have been reported as a contaminant in several antiseptics in human medicine, including chlorhexidine.20 Following isolation of BCC from the chlorhexidine bottle and dilutions, the clinic discarded these containers and ordered new antiseptic bottles from the same manufacturer. The incidents were reported by LC Chambers to the manufacturer, who tested a sample from the same lot of the 2% chlorhexidine scrub, and it did not yield bacterial growth. As additional precautions, the clinic cleaned the surgical and treatment areas with a 1:10 dilution of bleach with tap water and Roccal-D Plus (Pfizer, New York, NY) and switched to a surgical preparation of 3 alternating scrubs of iodine and alcohol. After 8 mo of performing this scrub protocol, the clinic returned to surgical preparation with 3 alternating scrubs of 2% chlorhexidine diluted with tap water and alcohol. They discontinued making pre-mixed dilutions of 2% chlorhexidine and instead poured directly from the manufacturer’s bottle to make fresh chlorhexidine scrubs for each individual case. They discarded these prepared scrub dilutions if unused for 30 min. Furthermore, the clinicians at this clinic in future cases are more likely to strongly recommend aerobic culture and susceptibility testing for every cat or dog that is presented because of a cutaneous or subcutaneous abscess. The clinic also sporadically submits samples from the 2% chlorhexidine bottle for aerobic culture testing, which has yielded no microbial growth. This set of incidents was also reported to the FDA by K Sakamoto.
It is important for pathologists and clinicians to be aware of the rare potential for BCC–associated subcutaneous abscesses and purulent cellulitis in cats. Delay of microbial culture and susceptibility was associated with increased time to recovery or death. In cases 1 and 2, it took 31 and 63 d for recovery, respectively, when bacterial culture and susceptibility testing was not initially utilized. In case 5, in which BCC infection was suspected, culture and susceptibility testing had been submitted on admittance. Effective antibiotics were administered, and the cat recovered in 11 d.
Because BCC can cause severe, potentially life-threatening infections in humans who are immunocompromised or who have CF,8 animals with the infection may pose a public health concern for these individuals. However, there are, to date, no reports of zoonotic transmission, to our knowledge. Regardless, pet owners and animal health workers should be aware of BCC, as well as other potentially zoonotic bacterial pathogens, when handling animals with purulent cellulitis, and should use appropriate personal protective equipment.
Acknowledgments
We thank Dr. Wilson Yau for technical assistance with the photomicrographs. We thank Dr. Claire Miller for insights and guidance in manuscript preparation. We thank the microbiology laboratory staff at the Athens Veterinary Diagnostic Laboratory for technical assistance with bacterial culture and PCR sequencing.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Akkoç A,et al. Burkholderia cepacia and Aeromonas hydrophila septicemia in an African grey parrot (Psittacus erithacus erithacus). Turk J Vet Anim Sci 2008;32:233–236. [Google Scholar]
- 2. Banovic F,et al. Deep pyoderma caused by Burkholderia cepacia complex associated with ciclosporin administration in dogs: a case series. Vet Dermatol 2015;26:287–e64. [DOI] [PubMed] [Google Scholar]
- 3. Bartlett SJ, et al. Bacterial contamination of commercial ear cleaners following routine home use. Vet Dermatol 2011;22:546–553. [DOI] [PubMed] [Google Scholar]
- 4. Berkelman RL, et al. Pseudobacteremia attributed to contamination of povidine-iodine with Pseudomonas cepacia. Ann Intern Med 1981;95:32–36. [DOI] [PubMed] [Google Scholar]
- 5. Berriatua E,et al. Outbreak of subclinical mastitis in a flock of dairy sheep associated with Burkholderia cepacia complex infection. J Clin Microbiol 2001;39:990–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 24th ed. Wayne, PA: CLSI, 2014. CLSI supplement M100. [Google Scholar]
- 7. Collobert C,et al. Bacteria and fungi isolated from equine tracheobronchial aspirates. Prat Vet Equin 1995;27:91–96. [Google Scholar]
- 8. Govan JRW, et al. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol 1996;45:395–407. [DOI] [PubMed] [Google Scholar]
- 9. Greene CE. Abscesses and pyogranulomatous inflammation caused by bacteria. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 3rd ed. Philadelphia: Elsevier Health Sciences, 2006:491–495. [Google Scholar]
- 10. Hancock RE. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin Infect Dis 1998;27:93–99. [DOI] [PubMed] [Google Scholar]
- 11. Jimenez L. Microbial diversity in pharmaceutical product recalls and environments. PDA J Pharm Sci Technol 2007;61:383–399. [PubMed] [Google Scholar]
- 12. Kaslow RA, et al. Nosocomial pseudobacteremia: positive blood cultures due to contaminated benzalkonium chloride. JAMA 1976;236:2407–2409. [DOI] [PubMed] [Google Scholar]
- 13. Krogh HV, Kristensen S. A study of skin diseases in dogs and cats. II. Microflora of the normal skin of dogs and cats. Nord Vet Med 1976;28:459–463. [PubMed] [Google Scholar]
- 14. Lambiase A,et al. Microbiology of airway disease in a cohort of patients with cystic fibrosis. BMC Infect Dis 2006;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahenthiralingam E,et al. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol 2005;3:144–156. [DOI] [PubMed] [Google Scholar]
- 16. Mortensen JE, et al. Recovery of Pseudomonas cepacia and other Pseudomonas species from the environment. Infect Control Hosp Epidemiol 1995;16:30–32. [DOI] [PubMed] [Google Scholar]
- 17. Murphy CP, et al. The prevalence of bacterial contamination of surgical cold sterile solutions from community companion animal veterinary practices in southern Ontario. Can Vet J 2010;51:634–636. [PMC free article] [PubMed] [Google Scholar]
- 18. Nasser RM, et al. Outbreak of Burkholderia cepacia bacteremia traced to contaminated hospital water used for dilution of an alcohol skin antiseptic. Infect Control Hosp Epidemiol 2004;25:231–239. [DOI] [PubMed] [Google Scholar]
- 19. Shyu JJ, et al. Study on diseases of primary specific pathogen free (SPF) piglets. J Chin Soc Vet Sci 1996;22:245–255. [Google Scholar]
- 20. Sobel JD, et al. Nosocomial Pseudomonas cepacia infection associated with chlorhexidine contamination. Am J Med 1982;73:183–186. [DOI] [PubMed] [Google Scholar]
- 21. Travers CW, van den Berg JS. Pseudomonas spp. associated vegetative endocarditis in two horses. J S Afr Vet Assoc 1995;66:172–176. [PubMed] [Google Scholar]


