Abstract
Routine testing of breeding herd oral fluid (OF) samples for porcine epidemic diarrhea virus (PEDV) IgG and/or IgA is used to track levels of PEDV immunity over time. However, OFs contain particles of feed, feces, and inorganic material that detract from the quality of the sample. We clarified swine OF samples using lyophilized chitosan-based formulas (A–C) tested by PEDV IgG and IgA ELISAs. To evaluate both the immediate and residual effects of treatment on antibody detection, samples were tested immediately post-treatment, then stored at 4°C and retested at 2, 4, and 6 days post-treatment (DPT). Formulations were shown to effectively clarify samples. Statistical analysis comparing treated to untreated OF samples at 0 DPT found that neither chitosan nor Tween 20 affected the OF ELISA IgA and IgG sample-to-positive (S/P) ratio results (p > 0.05). Furthermore, pairwise comparisons of 0 DPT to 2, 4, and 6 DPT results detected no significant differences (p > 0.05) in IgA and IgG S/P ratios (i.e., treated OF samples were stable over time). Therefore, chitosan efficiently clarified OF specimens without affecting the results of the PEDV IgG and IgA antibody ELISAs.
Keywords: Antibody, clarification, oral fluid, porcine epidemic diarrhea virus
Swine producers and veterinarians need a practical and efficient method to monitor population immunity in order to know when to take preventive action. Humoral immunity is key to the prevention of clinical outbreaks, and a variety of porcine epidemic diarrhea virus (PEDV; order Nidovirales, family Coronaviridae, subfamily Coronavirinae, genus Alphacoronavirus) and antibody assays have been developed for a variety of specimens (e.g., serum,2,5,9 oral fluid [OF],2 feces,6 and mammary secretions5,10,11,13). In the majority of cases, the focus has been on testing individual animal serum samples, but aggregate specimens, such as OFs, offer specific advantages and have come into common use for a variety of endemic pathogens, including PEDV.12 Swine OF does not inherently contain components that affect antibody-based testing (i.e., processing prior to testing is not mandatory). However, samples collected in the field routinely contain fine particulates (e.g., feces, soil, and feed particles) that potentially affect pipetting accuracy and/or test performance.
In brewing and winemaking, “clarification” using various chemicals removes suspended particles and improves the product. Applying that approach to OFs, we evaluated the effect of chemical clarification of OF specimens on PEDV antibody ELISA (IgG and IgA) responses over time. Three chemical treatments were evaluated using OF samples collected under experimental conditions (study 1) and under field conditions (study 2). In study 1, 7-wk-old PEDV-negative pigs (n = 16) were randomly assigned to negative control (n = 6 pigs, housed 2 pigs per pen) or PEDV-inoculated (n = 10 pigs, housed 2 pigs per pen) treatment groups. Serum samples were collected from all pigs at −7, 0, 3, 7, 10, 14, 17, 21, 28, 35, and 42 days post-inoculation (DPIs). Pen OF samples were collected at −3, 0, 5, 10, 15, 20, 25, 30, 35, and 42 DPI. In study 2, 10-wk-old PEDV-negative gilts (n = 20) housed in one pen were orally inoculated with PEDV-positive feces collected from clinically affected pigs in a commercial production system. Serum samples were collected from all pigs at −4, 0, 7, 14, 21, and 28 DPI. OF samples were collected from the pen at −4, 0, 3, 7, 10, 14, 17, 21, 24, 28, 31, 35, 38, and 42 DPI. OF samples (n = 104) collected in studies 1 and 2 were subdivided into 4 aliquots. Each aliquot was subjected to 1 of 3 chemical treatments, with the fourth aliquot serving as a non-treatment control. All aliquots were tested by PEDV IgG and IgA ELISAs (day 0), then kept at 4°C and tested again at 2, 4, and 6 days post-treatment (DPT).
Studies 1 and 2 were conducted under the approval of the Iowa State University Office for Responsible Research. In study 1, sixteen 7-wk-old pigs acquired from 1 commercial swine farm were housed in the Iowa State University Livestock Infectious Disease Isolation Facility (Ames, IA). Pigs were randomly assigned to 1 of 2 groups (negative control group, n = 6; PEDV-inoculated group, n = 10). PEDV inoculum was prepared as described elsewhere.11 Blood samples (n = 176) were collected at −7, 0, 3, 7, 10, 14, 17, 21, 28, 35, and 42 DPI. OF specimens were collected twice daily (0700 h and 1300 h) at −3, 0, 5, 10, 15, 20, 25, 30, 35 and 42 DPI.
In study 2, twenty 9-wk-old pigs were acquired from one commercial swine farm known to be free of PEDV infection on the basis of routine monitoring. The pigs were housed in one pen in a commercial production facility. To verify their PEDV-negative status, serum and OF specimens were collected from all pigs at −4 DPI and tested by PEDV ELISA and reverse-transcription real-time (RT-rtPCR). The pigs were inoculated with material prepared using clinical specimens collected from PEDV-infected piglets on commercial farms. The field specimens were thawed at room temperature, mixed with 500 mL of phosphate-buffered saline (PBS; 1×, pH 7.4, Sigma-Aldrich, St. Louis, MO), and then sprayed into the nares of each pig for 5 s using a garden sprayer (Chapin, Batavia, NY). Blood samples (n = 160) were collected at −4, 0, 7, 14, 21, 28, 35, and 42 DPI. OF specimens were collected once daily (0830 h) at −4, 0, 3, 7, 10, 14, 17, 21, 24, 28, 31, 35, 38, and 42 DPI.
Isotype-specific (IgG, IgA), in-house–developed, PEDV whole virus–based indirect ELISAs were performed as described elsewhere.10 Each OF sample was divided into 4 aliquots. Each aliquot was treated with 1 of 3 clarification formulations (A–C), with the fourth aliquot serving as an untreated control (D). Formula A consisted of 100 ppm chitosan oligosaccharide lactate (Sigma), 0.1% polysorbate 20 (Tween 20, Sigma), 0.5% bovine serum albumin (BSA; Jackson Immunoresearch, West Grove, PA), and 1 ppm xylene cyanol in PBS (1×, pH 7.4). Formula B was identical to formula A, minus Tween 20. Formula C was identical to formula A, except that it did not include chitosan oligosaccharide lactate. Formulations A and C contained Tween 20 (0.1%), a detergent known to block unoccupied protein-binding sites and dissolve unstable hydrophobic bonds.1,14 However, detergents can also adversely affect the attachment of proteins to polystyrene ELISA plates and/or interfere with antibody binding when used above the optimal working range.3,4,7,8 Therefore, one detergent-free treatment (formula B) was included to detect this response. A blue dye (xylene cyanol, 1 ppm) was added to each formulation in order to readily identify treated OF samples. BSA (0.5%) was added to all formulations to block nonspecific reactions (i.e., improve the specificity of the antigen–antibody reactions).14 Formulas A–C were lyophilized to avoid diluting the samples, minimize environmental contamination, improve stability, and to anticipate the use of this process in the field. This approach was effective (i.e., all formulations went into solution quickly and easily upon addition of OF).
To avoid diluting OF samples, formulations were lyophilized in 5-mL round-bottom polystyrene tubes (Falcon, Radnor, PA). For lyophilization, 1 mL of the formula was aliquoted into a tube, held at −80°C for 24 h, and then lyophilized (FreeZone, Labconco, Kansas City, MO) for 15 h. After lyophilization, tubes were sealed with polyethylene snap-caps (Falcon), and stored at room temperature in a vacuum-sealed plastic bag.
Prior to treatment, OF specimens were thawed by holding at 4°C for 16 h in an environmental chamber (Caron, Marietta, OH) and then 25°C for 2 h. Specimens were treated by adding 1 mL of each sample to 1 tube of each of the 3 formulations (A–C). Treated samples and the untreated control (D) were vortexed for 5 s and then centrifuged at 1,200 × g for 3 min at 4°C. The supernatant was then harvested and tested by PEDV IgA ELISA and PEDV IgG ELISA.
Immediately after initial testing, all OF samples (n = 104) and a subset of serum samples (n = 38; study 2, 0 and 42 DPI) were held at 4°C in an environmental chamber and retested at 2, 4, and 6 DPT. Samples were not vortexed or centrifuged prior to testing.
ELISA IgG and IgA results for serum (n = 38) and OF (n = 104) samples were analyzed for the effect of chemical treatment (OFs) and time (serum and OFs) using commercial software (SAS 9.4, SAS Institute, Cary, NC). The Shapiro–Wilk and Anderson–Darling tests (alpha level 0.05) rejected the assumption of normality for both serum and OF datasets; therefore, a nonparametric approach was utilized.
The effect of time on ELISA-detectable PEDV serum antibody was evaluated by comparing day 0 PEDV IgG and IgA ELISA sample-to-positive (S/P) ratios to results generated after 2, 4, and 6 d of storage at 4°C using the Kruskal–Wallis test. For OFs, the effect of “clean-up” formulation (A–C), and time (0, 2, 4, 6 DPT) on PEDV IgA and IgG ELISA S/P ratios were evaluated by comparing treated sample S/P ratios to time-matched untreated control S/P ratios using the Kruskal–Wallis test. Treatment and DPT were analyzed as fixed effects and sample as a random effect. Thereafter, the Dwass–Steel–Critchlow–Fligner method was used to make pairwise comparisons between combinations of treatments and DPTs. Finally, the effect of “clean-up” formulation (A–C), and time (0, 2, 4, 6 DPT) was evaluated separately for antibody-negative and antibody-positive samples. For this analysis, OF samples collected prior to 7 DPI were considered negative, and OF samples collected after 14 DPI were considered positive.
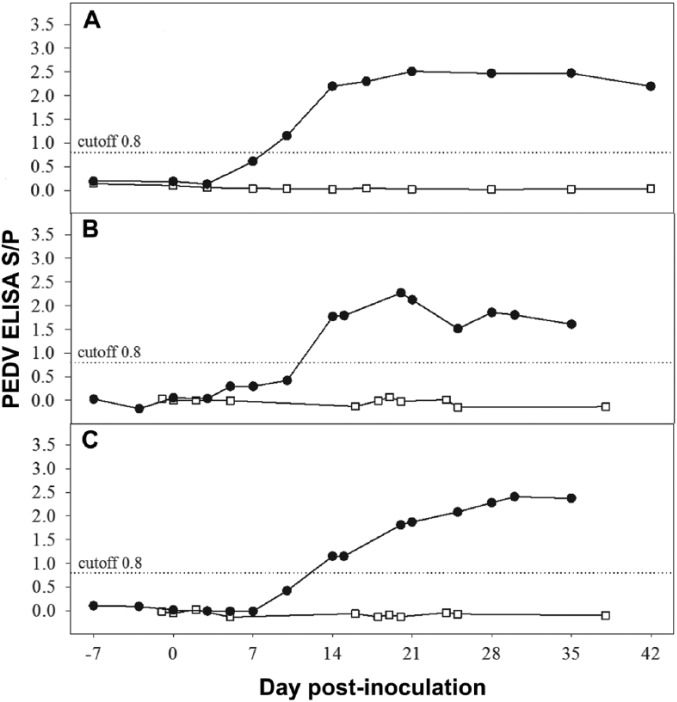
Using a stringent approach, samples with S/P values ⩾0.80 were considered positive in the serum IgG ELISA and the OF IgG and IgA ELISAs.2 All serum samples were negative at ⩽0 DPI, and all negative control animals (study 1) tested negative by PEDV IgG ELISA throughout the study (Fig. 1A). All virus-inoculated pigs tested positive by serum PEDV IgG ELISA at 14 DPI and thereafter.
Figure 1.
Porcine epidemic diarrhea virus (PEDV) isotype-specific antibody (mean ELISA sample-to-positive [S/P] response) by day post-inoculation in serum and oral fluid specimens collected from PEDV-inoculated (•) and negative control (□) groups in study 1: A. serum IgG; B. oral fluid IgG; C. oral fluid IgA.
All OF samples collected from negative control pigs tested negative by PEDV ELISA (IgG and IgA; Fig. 1B, 1C). OF samples from PEDV-inoculated pigs tested negative by PEDV IgG and IgA at ⩽0 DPI, but positive for both IgG and IgA at ⩾14 DPI.
The lyophilized formulations (A–C) were quickly resuspended by the addition of OF. The inclusion of chitosan at 100 ppm was found to effectively clarify OF. OFs treated with formulations A or B were more translucent than controls or OF treated with formulation C (Fig. 2). Analysis of serum IgG and IgA S/P ratios from samples held at 4°C and tested at 0, 2, 4, and 6 DPT did not detect a significant effect of DPT on the results (p > 0.05; Tables 1, 2). Further, pairwise comparisons of 0 DPT versus 2, 4, and 6 DPT results detected no differences (p > 0.05) in serum IgG or IgA ELISA S/P ratios over time. Analysis of OF IgG and IgA S/P ratios found that neither chemical treatment of the sample nor time held at 4°C significantly affected the results (p > 0.05). Pairwise comparisons of 0 DPT to 2, 4, and 6 DPT results detected no differences (p > 0.05) in OF IgG or IgA ELISA S/P ratios over time.
Figure 2.

Oral fluid specimens following treatment. NC = negative control; A = formula A (chitosan 100 ppm, Tween 20 0.1%, BSA 0.5%, xylene cyanol 1 ppm); B = formula B (chitosan 100 ppm, BSA 0.5%, xylene cyanol 1 ppm); C = formula C (Tween 20 0.1%, BSA 0.5%, xylene cyanol 1 ppm).
Table 1.
Effect of treatment and time* on porcine epidemic diarrhea virus IgG ELISA sample-to-positive ratios (mean, SD) on oral fluid specimens.
| Specimen and treatment† | Day post-treatment | |||
|---|---|---|---|---|
| Day 0 | Day 2 | Day 4 | Day 6 | |
| Untreated oral fluid | ||||
| Negative | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.0) | 0.0 (0.0) |
| Positive | 1.4 (1.2) | 1.5 (1.3) | 1.4 (0.1) | 1.1 (0.9) |
| Oral fluid treatment A | ||||
| Negative | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.1) |
| Positive | 1.0 (0.9) | 1.0 (1.0) | 1.1 (1.0) | 0.9 (1.1) |
| Oral fluid treatment B | ||||
| Negative | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.1) |
| Positive | 1.2 (1.1) | 1.4 (1.3) | 1.3 (1.1) | 1.1 (0.8) |
| Oral fluid treatment C | ||||
| Negative | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.1) |
| Positive | 1.0 (0.9) | 1.1 (1.0) | 1.0 (1.0) | 0.9 (0.9) |
| Serum (no treatment) | ||||
| Negative | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.2) |
| Positive | 1.9 (0.5) | 1.8 (0.5) | 1.7 (0.5) | 2.2 (0.7) |
Samples held at 4°C.
Pairwise comparisons of IgG S/P ratios at 0 days post-treatment (DPT) versus 2, 4, and 6 DPT results detected no differences in IgG ELISA S/P ratios over time (p > 0.05).
Table 2.
Effect of treatment and time* on porcine epidemic diarrhea virus IgA ELISA sample-to-positive ratios (mean, SD) on oral fluid specimens.
| Specimen and treatment† | Day post-treatment | |||
|---|---|---|---|---|
| Day 0 | Day 2 | Day 4 | Day 6 | |
| Untreated oral fluid | ||||
| Negative | 0.0 (0.1) | 0.0 (0.1) | −0.1 (0.1) | −0.1 (0.1) |
| Positive | 1.5 (1.1) | 1.4 (1.0) | 1.4 (1.0) | 1.4 (1.1) |
| Oral fluid treatment A | ||||
| Negative | 0.0 (0.1) | 0.0 (0.1) | −0.1 (0.1) | −0.1 (0.1) |
| Positive | 1.3 (0.9) | 1.2 (0.9) | 1.2 (0.9) | 1.2 (1.0) |
| Oral fluid treatment B | ||||
| Negative | −0.1 (0.1) | −0.1 (0.1) | −0.1 (0.1) | −0.1 (0.1) |
| Positive | 1.3 (1.0) | 1.3 (0.9) | 1.2 (1.0) | 1.3 (1.0) |
| Oral fluid treatment C | ||||
| Negative | 0.0 (0.1) | −0.1 (0.1) | −0.1 (0.1) | −0.1 (0.1) |
| Positive | 1.3 (0.9) | 1.2 (0.8) | 1.2 (0.9) | 1.2 (0.9) |
| Serum (no treatment) | ||||
| Negative | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.2) | 0.1 (0.1) |
| Positive | 2.7 (1.1) | 2.4 (1.1) | 2.6 (1.3) | 2.3 (1.0) |
Samples held at 4°C.
Pairwise comparisons of IgA S/P ratios at 0 days post-treatment (DPT) versus 2, 4, and 6 DPT results detected no differences in IgA ELISA S/P ratios over time (p > 0.05).
None of the treatments affected PEDV IgG or IgA ELISA S/P ratios, either in the short term (0 DPT) or over time (2, 4, 6 DPT) when compared to untreated controls (D). The absence of a residual effect of treatment is important because it implies that the treatment could be applied in the field. Likewise, it suggests that veterinary diagnostic laboratories could safely store treated OF samples at 4°C for a short time without affecting testing results.
Future work should verify the applicability of our results to other pathogens and other testing technologies. Given the variety and number of available coagulants, it would also be reasonable to speculate that other coagulants or formulations could provide equal or better results. From a wider perspective, our study should be interpreted as the initiation of a new line of research in OF testing.
Acknowledgments
We thank Drs. Qi Chen, Alexandra Henao, Ting-Yu Cheng, Juan Carlos Mora, Melisa Spadaro, and Elisa Gibert for technical assistance with animal experiments and/or laboratory assays.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was funded in part by Pork Checkoff Funds distributed by the Iowa Pork Producers Association through the National Pork Board (15-142; Des Moines, IA), Zoetis Animal Health (Parsippany, NJ), and the Iowa State University Veterinary Diagnostic Laboratory (Ames, IA).
References
- 1. Batteiger B, et al. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods 1982;55:297–307. [DOI] [PubMed] [Google Scholar]
- 2. Bjustrom-Kraft J, et al. Porcine epidemic diarrhea virus (PEDV) detection and antibody response in commercial growing pigs. BMC Vet Res 2016;12:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Engvall E, Perlmann P. Enzyme-linked immunosorbent assay, ELISA. J Immunol 1972;109:129–135. [PubMed] [Google Scholar]
- 4. Gardas A, Lewartowska A. Coating of proteins to polystyrene ELISA plates in the presence of detergents. J Immunol Methods 1988;106:251–255. [DOI] [PubMed] [Google Scholar]
- 5. Gerber PF, et al. Detection of antibodies against porcine epidemic diarrhea virus in serum and colostrum by indirect ELISA. Vet J 2014;202:33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerber PF, Opriessnig T. Detection of immunoglobulin (Ig) A antibodies against porcine epidemic diarrhea virus (PEDV) in fecal and serum samples. MethodsX 2015;2:368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffman WL, Jump AA. Tween 20 removes antibodies and other proteins from nitrocellulose. J Immunol Methods 1986;94:191–196. [DOI] [PubMed] [Google Scholar]
- 8. Julián E, et al. An ELISA for five glycolipids from the cell wall of Mycobacterium tuberculosis: Tween 20 interference in the assay. J Immunol Methods 2001;251:21–30. [DOI] [PubMed] [Google Scholar]
- 9. Okda F, et al. Development of an indirect ELISA, blocking ELISA, fluorescent microsphere immunoassay and fluorescent focus neutralization assay for serologic evaluation of exposure to North American strains of Porcine Epidemic Diarrhea Virus. BMC Vet Res 2015;11:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poonsuk K, et al. Does circulating antibody play a role in the protection of piglets against porcine epidemic diarrhea virus? PloS One 2016;11:e0153041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poonsuk K, et al. Quantifying the effect of lactogenic antibody on porcine epidemic diarrhea virus infection in neonatal piglets. Vet Microbiol 2016;197:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramirez A, et al. Efficient surveillance of pig populations using oral fluids. Prev Vet Med 2012;104:292–300. [DOI] [PubMed] [Google Scholar]
- 13. Song Q, et al. Characterization of anti-porcine epidemic diarrhea virus neutralizing activity in mammary secretions. Virus Res 2016;226:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Steinitz M. Quantitation of the blocking effect of tween 20 and bovine serum albumin in ELISA microwells. Anal Biochem 2000;282:232–238. [DOI] [PubMed] [Google Scholar]