Abstract
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was compared to conventional biochemical testing methods and nucleic acid analyses (16S rDNA sequencing, hippurate hydrolysis gene testing, whole genome sequencing [WGS]) for species identification of Campylobacter isolates obtained from chickens (Gallus gallus domesticus, n = 8), American crows (Corvus brachyrhynchos, n = 17), a mallard duck (Anas platyrhynchos, n = 1), and a western scrub-jay (Aphelocoma californica, n = 1). The test results for all 27 isolates were in 100% agreement between MALDI-TOF MS, the combined results of 16S rDNA sequencing, and the hippurate hydrolysis gene PCR (p = 0.0027, kappa = 1). Likewise, the identifications derived from WGS from a subset of 14 isolates were in 100% agreement with the MALDI-TOF MS identification. In contrast, biochemical testing misclassified 5 isolates of C. jejuni as C. coli, and 16S rDNA sequencing alone was not able to differentiate between C. coli and C. jejuni for 11 sequences (p = 0.1573, kappa = 0.0857) when compared to MALDI-TOF MS and WGS. No agreement was observed between MALDI-TOF MS dendrograms and the phylogenetic relationships revealed by rDNA sequencing or WGS. Our results confirm that MALDI-TOF MS is a fast and reliable method for identifying Campylobacter isolates to the species level from wild birds and chickens, but not for elucidating phylogenetic relationships among Campylobacter isolates.
Keywords: Campylobacter, chickens, matrix-assisted laser desorption/ionization mass spectrometry, wild birds
Introduction
Campylobacter infections are a leading cause of bacterial enterocolitis in humans in North America.31 Approximately 85% of all human cases are caused by Campylobacter jejuni, with most of the remainder involving C. coli.14,15 Humans can be exposed to C. jejuni by handling or ingesting contaminated chicken,3 or by contact with chicken feces.13 As backyard chicken production increases throughout North America, people may be at increased risk of exposure to chicken feces or contaminated meat containing pathogenic organisms.25 In some cases, contamination of backyard poultry flocks might originate through contact with wild animals, particularly wild birds, among which Campylobacter prevalence can be high.8,9,33,34
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is increasingly used as a front-line laboratory tool for bacterial identification and differentiation at the genus, species, and strain level.11 A limited number of earlier studies suggest that MALDI-TOF MS is useful to identify Campylobacter isolated from wild birds to the genus,12 species,6,23 and even the subspecies level.20 One study has suggested that MALDI-TOF MS may be able to separate Campylobacter isolates according to the species of animal that originally shed the bacteria,23 while another study concluded otherwise.20 However, none of these studies utilized MALDI-TOF MS to identify and compare Campylobacter isolates from wild and domestic birds sharing habitat to determine the extent to which they are colonized by similar strains of bacteria and directly compare identification methods.
In our study area in Yolo County, California, backyard chickens overlap and co-mingle with many wild birds, including American crows (Corvus brachyrhynchos), which are known carriers of C. jejuni,34,36 western scrub-jays (Aphelocoma californica), and urban wild turkeys (Meleagris gallopavo). These 3 species overlap in their habitat use with wild and domestic mallard ducks (Anas platyrhynchos), which are also common in the area. We compared MALDI-TOF MS, conventional biochemical testing, 16S ribosomal (r)DNA sequence analysis, hippurate hydrolysis gene testing, and whole genome sequence (WGS) analysis for species-level identification of Campylobacter isolates obtained from chickens and wild birds in Yolo County, California. We also compared the protein dendrograms produced by MALDI-TOF MS to analyses of 16S rDNA and WGS data to see if isolates were classified into similar phylogenomic groups using the different methods.
Materials and methods
Amies clear gel collection swabs (Hardy Diagnostics, Santa Maria, CA) were used to sample feces from live mallard ducks (n = 24), western scrub-jays (n = 44), American crows (n = 88), and peri-urban wild turkeys (n = 31) in Davis, CA, from February to July 2014. Samples from backyard chickens (n = 22) were collected from live birds and from chickens submitted for autopsy to the California Animal Health and Food Safety Laboratory in Davis, CA. All capture and handling activities were conducted under permits from the U.S. Geological Survey Bird Banding Laboratory, California Department of Fish and Wildlife, and University of California, Davis (UC Davis).
Fecal swabs were refrigerated until plated onto Campylobacter CVA agar (Hardy Diagnostics) within 24 h of collection. Plates were incubated at 35°C in microaerophilic conditions (MicroAero AnaeroPak system, Mitsubishi Gas Chemical America, New York, NY) for 72 h, and bacteria from isolated colonies were Gram-stained. The colonies possessing gram-negative curved rods were subcultured onto 5% sheep blood agar (Hardy Diagnostics), and those isolates showing pure growth were subjected to biochemical, MALDI-TOF MS, and PCR testing. The isolates were archived (Microbank bacterial and fungal preservation system porous beads, Pro-lab Diagnostics, Richmond Hill, ON, Canada) and stored at −80°C.
Biochemical testing of isolates tentatively identified as Campylobacter (gram-negative curved rods) included determining resistance to cephalothin (30 μg discs; Becton Dickinson, Franklin Lakes, NJ) and nalidixic acid (30 μg discs; Becton Dickinson), catalase activity (Hardy Diagnostics), and the ability to hydrolyze hippurate (Dalynn Biologicals, Calgary, AB, Canada). Nitrate reduction tests (Mast Diagnostics Mastidiscs ID, Hardy Diagnostics; BioMérieux, Durham, NC) were performed on C. jejuni isolates to differentiate C. jejuni subsp. doylei (negative nitrate reduction) from C. jejuni subsp. jejuni (positive nitrate reduction).32
We used MALDI-TOF MS to identify isolates, and to create new reference spectra (Biotyper solution preparation V.1 instructions, August 29, 2011, Bruker Daltonics, Bremen, Germany) for each procedure. To identify isolates, colonies were spotted in duplicate onto the MALDI-TOF MS target plate, overlaid with 1 μL of 70% formic acid; 1 μL of matrix α-cyano-4-hydroxycinnamic acid (HCCA; Bruker Daltonics) dissolved in 50% acetonitrile and 2.5% trifluoroacetic acid was applied after the formic acid dried. Each MS run included a bacterial test standard (Bruker Daltonics) that contained Escherichia coli and 8 proteins for calibration of the apparatus. The spectra of the isolates were then compared (Real-time Classification software, MALDI Biotyper 3.1, Bruker Daltonics) to reference protein spectra using the default settings, yielding similarity scores that indicated a species-level match (2.3–3.0), a genus-level and probable species-level match (2.0–2.3), a probable genus-level match (1.7–2.0), or no identification (<1.7). To determine similarity, the Bruker software aligned peaks of the spectra, and those peaks with a mass-to-charge ratio difference <250 ppm were considered identical. Then, the software algorithms compared and matched test samples to reference samples in the reference library.6 At the time of our study, there were 22 Campylobacter species reference spectra. To create new reference spectra using our isolates, pure bacterial colonies were suspended in 300 μL of high-performance liquid chromatography–grade water, vortexed, and then 900 μL of ethanol was added and the mixture centrifuged for 2 min at 12,000 × g. The supernatant was discarded, 50 μL of 70% formic acid and 50 μL of acetonitrile were added to the pellet, and the mixture vortexed and then centrifuged at 12,000 × g for 2 min. The supernatant was collected, and a 1-μL sample (n = 8) of each protein extract was added to the target, dried, and HCCA matrix added. Each spot was read 3 times to create 24 protein spectra for each isolate. The spectra were imported (Custom MSP and Library Creation software, Bruker Daltonics; main spectrum profile [MSP]), up to 4 spectra were removed as needed to create the most harmonious combination, and the remaining spectra were combined to form the MSP, all according to the manufacturer’s instructions and default settings.
For 16S rDNA and hippurate hydrolysis gene (hipO) amplification, DNA was extracted from isolates (DNeasy blood and tissue kit, Qiagen, Hilden, Germany), and PCR assays2,5 were performed using primers that amplify the 16S rDNA gene (F: 5’-CTGCAGAGTTTGATCCTGGCTCAG-3’, R: 5’-CGGGTTACCTTGTTACGACTT-3’) and the hipO gene (F: 5’-GAAGAGGGTTTGGGTGGTG-3’, R: 5’-AGCTAGCTTCGCATAATAACTTG-3’; Integrated DNA Technologies, San Diego, CA). For the 16S rDNA gene, 3 μL of the forward and reverse primers (25 pmol/μL), 25 μL of FideliTaq (Affymetrix, Thermo Fisher Scientific, Santa Clara, CA), and 17 μL of PCR-grade water were mixed per reaction. The samples then underwent a PCR protocol of 94°C for 10 min, 35 cycles of 94°C for 1 min, 63.1°C for 1 min and 72°C for 2 min, followed by a single 10-min incubation at 72°C. For the hipO gene amplification, 0.5 μL of forward and reverse primer (50 pmol/μL), 2.5 μL of MgCl2 (Applied Biosystems, Thermo Fisher Scientific), 2.5 μL of 10× buffer (Applied Biosystems, Thermo Fisher Scientific), 4 μL of dNTPs (Invitrogen, Carlsbad, CA), 0.5 μL of Taq polymerase (Invitrogen), 13.5 μL of water, and 1 μL of template DNA were combined per reaction. The samples were then amplified as previously described21 with the cycling conditions modified as follows: 35 cycles of 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min. If no amplification product was obtained, the PCR was repeated to ensure that an error in the original process was not the cause of the lack of product.
The products from the 16S rDNA PCR were adjusted to concentrations of 30 ng/mL and sequenced at the UC Davis DNA Genome Center. The sequences were trimmed to equal length (1,231 bp), and the Basic Local Alignment Search Tool (BLAST) was used to compare each sequence with sequences in GenBank. Species level identification was based on the criteria of ≥99% nucleotide similarity.18 In cases in which isolates had >99% similarity to both C. jejuni and C. coli, species identification was determined by the presence (C. jejuni) or absence (C. coli) of a 735-bp10 product in the hipO PCR described above.
WGS was carried out on 14 samples revived from storage, following previously published protocols.29,35,36 Briefly, isolates were sequenced as part of the 100K Pathogen Genome Project (http://www.100kgenomes.org) in the laboratory of Dr. Bart Weimer (UC Davis). As described previously,22 isolates were checked for purity, genomic DNA (gDNA) was extracted from cultures grown on 5% blood agar plates (UC Davis, VetMed Biological Services, Davis, CA) for 1–2 d, lysed (Agilent Technologies application note, doi:10.13140/RG.2.1.3354.6961, https://goo.gl/N5EVcZ), purified with the QIAamp DNA mini kit (catalog 51306, Qiagen), and analyzed (2200 TapeStation system, Genomic DNA ScreenTape assay, Agilent Technologies, Santa Clara, CA) to ensure gDNA integrity (Agilent Technologies application note, doi:10.13140/RG.2.1.3616.8409, https://goo.gl/VW9a6F). Isolated gDNA was used to construct sequencing libraries (Hyper Plus kit, KR1145 v3.16, Kapa Biosystems, Wilmington, MA) with dual-SPRI size selection (Kong N, et al. Quality control of high-throughput library construction pipeline for KAPA HTP library using an Agilent 2200 TapeStation. Application note. Santa Clara, CA: Agilent Technologies, 2014, https://goo.gl/CxCUQR). Libraries were constructed (Sciclone NGS workstation, Perkin Elmer, Hopkinton, MA). Library quantitation was performed (SYBR FAST qPCR kits, Kapa Biosystems) to ensure the starting concentration of 400 ng and a fragment insert size of 350–450 bp (https://goo.gl/CxCUQR). Libraries were indexed (Weimer 384 TS-LT DNA Barcodes, Integrated DNA Technologies) to allow multiplexing up to 384 isolates in a single sequencing lane. Sequencing was performed at the UC Davis Genome Center (HiSeq 3000 instrument, paired-end 150-bp protocol, Illumina, San Diego, CA; https://goo.gl/CxCUQR; Miller B, et al. A novel, single tube enzymatic fragmentation and library construction method enables fast turnaround times and improved data quality for microbial whole-genome sequencing. Application note. Wilmington, MA: Kapa Biosystems, 2015, https://goo.gl/TC55Wx).
Genome analysis was done as described previously.36 Briefly, paired-end reads were assembled (ABySS 1.5.2 at kappa = 64),5 and annotations were carried out (Prokka pipeline).28 Genomic distances were determined using the Genome-to-Genome Distance Calculator, an in silico DNA-DNA hybridization technique (http://ggdc.dsmz.de/distcalc2.php).4,24 The DDH model “Formula 2” was used as recommended for draft genomes. Distance matrices were built into the Newick tree format using T-REX webserver software using the neighbor-joining method to generate phylogenetic trees.7,27 Trees were edited using Dendroscope 3.0.17 Multilocus sequence typing (MLST) was performed in silico using the Campylobacter MLST database.19 All sequences are available in the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra). Accessions numbers are found in the supplementary material section of this article.
Kappa statistics were calculated (R software v.3.1.1, https://www.r-project.org/) to compare results among biochemical testing, 16S rDNA and hippurate hydrolysis gene testing, and MALDI-TOF MS. We also examined MALDI-TOF MS protein dendrograms, 16S rDNA sequences, and WGS (subset of isolates) to determine if there was agreement in the relationships identified by these phenotypic (protein) and genotypic (16S rDNA and WGS) approaches. The MSPs created for our isolates were used to create minimum spanning trees (dendrograms; Biotyper OTC software, Bruker Daltonics), and Mega 6.6 software was used to calculate percent nucleotide similarities among 16S rDNA sequences.30
Results
Twenty-seven Campylobacter isolates were obtained from chickens (n = 8), American crows (n = 17), mallards (n = 1), and western scrub-jays (n = 1); no isolates were obtained from turkeys. All 27 isolates were identified as either C. jejuni or C. coli using biochemical techniques, MALDI-TOF MS, or 16S rDNA sequencing (Table 1), but species-level assignments varied considerably among the 3 methods, especially between 16S rDNA sequencing and MALDI-TOF MS (p = 0.1573, kappa = 0.0857; poor agreement). Analysis of 16S rDNA sequences using BLAST revealed that 11 of 27 Campylobacter sequences were ≥99% similar to both C. jejuni and C. coli sequences in GenBank. When these 11 isolates were tested by hipO PCR, 9 were determined to be C. jejuni, whereas 2 were C. coli. When the results of 16S rDNA sequencing and hipO testing were combined, the resulting species assignments (C. jejuni: n = 25, C. coli: n = 2) were identical to MALDI-TOF MS (p = 0.0027, kappa = 1), and to the subset of isolates analyzed by WGS (n = 14). All 25 C. jejuni isolates reduced nitrate to nitrite, indicating that they belonged to the subspecies C. jejuni subsp. jejuni, and not C. jejuni subsp. doylei.
Table 1.
Identification of Campylobacter isolates from wild birds and chickens using different methods.
| Method | Total (n =
27) |
Crows (n =
17) |
Chickens (n =
8) |
Scrub jay (n =
1) |
Mallard (n =
1) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| C. jejuni | C. coli | C. jejuni | C. coli | C. jejuni | C. coli | C. jejuni | C. coli | C. jejuni | C. coli | |
| Biochemical testing | 20 | 7 | 14 | 3 | 5 | 3 | 0 | 1 | 1 | 0 |
| MALDI-TOF MS | 25 | 2 | 17 | 0 | 6 | 2 | 1 | 0 | 1 | 0 |
| 16S rDNA sequencing* | 16 | 0 | 14 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| 16S rDNA sequencing plus hipO gene PCR | 25 | 2 | 17 | 0 | 6 | 2 | 1 | 0 | 1 | 0 |
Biochemical tests were catalase testing, susceptibility to nalidixic acid and cephalothin, and hippurate hydrolysis. MALDI-TOF MS = matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.
Eleven of 27 isolates showed >99% similarity to both C. jejuni and C. coli sequences in GenBank.
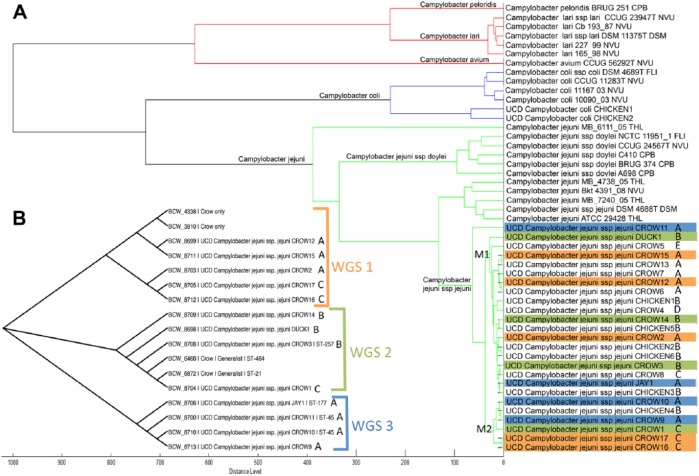
The MALDI-TOF MS dendrogram clades containing our 27 isolates indicated complete agreement with the species assignments described above. Figure 1A shows the MALDI-TOF MS protein dendrogram for the 27 Campylobacter isolates, as well as the Bruker Daltonik reference library (Bruker Daltonics) spectra for C. jejuni, C. coli, C. lari, C. peloridis, and C. avium. The 2 C. coli isolates were most closely related to the reference C. coli samples, whereas the 25 C. jejuni subsp. jejuni isolates were grouped within a single clade containing all of the C. jejuni reference samples. There was some evidence of subdivision within the C. jejuni clade—the C. jejuni subsp. doylei reference spectra formed a single group that did not contain any of our C. jejuni subsp. jejuni isolates, and our isolates all belonged to a subgroup that contained the only available reference spectrum for C. jejuni subsp. jejuni. Our C. jejuni subsp. jejuni isolates were further subdivided into 2 major groups (designated M1, M2), and both of these groups contained isolates from wild birds and chickens.
Figure 1.
A. Dendrogram showing distances and grouping of Campylobacter isolates based on protein phenotypes created using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). The 2 major subclades of C. jejuni subsp. jejuni are designated M1 and M2. Isolates with similar letters (A–E) had identical 16S ribosomal (r)DNA sequences. Color-coding refers to 3 clades seen in the distance matrix tree. B. Phylogenetic relationships of 13 C. jejuni subsp. jejuni isolates determined by whole genome sequence analysis. Three clades are identified (WGS1, WGS2, WGS3) in the distance matrix tree, and isolates with identical 16S rDNA sequences are identified as above (A–E).
The 16S rDNA sequences did not show any correlation to the MALDI-TOF MS dendrogram grouping of isolates. Examination of the 16S rDNA amplicon sequences showed that the 25 C. jejuni subsp. jejuni isolates were classified into 5 groups, with each group containing isolates having identical sequences. These groups were designated A–E (see Table 2 for reference, and Fig. 1 for comparison to the MALDI-TOF MS dendrogram). Although all of the C. jejuni subsp. jejuni isolates from chickens had identical 16S rDNA sequences (group B), there was no apparent relationship between 16S rDNA sequences and the grouping of isolates on the MALDI-TOF MS dendrogram (Fig. 1A). For example, the MALDI-TOF MS clade M1 contained isolates belonging to 16S rDNA sequence groups A–E, and MALDI-TOF MS clade M2 contained groups A–C.
Table 2.
Five groups of 25 Campylobacter jejuni subsp. jejuni isolates that contain isolates with identical 16S ribosomal DNA sequences.
| Group A | Group B | Group C | Group D | Group E |
|---|---|---|---|---|
| CROW2 | DUCK1 | CROW1 | CROW4 | CROW5 |
| CROW6 | CHICKEN1 | CROW8 | ||
| CROW7 | CHICKEN2 | CROW16 | ||
| CROW9 | CHICKEN3 | CROW17 | ||
| CROW10 | CHICKEN4 | |||
| CROW11 | CHICKEN5 | |||
| CROW12 | CHICKEN6 | |||
| CROW13 | CROW3 | |||
| CROW15 | CROW14 | |||
| JAY1 |
Likewise, the dendrogram based on WGS differed substantially from the MALDI-TOF MS dendrogram and 16S rDNA groups of identical sequences. Whole genome sequences were generated for 14 wild bird isolates (13 C. jejuni and 1 C. coli), and phylogenetic analysis of the 13 C. jejuni sequences and the 4 reference sequences yielded a distance matrix tree (Fig. 1B) with 3 distinct lineages or clades (designated WGS1, WGS2, WGS3). Although only a subset of our C. jejuni subsp. jejuni isolates had their complete genomes sequenced, it was clear that there was no relationship between WGS and the grouping of isolates on the MALDI-TOF MS dendrogram. For example, both of the major MALDI-TOF MS clades (M1, M2) contained isolates belonging to whole genome sequence groups WGS1, WGS2, and WGS3. Isolates with identical 16S rDNA sequences were found to belong to separate lineages when their whole genomes were analyzed (Fig. 1B).
MLST genotyping was successful for 7 of the 14 wild bird isolates that were fully sequenced (13 C. jejuni and 1 C. coli isolate; Table 3). Seven sequences were novel alleles or novel allele combinations and had no match within the existing MLST database. These novel sequences have been submitted to the MLST database for inclusion. Sequence types could only be determined for 7 isolates, and 4 of these were further classified into clonal complexes. In addition, the tetO locus was identified in 3 of 13 C. jejuni isolates.
Table 3.
Sequence type, clonal complex, and tetO gene presence in fully sequenced Campylobacter isolates.
| Isolate | Whole genome sequence clade | Sequence type | Clonal complex | tetO |
|---|---|---|---|---|
| CROW2 | WGS1 | 5473 | NAS | − |
| CROW12 | WGS1 | NAS | NAS | + |
| CROW15 | WGS1 | NAS | NAS | − |
| CROW17 | WGS1 | 1224 | NAS | − |
| CROW16 | WGS1 | NAS | NAS | − |
| CROW1 | WGS2 | NAS | NAS | + |
| DUCK1 | WGS2 | NAS | NAS | − |
| CROW3 | WGS2 | 929 | ST-257 | + |
| CROW14 | WGS2 | 1962 | NAS | − |
| JAY 1 | WGS3 | 177 | ST-177 | − |
| CROW9 | WGS3 | NAS | NAS | − |
| CROW10 | WGS3 | 782 | ST-45 | − |
| CROW11 | WGS3 | 782 | ST-45 | − |
| UCD C. coli CHICKEN2 | NA | NAS | NAS | − |
Determined by analysis of full genome sequences of 13 C. jejuni subsp. jejuni isolates and 1 C. coli isolate. NA = not applicable; NAS = not assigned; ST = sequence type; UCD = University of California, Davis.
Discussion
Our major finding was that MALDI-TOF MS accurately identified all 27 Campylobacter isolates from wild birds and chickens to the species level, whereas limited conventional biochemical testing and analysis of 16S rDNA sequences produced inconclusive or inaccurate species assignments, which agreed with previous observations.29,34,36 The major problems with biochemical testing and 16S rDNA sequence analysis were their inability to accurately determine the hippurate hydrolysis gene phenotype (biochemical testing) or to clearly assign to a species (16S rDNA sequencing). Although all C. jejuni possess the hippurate hydrolysis gene, it is well known that the hydrolyzing reaction does not always occur during biochemical testing.1,26 In our study, hippurate hydrolysis gene amplification revealed that 5 of the 7 isolates classified as C. coli based on a negative hippurate hydrolysis test actually possessed the hippurate hydrolysis gene.
Although 16S rDNA gene sequences are widely used in studies of bacterial taxonomy and phylogenetics, their value is somewhat limited when attempting to identify and differentiate closely related strains and species. In particular, there is no universally recognized threshold (i.e., ≥98.5% similarity) for definitive identification of a species based on its similarity to sequences in public databases.18 In our study, 11 of 27 isolates could not be definitively identified as either C. coli or C. jejuni by 16S rDNA sequence analysis, and the isolates were only correctly identified as C. jejuni when hipO PCR testing showed that all 11 isolates possessed the hippurate hydrolysis gene.
The 100% agreement in species assignments among MALDI-TOF MS, sequencing or PCR identification (combined 16S rDNA sequencing and hippurate hydrolysis gene presence), and WGS identification (for a subset of isolates), confirmed that MALDI-TOF MS is highly accurate for C. jejuni and C. coli identification. Furthermore, MALDI-TOF MS can be performed on bacteria isolated from selective media and thus has the advantage of not requiring preemptive knowledge of genus or species prior to testing. Given that the MALDI-TOF MS dendrogram was able to separate C. jejuni subsp. jejuni isolates from the reference spectra for C. jejuni subsp. doylei, it also may be possible to classify C. jejuni isolates to the subspecies level. However, further sampling and analyses are needed to confirm this hypothesis because we did not isolate any C. jejuni subsp. doylei in our study, and there was only a single reference spectrum for which C. jejuni ssp. jejuni was designated in the MALDI-TOF MS database utilized in our study.
Although MALDI-TOF MS accurately identified isolates to the species level, the 2 major subgroups of C. jejuni subsp. jejuni visualized on the protein dendrogram (M1, M2) were not congruent with the clades identified by either 16S rDNA or WGS (Fig. 1). This lack of agreement is likely because of the fact that MALDI-TOF MS MSPs represent a phenotype based on ribosomal proteins and other abundant proteins in the bacterium, whereas sequencing methods identify genotypes based on a DNA fragment (1,231 bp) or whole (1.6 Mbp) genome sequences. Given our results, we conclude that MALDI-TOF MS, similar to analysis of 16S rDNA sequences alone,16 is unlikely to be useful for assessing phylogenetic relationships among isolates of C. jejuni.
MLST analysis has been widely used to genotype bacterial isolates and identify pathogenic phenotypes; however, identification requires that a sample be matched to an isolate or sequence type already in a database.19 Using the PubMLST database, our MLST analysis was able to genotype only half of the fully sequenced wild bird isolates (Table 3). Four clonal complexes were identified, including 2 crow isolates that assigned to clonal complex ST-45, a marker reportedly associated with human pathogens.36
Several studies have shown that Campylobacter is a frequent inhabitant of the gastrointestinal tract of birds8,9,33,34 and, although our study was not designed to estimate the comparative prevalence of Campylobacter, it was striking that isolates were obtained from 36% of backyard chickens (8 of 22), whereas no isolates were obtained from wild turkeys (0 of 31) living in the same area. Likewise, the relatively high number of isolates from American crows (17 of 88, 19%) contrasted with the recovery of a single isolate from a mallard duck (1 of 24, 4%) and a western scrub-jay (1 of 44, 2%). C. coli was only detected in chickens. However, we did not find any relationship between host species and the clustering of C. jejuni subsp. jejuni isolates on the MALDI-TOF MS protein dendrogram. Wild bird and chicken isolates occurred in both C. jejuni subsp. jejuni subgroups (M1, M2; Fig. 1A), suggesting that MALDI-TOF MS phenotypes were not restricted by or limited to particular host species in our study area.
In contrast, there was some evidence for a relationship between host species and Campylobacter genotypes. A WGS analysis36 of Campylobacter isolates from multiple animal hosts found evidence for a crow-adapted clade, as well as a generalist clade that included isolates from wild birds, domestic poultry, and mammals. Analysis of the WGS of a subset of our isolates, including reference sequences from the previous study,36 yielded sequences that could be classified into the previously identified crow-only clade (WGS1), as well as the generalist clade (WGS2; Fig. 1B). The generalist strains have been associated with livestock abortion and human gastroenteritis,36 whereas the strains of C. jejuni in the crow-only clade are yet to be linked to disease in crows or other animal species.
Our results confirm that MALDI-TOF MS is a fast, reliable method for identifying Campylobacter species from wild birds and chickens. Nevertheless, our results suggest that MALDI-TOF MS will not be useful for elucidating phylogenetic relationships among Campylobacter isolates, or identifying strains associated with particular host species. An important caveat is that we performed MALDI-TOF MS without any prior attempt to purify or enhance specific proteins present in our isolates. A more selective protocol might well increase the utility of MALDI-TOF MS for addressing phylogenetic or epidemiologic research questions.
Supplemental Material
Supplemental material, DS1_JVDI_10.1177_1040638718762562 for Comparative analysis of Campylobacter isolates from wild birds and chickens using MALDI-TOF MS, biochemical testing, and DNA sequencing by Samantha J. Lawton, Allison M. Weis, Barbara A. Byrne, Heather Fritz, Conor C. Taff, Andrea K. Townsend, Bart C. Weimer, Asli Mete, Sarah Wheeler and Walter M. Boyce in Journal of Veterinary Diagnostic Investigation
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This project was supported in part by a STAR Fellowship from the UC Davis School of Veterinary Medicine to the first author (SJ Lawton) and by the Agriculture and Food Research Initiative Competitive Grant 2014-67012-21622 to CC Taff from the USDA National Institute of Food and Agriculture. The nitrate testing was supported by Byrne Faculty allotment funds, University of California, Davis. Isolates were sequenced as part of the 100K Pathogen Genome Project in the Weimer lab.
References
- 1. Adzitey F, Corry JA. Comparison between hippurate hydrolysis and multiplex PCR for differentiating Campylobacter coli and Campylobacter jejuni. Trop Life Sci Res 2011;22:91–98. [PMC free article] [PubMed] [Google Scholar]
- 2. Al Amri A, et al. Multiplex PCR for direct identification of Campylobacter ssp. in human and chicken stools. J Med Microbiol 2007;56:1350–1355. [DOI] [PubMed] [Google Scholar]
- 3. Altekruse SF, et al. Campylobacter jejuni—an emerging foodborne pathogen. Emerg Infect Dis 1999;5:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Auch AF, et al. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Standards Genomic Sci 2010;2:117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bang DD, et al. PCR detection of seven virulence and toxin genes of Campylobacter jejuni and Campylobacter coli isolates from Danish pigs and cattle and cytolethal distending toxin production of the isolates. J Appl Microbiol 2003;94:1003–1014. [DOI] [PubMed] [Google Scholar]
- 6. Bessède E, et al. Identification of Campylobacter species and related organisms by matrix assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry. Clin Microbiol Infect 2011;17:1735–1739. [DOI] [PubMed] [Google Scholar]
- 7. Boc A, et al. T-REX: a web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Res 2012;40:W573–W579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Broman T, et al. Campylobacter jejuni in black-headed gulls (Larus ridibundus): prevalence, genotypes, and influence on C. jejuni epidemiology. J Clin Microbiol 2002;40:4594–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Broman T, et al. Diversities and similarities in PFGE profiles of Campylobacter jejuni isolated from migrating birds and humans. J Appl Microbiol 2004;96:834–843. [DOI] [PubMed] [Google Scholar]
- 10. Burnett TA, et al. Speciating Campylobacter jejuni and Campylobacter coli isolates from poultry and humans using 6 PCR-based assays. FEMS Microbiol Lett 2002;216:201–209. [DOI] [PubMed] [Google Scholar]
- 11. De Bruynea K, et al. Bacterial species identification from MALDI-TOF mass spectra through data analysis and machine learning. Syst Appl Microbiol 2011;34:20–29. [DOI] [PubMed] [Google Scholar]
- 12. Dudzic A, et al. Isolation, identification and antibiotic resistance of Campylobacter strains isolated from domestic and free-living pigeons. Br Poult Sci 2016;57:172–178. [DOI] [PubMed] [Google Scholar]
- 13. El-Tras WF, et al. Campylobacter infections in children exposed to infected backyard poultry in Egypt. Epidemiol Infect 2014;28:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friedman CR, et al. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser MJ, eds. Campylobacter. 2nd ed. Washington, DC: American Society for Microbiology, 2000:121–138. [Google Scholar]
- 15. Gillespie IA, et al. A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: A tool for generating hypotheses. Emerg Infect Dis 2002;8:937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hansson I, et al. Identification of nine sequence types of the 16S rRNA genes of Campylobacter jejuni subsp. jejuni isolated from broilers. Acta Vet Scand 2008;50:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huson DH, Scornavacca C. Dendroscope 3: an interactive tool for rooted phylogenetic trees and networks. Syst Biol 2012;61:1061–1067. [DOI] [PubMed] [Google Scholar]
- 18. Janda JM, Abbott SL. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils and pitfalls. J Clin Microbiol 2007;45:2761–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jolley KA, Maiden MCJ. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinform 2010;11:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kolínská R, et al. Species identification of Campylobacter jejuni ssp. jejuni and C. coli by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and PCR. Folia Microbiol (Praha) 2008;53:403–409. [DOI] [PubMed] [Google Scholar]
- 21. Lawson AJ, et al. Detection of Campylobacter in gastroenteritis: comparison of direct PCR assay of faecal samples with selective culture. Epidemiol Infect 1998;121:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ludeke CH, et al. Complete genome sequences of a clinical isolate and an environmental isolate of Vibrio parahaemolyticus. Genome Announc 2015;3:e00216–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mandrell RE, et al. Speciation of Campylobacter coli, C. jejuni, C. helveticus, C. lari, C. sputorum, and C. upsaliensis by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl Environ Microbiol 2005;71:6292–6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meier-Kolthoff JP, et al. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform 2013;14–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mete A, et al. Causes of mortality in backyard chickens in northern California: 2007–2011. Avian Dis 2013;57:311–315. [DOI] [PubMed] [Google Scholar]
- 26. Nakari UM, et al. Correct identification and discrimination between Campylobacter jejuni and C. coli by a standardized hippurate test and species-specific polymerase chain reaction. Eur J Clin Microbiol Infect Dis 2008;27:513–518. [DOI] [PubMed] [Google Scholar]
- 27. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 1987;4:406–425. [DOI] [PubMed] [Google Scholar]
- 28. Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 2014;30:2068–2069.24642063 [Google Scholar]
- 29. Taff CC, et al. Influence of host ecology and behavior on Campylobacter jejuni prevalence and environmental contamination risk in a synanthropic wild bird. Appl Environ Microbiol 2016;82:4811–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tamura K, et al. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol 2011;28:2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tortora GJ, et al. A survey of the microbial world. In: Tortora GJ, et al., eds. Microbiology: An Introduction. 10th ed. San Francisco, CA: Benjamin Cummings, 2010:312. [Google Scholar]
- 32. UK Standards for Microbiology Investigations. Identification of Campylobacter species. London, England: Standards Unit, Microbiology Services, Public Health England, 2015. Document ID 23, issue 3. [Google Scholar]
- 33. Waldenstrom J, et al. Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in different ecological guilds and taxa of migrating birds. Appl Environ Microbiol 2002;68:5911–5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weis AM, et al. Prevalence and pathogenic potential of Campylobacter isolates from free-living, human-commensal American crows. Appl Environ Microbiol 2014;80:1639–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weis AM, et al. Campylobacter jejuni strains that cause abortion in livestock. Genome Announc 2016;4:e01324–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weis AM, et al. Genomic comparison of Campylobacter spp. and their potential for zoonotic transmission between birds, primates and livestock. Appl Environ Microbiol 2016;82:7165–7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS1_JVDI_10.1177_1040638718762562 for Comparative analysis of Campylobacter isolates from wild birds and chickens using MALDI-TOF MS, biochemical testing, and DNA sequencing by Samantha J. Lawton, Allison M. Weis, Barbara A. Byrne, Heather Fritz, Conor C. Taff, Andrea K. Townsend, Bart C. Weimer, Asli Mete, Sarah Wheeler and Walter M. Boyce in Journal of Veterinary Diagnostic Investigation