Abstract
Protein p72 is the major capsid protein of African swine fever virus (ASFV) and is an important target for test and vaccine development. Monoclonal antibodies (mAbs) were prepared against a recombinant antigenic fragment, from amino acid (aa) 20–303, expressed in baculovirus. A total of 29 mAbs were recovered and tested by immunofluorescent antibody (IFA) staining on ASFV Lisbon-infected Vero cells. Six antibodies were IFA-positive and selected for further characterization. Epitope mapping was performed against overlapping polypeptides expressed in E. coli and oligopeptides. Based on oligopeptide recognition, the mAbs were divided into 4 groups: mAb 85 (aa 165–171); mAbs 65-3 and 6H9-1 (aa 265–280); mAbs 8F7-3 and 23 (aa 280–294); and mAb 4A4 (aa 290–303). All mAbs were located within a highly conserved region in p72. This panel of antibodies provides the opportunity to develop new assays for the detection of ASFV antibody and antigen.
Keywords: African swine fever virus, monoclonal antibodies, p72
Introduction
African swine fever virus (ASFV) is the etiologic agent of African swine fever (ASF), a disease of swine characterized by high morbidity and mortality, and for which commercial vaccines are not available. ASFV is a large DNA virus and the only member of family Asfarviridae.5 Up to 54 polypeptides constitute its multilayered virion6 with p72 being the major capsid component.13
In the absence of vaccines, control measures are heavily dependent on detection of nucleic acid, antigen, and antibodies. For the purpose of serologic detection, p72 antigen is one of the most immunogenic ASFV proteins.8 The availability of the full-length nucleotide sequence of p72 has allowed its expression in vitro and use as antigen in assay development.7,10 Moreover, the C-terminal nucleotide sequence of p72 has been traditionally used for the genotyping of ASFV isolates.1,2,11 However, the variability at the nucleotide level does not translate into variability at the aa level, given that variation occurs in the third nucleotide position. Therefore, p72 has been used as antigen in indirect ELISAs and a pen-side assay.7,12,15,17
Monoclonal antibodies (mAbs) have emerged as important reagents for blocking ELISAs and antigen-capture assays. Anti-ASFV mAbs, using the whole ASFV virion as antigen, have been produced previously, and the majority of them recognized the structural protein p72.14 A commercial blocking ELISA for the detection of anti-ASFV antibodies (INGEZIM PPA Compac, Ingenasa, Madrid, Spain) has been produced, and shown to possess high specificity and sensitivity. Moreover, commercially produced anti-p72 mAbs (Ingenasa) have been used as reagents in antigen-capture17 and pen-side16 assays. Although anti-p72 mAbs have been successfully produced, there is limited information on specific epitopes. Previous work identified a conformational epitope, covering the region aa 393–442.3
We prepared a panel of mAbs from mice immunized with a recombinant baculovirus-expressed p72 protein fragment aa 20–303 of the 646 aa protein. Epitope mapping was conducted against recombinant polypeptide fragments and synthesized oligopeptides. The resulting panel recognized 4 separate regions on p72.
Material and methods
Preparation of p72 recombinant protein and mAbs
For the production of mAbs, ASFV Georgia 2007 strain genome was used as a template to amplify partial open reading frame B646L (encoding for p72) via conventional PCR using the following primers: forward 5′-TTGGCCCAAGACTTGCTGAATAGC-3′, and reverse 5′-ATACGTTGCGTCCGTGATAGGAGT-3′. The amplified region covered aa 20–303 of p72. The amplicon was cloned (Invitrogen Bac-to-Bac HBM TOPO secreted expression system, Thermo Fisher Scientific, Waltham, MA) into the expression cassette of pFastBac/HBM TOPO vector, and then shuttled into the baculovirus shuttle vector (bacmid). The recombinant bacmid DNA was then used to transfect SF9 and Hi-Five insect cells to express p72 fusion protein with 6× His-tag attached to the C-terminus, according to the manufacturer’s instructions. The recombinant protein was purified (HisPur cobalt chromatography cartridge, Thermo Fisher Scientific), and solubilized using 4 M urea buffer. The successful protein purification was assessed by western blot using anti-histidine tag antibody (Aviva System Biology, San Diego, CA) according to the manufacturer’s instructions.
The following animal work was approved by Plum Island Animal Disease Center Institutional Animal Care and Use Committee (Protocol 138). To prepare the immunogen, the purified protein (100 µg/mL) was emulsified with adjuvant (Montanide ISA 206 VG, Seppic, Fairfield, NJ) at 1:1 ratio. Each of the 6–8-wk-old BALB/c mice was immunized with 0.2 mL of immunogen by intraperitoneal (IP) injection. Mice were boosted twice or more (depending on the antibody titer) at 2-wk intervals. The antibody titer of the immunized mice was assessed by indirect ELISA using the purified p72 protein as antigen. The final immunization was done with the same amount of purified protein by IP route 3 d prior to fusion. Spleen lymphocytes from mice with the highest antibody titer were isolated and fused to Sp2/O myeloma cells (kindly provided by Dr. FL Lucy, Avian Disease Oncology Laboratory, Agriculture Research Service, USDA) to prepare hybridomas. The fused cells were suspended in hybridoma serum-free medium (Gibco, Thermo Fisher Scientific) with 10% fetal bovine serum and 1× hypoxanthine–aminopterin–thymidine (HAT), and seeded to 96-well tissue culture plates. After 10–14 d incubation, the hybridomas were screened by indirect ELISA and immunofluorescent antibody (IFA).
For the ELISA, baculovirus-expressed p72 was used as antigen. Briefly, 96-well plates were coated with the recombinant p72 protein at 1:2,000 dilution (50–100 ng/mL) in carbonate–bicarbonate coating buffer (pH 9.6). Plates were incubated at 37°C for 1 h, or 4°C overnight. After 4 washes with phosphate-buffered saline with 0.05% Tween-20 (PBST), the plates were blocked with blocking buffer (1× fish gelatin in PBST) for 1 h at 37°C. After 4 washes, 100 µL of hybridoma supernatants were added to the plate and incubated at 37°C for 1 h. Following 4 washes, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Thermo Scientific), diluted at 1:2,000 in blocking buffer, was added to the plate (100 µL/well) and incubated at 37°C for 45 min. After 4 washes, 100 µL/well of o-phenylenediamine dihydrochloride (OPD) at pH 5.0 with hydrogen peroxide was added and incubated at room temperature for 15 min. The reaction was stopped by adding 100 µL of 1 M sulfuric acid. The absorbance at 492 nm was read using a spectrophotometer.
For the IFA, ASFV Lisbon-infected Vero cells were fixed with cold (4°C) acetone:methanol (50:50%, v/v) for 5 min at room temperature. The monolayers were rinsed once with PBS, then incubated with hybridoma supernatant at 37°C for 30 min. After washes, the Alexa Fluor 488–conjugated goat anti-mouse IgG (Life Technologies, Thermo Fisher Scientific), diluted 1:500 in PBS, was added to the plate and incubated at 37°C for 30 min. Cells were viewed under a fluorescence microscope. Positive clones were amplified and sub-cloned to produce monoclonal hybridoma cell lines and mAbs.
Monoclonal antibodies were isotyped, following the manufacturer’s instructions, using the following kits: IsoStrip mouse monoclonal antibody isotyping (Roche Diagnostic, Indianapolis, IN); Pierce rapid antibody isotyping kit plus kappa and lambda–mouse (Thermo Fisher Scientific); and Mouse Ig isotyping ready-set-go (eBioscience, Affimetrix, Thermo Fisher Scientific). The kits contain an anti-mouse positive control and identify immunoglobulins IgA, IgM, IgG1, IgG2a, IgG2b, IgG3 heavy chains, and kappa and lambda light chains.
Production of recombinant p72 fragments and oligopeptides
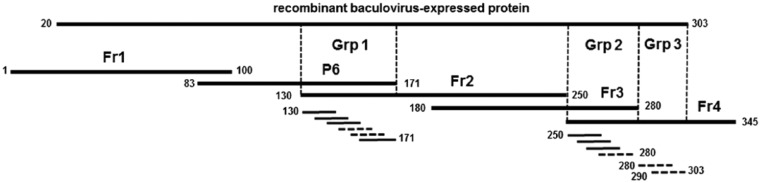
The ASFV p72 gene sequence of aa 1–345, based on ASFV BA71V strain (GenBank accession U18466.1), was used for the preparation of the p72 fragments. The aa sequence of the protein was divided into 5 overlapping fragments (Fr) of ~100 aa in length (Fig. 1): Fr1 (aa 1–100), P6 (aa 83–171), Fr2 (aa 130–250), Fr3 (aa 180–280), Fr4 (aa 250–345). A clone containing pHUE with the p72 whole protein (based on the BA71V strain) was also used in our study. The corresponding nucleotide sequences of the fragments were codon-optimized for expression in E. coli, and synthesized by Integrated DNA Technologies (Coralville, IA). SacII and EcoRI were added to the 5′- and 3′-end, respectively. The fragments were then cloned into pCR2.1-TOPO vector (TOPO TA cloning kit, Invitrogen), following the manufacturer’s instructions, and transformed into NEB 10-beta E. coli chemically competent cells (New England Biolabs, Ipswich, MA). Transformed cells were transferred into Luria-Bertani broth with 100 µg/mL of ampicillin (Thermo Fisher Scientific), and incubated overnight at 37°C on a shaker. The plasmids were extracted from the overnight culture (PureYield plasmid miniprep system, Promega, Madison, WI), following the manufacturer’s protocol. The plasmids, double-digested with SacII (New England Biolabs) and EcoRI-HF (New England Biolabs), were cloned in frame into the SacII-EcoRI sites on the pHUE expression vector.4 Plasmids were transformed into NEB BL21 (DE3) E. coli chemically competent cells (New England Biolabs). Three clones per sample were selected from the overnight plates and transferred into Luria-Bertani broth with 100 µg/mL of ampicillin. Clones were screened by double digestion with SacII and EcoRI for the presence of the target insert.
Figure 1.
Schematic representation of African swine fever virus p72 overlapping fragments and oligomers. The dotted lines indicate the oligopeptides recognized by the monoclonal antibodies (mAbs). Fr1, P6, Fr2–Fr4, and Grp1, 2, and 3 identify the location of binding by the different mAbs. Oligopeptides were ~15 amino acids (aa) in length and overlapped by 8–9 aa. The aa 20–303 recombinant protein is also shown.
For expression of p72 protein fragments and whole protein, selected clones were grown overnight in Luria-Bertani broth with 100 µg/mL of ampicillin. Two milliliters of overnight culture were added to 100 mL of Luria-Bertani broth in a Fernbach flask and shaken at 37°C in a shaker incubator. Once the optical density was 0.4–0.6, protein expression was induced by adding 1 mL of 0.1 M isopropyl β-D-1-thiogalactopyranoside (IPTG) to the culture, and incubation was continued for 4 h. Bacteria were pelleted by centrifugation at 4,000 × g for 10 min and stored at −20°C. Soluble proteins were purified from the bacterial pellet (USB PrepEase histidine-tagged protein purification kit—high yield, Thermo Fisher Scientific), following the manufacturer’s protocol for native conditions. Insoluble proteins were purified using the same kit with 2 different protocols: one was the manufacturer’s protocol for denaturing conditions; the other was a modified protocol with 0.5 M 3-(cyclohexylamino)-1-propane-sulfonic acid (CAPS)–0.3% Sarkosyl buffer. The latter protocol was performed as follows: the bacterial pellet was resuspended in 1× lysis–equilibrium wash (LEW) buffer (50 mM NaH2PO4, 300 mM NaCl, pH 8.0), and then Halt protease inhibitor cocktail, EDTA-free (Thermo Fisher Scientific) and lysozyme (Sigma-Aldrich, St. Louis, MO) were added. The resuspended pellet was placed on ice for 30 min, and then bacterial cells were disrupted by sonication, followed by centrifugation at 10,000 × g for 30 min. The pellet post-centrifugation was resuspended in 0.5 M CAPS–0.3% Sarkosyl and subjected to 4 cycles of freeze (–80°C) and thaw (37°C), followed by a centrifugation step at 10,000 × g for 30 min. The supernatant was filtered and applied to the column following the manufacturer’s protocol using as wash and elution buffers, 0.5 M CAPS–0.3% Sarkosyl and 0.5 M CAPS–0.3% Sarkosyl–250 mM imidazole, respectively. The purity of each protein was assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).9
For the fine mapping, the protein regions recognized by the mAbs where subdivided into fragments of 15–17 aa and overlapping by 8–9 aa (Fig. 1). The aa sequence was commercially synthesized (21st Century Biochemicals, Marlboro, MA). The oligopeptides were synthesized, coupled with ovalbumin, and were then used as antigen in ELISA to test the reactive mAbs.
Swine polyclonal sera
A total of 5 polyclonal sera were used for the mapping. Sera were obtained at 57 days postinoculation (dpi) from pigs 5 and 9 that had been immunized with RP-sHA-p72, a defective alphavirus replicon particle expressing a chimeric protein composed of the extracellular domain of the ASFV CD2-like protein (based on the ASFV E75 strain) upstream to the whole p72 protein (based on the ASFV BA71V strain). Sera were collected at 17 dpi from pigs 18, 73, and 75 that had been experimentally infected with ASFV OURT88/3 strain.
Indirect ELISAs
For the testing of anti-p72 hybridoma supernatants and swine polyclonal sera, 96-well plates were coated with ASFV p72 fragments. The protein concentrations were adjusted to 4 µg/mL in carbonated coating buffer (pH 9.6), and 100 μL were used to coat flat-bottom polystyrene plates. Plates were incubated for 1 h at 37°C, followed by 3 washes with PBST. The plates were then blocked for 1 h at 37°C using 10% goat serum in PBS (PBS-GS), followed by washes. One hundred microliters of primary antibody (polyclonal swine serum or mAbs), 2-fold serially diluted in PBS-GS, were added to each well and incubated for 1 h at 37°C. The plates were then washed, and a secondary antibody was added to each well, and incubated for 1 h at 37°C. The secondary antibodies (ICN Biomedicals, Irvine, CA) were HRP-conjugated goat anti-swine IgG for the swine sera and HRP-conjugated goat anti-mouse IgG for the mAbs, diluted 1:2,000 or 1:500 in PBS-GS, respectively. The plates were washed, and ABTS 1-component microwell peroxidase substrate (KPL, SeraCare Life Sciences, Milford, MA) was added. The plates were then incubated at room temperature in the dark for 20 min. The reaction was stopped by adding 1% SDS in double-distilled water and the absorbance measured (FLUOstar omega microplate reader, BMG Labtech, Cary, NC). The absorbance at 405 nm and 650 nm was recorded, and results were reported as the absorbance at 405–650 nm.
Western blotting
Protein fragments were separated by SDS-PAGE and then transferred to 0.2 µM polyvinylidene fluoride membranes (Amersham, GE Healthcare Life Sciences, Pittsburg, PA) using the Mini Trans-Blot electrophoretic transfer cell (Bio-Rad, Hercules, CA), following the manufacturer’s instructions. Membranes were incubated overnight at 4°C in blocking buffer followed by 3 washes with PBST. The blocking buffer for swine sera was protein-free buffer (G-Biosciences, St. Louis, MO) or 1% fish gelatin in PBS with 0.1% Tween-20; for the mAbs, the buffer was 5% non-fat dry milk in PBS with 0.1% Tween-20 (NFDM). Sera from RP-sHA-p72–vaccinated pigs, diluted 1:300 in blocking buffer, were added to the membranes and incubated for 1 h at room temperature with shaking. After 3 washes with PBST, HRP-conjugated goat anti-swine IgG (ICN Biomedicals), diluted 1:3,000 in blocking buffer, was added and incubated for 1 h 15 min at room temperature with shaking. Peroxidase activity was detected with 4-chloro-1-naphtol/3,3’-diaminobenzidine, tetrahydrochloride substrate (Thermo Fisher Scientific). After incubation for 2–10 min at room temperature, the reaction was stopped by rinsing the membrane with double-distilled water.
The p72 mAbs and Penta-His antibody, BSA-free (Qiagen, Germantown, MD) were diluted 1:100 and 1:1,000 in NFDM, respectively, and incubated for 1 h at room temperature with shaking. The secondary antibody was HRP-conjugated goat anti-mouse IgG (ICN Biomedicals) diluted 1:500 in NFDM and incubated for 1 h at room temperature. The subsequent steps were the same as for the polyclonal sera.
Results
Preliminary screening of mAbs against p72 antigens
A total of 29 mAbs reacted by indirect ELISA using the baculovirus-expressed p72 protein as the target antigen (data not shown). The mAbs were further screened for reactivity by IFA on Vero cells infected with the Lisbon ASFV isolate along with the entire p72 protein expressed in E. coli by western blot and ELISA. Only 6 mAbs reacted with cell and E. coli–expressed antigens: 23, 4A4, 85, 65-3, 6H9-1, and 8F7-3 (Table 1). Fourteen of the remaining 23 mAbs recognized the recombinant protein in both ELISA and western blot formats, 9 reacted with the recombinant protein in only 1 of the 2 assay formats, and 2 did not react in either format. The 6 mAbs that recognized the p72 protein in all assay formats were selected for further study.
Table 1.
Summary of screening of monoclonal antibodies (mAbs) to African swine fever virus p72 capsid protein.
| mAb | Isotype | Lisbon-infected Vero cells |
E.
coli–expressed antigen |
|
|---|---|---|---|---|
| IFA | ELISA | WB | ||
| 10C7 | IgA-k | − | + | + |
| 23 | IgG1-k/λ | + | + | + |
| 2B6 | IgG1-k/λ | − | + | + |
| 34 | IgG1-k | − | + | + |
| 4 | IgG1-k | − | + | − |
| 4A4 | IgG1-k | + | + | + |
| 54 | IgG1-k | − | + | + |
| 58 | IgG1-k | − | + | + |
| 5B10 | IgG1/IgG3-k | − | − | + |
| 5G1 | IgG1-k | − | + | + |
| 61 | IgG1-k | − | + | + |
| 7C5 | IgG1-k | − | − | + |
| 7H4 | IgG1-k | − | + | + |
| 81 | IgG1-k | − | + | + |
| 85 | IgG1-k | + | + | + |
| 89 | IgG1-k | − | + | + |
| 8B12 | IgG1/IgM-k | − | + | − |
| 8C11 | IgG1-k | − | + | + |
| 8H8 | IgG1-k | − | + | − |
| 94 | IgG1-k | − | + | + |
| 9G11 | IgG1-k | − | − | + |
| 9G6 | IgG1-k | − | − | + |
| 65-3 | IgG1-k | + | + | + |
| 6F9-10 | IgG1-k | − | + | + |
| 6H9-1 | IgG1-k | + | + | + |
| 8F7-3 | IgG1-k | + | + | + |
| 8H2-1 | IgG1-k | − | + | + |
IFA = immunofluorescent antibody; WB = western blot.
Epitope mapping using monoclonal and polyclonal antibodies
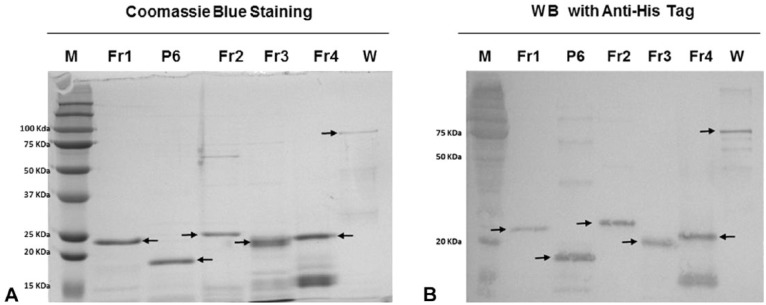
E. coli–expressed polypeptide fragments were used to identify the p72 region recognized by the mAbs (Fig. 1). The fragments P6, Fr2, and Fr3 were soluble and purified under native conditions. The full-length p72 protein and Fr1 were insoluble and were maintained in 4 M urea during purification. Fr4 was also insoluble but was easily purified in the presence of CAPS–Sarkosyl. All recombinant polypeptides were easily detected on SDS-PAGE and by western blotting using anti-6× His antibody (Fig. 2). All polypeptides migrated at the predicted formula weight.
Figure 2.
Purification of African swine fever virus p72 polypeptide fragments. A. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie blue staining. B. Western blot (WB) staining with anti-histidine monoclonal antibody. M = protein ladder; Fr1–Fr4 = polypeptide fragments; W = whole protein. Arrows indicate band showing the predicted size.
The 6 selected mAbs reacted in ELISA and western blots against the recombinant polypeptides. The mAbs could be placed into 3 distinct groups (Table 2, Fig. 1). The single group 1 antibody, mAb 85, recognized polypeptides P6 (aa 83–171) and Fr2 (aa 130–250), which included the overlapping region aa 130–171 (Fig. 1). The mAb was then used to screen a group of 6 overlapping oligopeptides within the aa 130–171 region. Two oligopeptides, aa 150–165 and aa 156–171 reacted with mAb-85 (Fig. 1). The overlapping region covered by the 2 oligopeptides was the peptide sequence 156-TLVDPFGRPI-165 (Table 3). Two mAbs, 65-3 and 6H9-1, reacted with Fr3 (aa 180–280) and Fr4 (aa 250–345), which overlapped the region covered by aa 250–280. These mAbs were assigned to group 2. Those mAbs were tested against 4 oligopeptides within the aa 250–280 region, and showed reactivity with only 1 oligopeptide, 265-QRTCSHTNPKFLSQHF-280. Three mAbs, 4A4, 8F7-3, and 23, reacted with the single polypeptide Fr4 (aa 250–345), which recognized the aa 250–303 region and were assigned to group 3. Recognition of 2 oligopeptides within the aa 250–303 region showed that mAbs could be further divided into 2 subgroups. Subgroup 3a, composed of mAbs 8F7-3 and 23, recognized the oligopeptide aa 280–294; subgroup 3b, composed of mAb 4A4, recognized the oligopeptide aa 290–303 (Table 3). The aa sequences of the epitopes of the mAbs belonging to groups 3a and 3b are 280-FPENSHNIQTAGKQD-294 and 290-AGKQDITPITDATY-303, respectively (Table 3).
Table 2.
Summary of monoclonal antibody (mAb) reactivity against African swine fever virus p72 fragments and oligomers.
| mAb | Antigen |
|||||
|---|---|---|---|---|---|---|
|
E.
coli–expressed recombinant protein |
Oligomer(s) | |||||
| Fr1 (1–100) |
P6 (83–171) |
Fr2 (130–250) |
Fr3 (180–280) |
Fr4 (250–345) |
||
| Grp1 (aa 130–171) | ||||||
| 85 | − | + | + | − | − | 150–165, 156–171 |
| Grp2 (aa 250–280) | ||||||
| 65-3 | − | − | − | + | + | 265–280 |
| 6H9-1 | − | − | − | + | + | 265–280 |
| Grp3 (aa 250–303) | ||||||
| 8F7-3 | − | − | − | − | + | 280–294 |
| 23 | − | − | − | − | + | 280–294 |
| 4A4 | − | − | − | − | + | 290–303 |
| Polyclonal sera | ||||||
| P18 | + | − | − | − | − | ND |
| P73 | + | − | − | − | − | ND |
| P75 | + | − | − | − | + | ND |
| P5 | + | − | − | + | + | ND |
| P9 | + | − | − | + | + | ND |
aa = amino acid; ND = not done.
Table 3.
Summary of African swine fever virus p72 epitopes recognized by monoclonal antibodies (mAbs).
| mAb | Group | Epitope |
|
|---|---|---|---|
| Location | aa sequence | ||
| 85 | 1 | aa 156–165 | 156-TLVDPFGRPI-165 |
| 65-3 and 6H9-1 | 2 | aa 265–280 | 265-QRTCSHTNPKFLSQHF-280 |
| 8F7-3 and 23 | 3a | aa 280–294 | 280-FPENSHNIQTAGKQD-294 |
| 4A4 | 3b | aa 290–303 | 290-AGKQDITPITDATY-303 |
aa = amino acid.
We took advantage of archived sera from pigs immunized with an alphavirus replicon particle expressing p72 (2 sera) and infected with ASFV (3 sera) to identify p72 antigenic regions in swine and compare them with the regions identified using the mAbs. Serum samples P18, P73, and P75, from pigs infected with ASFV OURT88/3, were obtained at 17 d after infection. The results showed reactivity of all pigs with Fr1 (aa 1–100), and a single pig that recognized Fr4 (aa 250–345; see Table 2). Overlap between polyclonal and monoclonal antibody recognition was found in the mAb group 2 and 3 region, aa 250–345. Similar results were obtained in sera from pigs immunized with a construct that expressed p72. Samples P5 and P9 reacted with Fr1 (aa 1–100), Fr3 (aa 180–280), and Fr4 (aa 250–345), which also cover regions corresponding to groups 2 and 3. Differences in the reactivity of polyclonal sera and mAbs was noted for Fr1, which was recognized by all swine sera and none of the mAbs, and fragments P6 and Fr2, which were recognized by mAb-85, but none of the swine sera. Pig sera were not further tested for reactivity against the panel of oligopeptides.
Discussion
We produced a panel of mAbs against a p72 polypeptide antigen, which covered aa 20–303. A total of 29 mAbs were produced that recognized the original immunogen, but only 6 recognized the native form of the protein as determined by IFA on ASFV-infected cells. Epitope mapping divided the mAbs into 4 groups: group 1 recognized the epitope aa 156–165, group 2 the epitope aa 265–280, group 3a the epitope aa 280–294, and group 3b the epitope aa 290–303. In a previous study,3 a partially neutralizing mAb was identified that recognized a conformational epitope on p72, located between aa 393 and 442, a region downstream of the linear epitope of the group 3b mAb produced in our study. In order to determine if the protein regions recognized by the mAbs were also recognized by swine sera, 5 sera from pigs that we had immunized with a construct expressing p72, or infected with ASFV OURT88/3, were tested against the p72 protein fragments. Three polyclonal sera reacted with the same protein region as mAbs in groups 2 and 3, indicating that the mouse mAbs and pig antibodies recognize similar regions. The results suggests that the mAbs are good candidates as blocking antibody.
Three of the mAbs (85, 8F7-3, and 23) bind to epitopes conserved among at least 10 different p72 genotypes. Moreover, mAb 4A4 is conserved in at least 9 genotypes. These results suggest that they are potential candidates for assay development. The remaining 2 mAbs recognized an epitope not fully conserved (1 aa difference) among those genotypes. The amino acid difference was serine to threonine, which are both amino acids belonging to the same group. Therefore, that change most likely will have minimal or no effect on the binding of the mAbs.
The mAbs produced in our study recognize conserved areas, and could improve the sensitivity of antigen-based and antibody assays by allowing the detection of isolates belonging to a wide range of genotypes. Moreover, the knowledge of their binding epitope permits the identification of antibody pairs with distinct binding sites to be used as capture and detection antibodies in antigen-based assays, which in turn would improve antigen detection. The p72 mAbs produced in our study appear to be good candidates for the development of antigen-capture and blocking ELISAs.
Acknowledgments
We thank Dr. RT Baker (Clinical Genomics Pty Ltd., Australia) for the contribution of the pHUE expression vector.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received financial support for the research, authorship, and/or publication of this article by the Kansas National Bio and Agro-Defense Facility Transition Fund and the Kansas Bioscience Authority through a matching grant to Kansas State University’s Center of Excellence for Emerging and Zoonotic Animal Diseases, Award 15-130. The monoclonal antibody development project was funded by the U.S. Department of Homeland Security under IAA, Award HSHQDC-12-X-00122.
References
- 1. Achenbach JE, et al. Identification of a new genotype of African swine fever virus in domestic pigs from Ethiopia. Transbound Emerg Dis 2017;64:1393–1404. [DOI] [PubMed] [Google Scholar]
- 2. Bastos A, et al. Genotyping field strains of African swine fever virus by partial p72 gene characterization. Arch Virol 2003;148:693–706. [DOI] [PubMed] [Google Scholar]
- 3. Borca MV, et al. African swine fever virus structural protein p72 contains a conformational neutralizing epitope. Virology 1994;201:413–418. [DOI] [PubMed] [Google Scholar]
- 4. Catanzariti, et al. An efficient system for high level expression and easy purification of authentic recombinant proteins. Protein Sci 2004;13:1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dixon LK, et al. African swine fever virus replication and genomics. Virus Res 2013;173:3–14. [DOI] [PubMed] [Google Scholar]
- 6. Esteves A, et al. Two-dimensional analysis of African swine fever virus proteins and proteins induced in infected cells. Virology 1986;152:192–206. [DOI] [PubMed] [Google Scholar]
- 7. Freije JMP, et al. High-level expression in Escherichia coli of the gene coding for the major structural protein (p72) of African swine fever virus. Gene 1993;123:259–262. [DOI] [PubMed] [Google Scholar]
- 8. Kollnberger SD, et al. Identification of the principal serological immunodeterminants of African swine fever virus by screening a virus cDNA library with antibody. J Gen Virol 2002;83:1331–1342. [DOI] [PubMed] [Google Scholar]
- 9. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685. [DOI] [PubMed] [Google Scholar]
- 10. López-Otín C, et al. Mapping and sequence of the gene coding for protein p72, the major capsid protein of African swine fever virus. Virology 1990;175:477–484. [DOI] [PubMed] [Google Scholar]
- 11. Lubisi BA, et al. Molecular epidemiology of African swine fever in East Africa. Arch Virol 2005;150:2439–2452. [DOI] [PubMed] [Google Scholar]
- 12. Pastor MJ, et al. Comparison of two antigens for use in an enzyme-linked immunosorbent assay to detect African swine fever antibody. Am J Vet Res 1990;51:1540–1543. [PubMed] [Google Scholar]
- 13. Salas ML, Andrés G. African swine fever virus morphogenesis. Virus Res 2013;173:29–41. [DOI] [PubMed] [Google Scholar]
- 14. Sanz A, et al. Monoclonal antibodies specific for African swine fever virus proteins. J Virol 1985;54:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sastre P, et al. Development of a duplex lateral flow assay for the simultaneous detection of antibodies against African and Classical swine fever viruses. J Vet Diagn Invest 2016;28:543–549. [DOI] [PubMed] [Google Scholar]
- 16. Sastre P, et al. Development of a novel lateral flow assay for detection of African swine fever in blood. BMC Vet Res 2016;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vidal MI, et al. A solid-phase enzyme linked immunosorbent assay using monoclonal antibodies, for the detection of African swine fever virus antigens and antibodies. J Virol Methods 1997;66:211–218. [DOI] [PubMed] [Google Scholar]