Abstract
We describe lipid pneumonia in 5 of 24 Arctic foxes (Vulpes lagopus) in association with morbillivirus infection, and lymphoid depletion in 3 of these 5 foxes. Canine distemper virus (CDV) immunohistochemistry yielded positive staining in lung, lymph nodes, spleen, adipose tissue, and renal pelvic urothelial cells in 5 cases. Liver and bone marrow samples collected from these cases tested positive for morbillivirus by reverse-transcription PCR assay. Strains belonged to the CDV Arctic lineage based on sequencing of the hemagglutinin gene followed by phylogenetic analysis. Phylogenetic analysis of the phosphoprotein gene showed that the identified CDV strains were not closely related to any previously documented strains responsible for outbreaks in different animals in other parts of the world.
Keywords: Alaska, Arctic foxes, Arctic lineage, canine distemper virus, novel strain
Morbillivirus has been identified in domesticated and wild carnivores and other non-carnivore species.14 All fox species (Vulpes spp.) are considered susceptible to canine distemper virus (CDV; order Mononegavirales, family Paramyxoviridae, genus Morbillivirus, species Canine morbillivirus) infections, and infected foxes may show abnormal behavior (e.g., loss of fear of humans, disorientation, etc.) and/or respiratory signs. Typical pathologic manifestations in foxes and other carnivores include lymphoid depletion, bronchointerstitial pneumonia, encephalitis, syncytial cell formation, intranuclear and intracytoplasmic inclusion bodies, and secondary bacterial, viral, fungal, and/or protozoal infection as a result of immunosuppression.3 The significance of CDV infection in circumpolar arctic fox (Vulpes lagopus) populations is not well understood, and gross and histopathologic findings of morbillivirus infection in Arctic foxes have not been described; we found no published reports. CDV infections were causally implicated in several large-scale mortality events among Arctic sled dogs, Arctic foxes, and wolves (Canis lupus) in Canada and Alaska.8,16 However, surveillance for CDV in young Arctic foxes from the North Slope of Alaska had detected no antibody-positive animals, whereas comparative studies from Svalbard (Norway) indicate that CDV appears to be widespread in the Arctic fox population in Svalbard with a seroprevalence of 9.6–12.3%.1,2 Interestingly, there are no records of periodic CDV disease outbreaks in arctic foxes in Svalbard, but there are for red foxes (Vulpes vulpes). Although no recent serologic surveillance data for CDV in Alaskan wildlife are available, previous studies suggest that canine morbillivirus is present among northern carnivore populations including wolves and polar bears (Ursus maritimus).11,19
Twenty-four fresh or frozen Arctic fox carcasses (2012, n = 15; 2013, n = 9) were submitted to the North Slope Borough Department of Wildlife Management Utqiagvik (Barrow), Alaska (71.3°N, 156.8°W) for autopsy. Carcasses originated from predator management efforts of Arctic fox culling conducted in May, June, and July within and near the Barrow Steller’s Eider Conservation Planning Area (U.S. Fish and Wildlife Service. 2002. Steller’s Eider Recovery Plan. Fairbanks, AK. Available at: https://www.fws.gov/alaska/fisheries/endangered/pdf/Steller’s%20Eider%20Recovery%20Plan.pdf). Methods of fox removal included trapping and shooting as permitted by the Alaska Department of Fish and Game (ADF&G) under Fox Control Permit 12-093 and amendment 12-093-A1. Arctic foxes were classified as kits (<3 mo), juveniles (<1 y), and adults (>1 y) based on body size measurements and tooth eruption and wear. Autopsies were performed, and a full set of tissues except for brain was collected and fixed in 10% buffered formalin, routinely processed for histopathology, and stained with hematoxylin and eosin. Brains were not examined because the whole head was submitted to the ADF&G for rabies diagnostic testing. Sections of lung were stained with Grocott–Gomori methenamine silver (GMS), Ziehl–Neelsen acid-fast (ZN), and Gram stains.
CDV immunohistochemistry (IHC) was performed on formalin-fixed, paraffin-embedded lung and lymph node from 7 Arctic foxes, including 5 with lipid pneumonia, 1 with mild alveolar histiocytosis, and 1 with no pulmonary histopathologic changes. Sections also included additional organs that were evaluated (Table 1). CDV was identified using a mouse monoclonal antibody IgG2B (VMRD, Pullman, WA).18
Table 1.
Canine distemper virus immunohistochemistry results for arctic foxes (Vulpes lagopus) with lipid pneumonia (n = 5) and without lipid pneumonia (n = 2).
| Case | Lipid pneumonia | Lymphoid depletion | Lung | Lymph node | Spleen | Thymus | Urinary bladder | Kidney | Adipose tissue | Liver |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | No | No | – | NE | – | NE | NE | NE | – | – |
| 2 | Yes | No | + | NE | + | NE | + | NE | + | NE |
| 3 | Yes | No | + | + | + | NE | + | NE | NE | + |
| 4 | No* | No | – | +/– | – | NE | NE | NE | +/– | – |
| 5 | Yes | L, S, T | + | + | NE | + | NE | + | + | NE |
| 6 | Yes | L | + | + | + | + | + | NE | NE | + |
| 7 | Yes | L | + | + | + | NE | NE | NE | NE | NE |
L = lymph node; NE = not examined; S = spleen; T = thymus; – = not stained; + = stained; +/– = suspect positive staining.
This Arctic fox had mild alveolar histiocytosis.
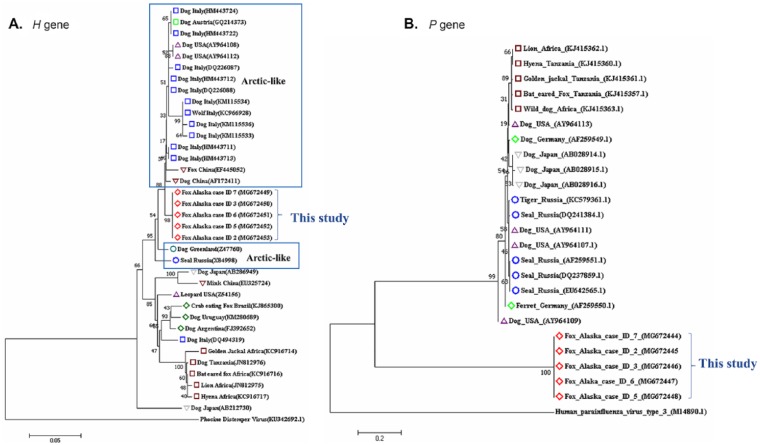
Viral RNA was extracted from liver and bone marrow samples from 5 of 6 foxes (cases 2, 3, 5–7; QIAamp cador pathogen kit, Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Using published primers,10,17 613 bp of the hemagglutinin (H gene) and 300 bp of the phosphoprotein (P gene) were amplified. Reverse-transcription PCR (RT-PCR) reaction to amplify the H gene was carried out (OneStep RT-PCR kit, Qiagen, Hilden, Germany) using 8 μL of template RNA, 5 μL of 5× buffer, 1.2 μL of MgCL2 (25 Mm), 0.4 mM of each dNTP, 0.5 μL of RNase inhibitor 13 U/μL (Promega, Madison, WI), 10 µM of each forward and reverse primer, and 1 μL of enzyme mix. The thermocycling conditions were as follows: 30 min at 50°C then 15 min at 95°C (initial denaturation), followed by 40 cycles at 95°C, 30 s at 50°C, and 30 s at 72°C. Conventional semi-nested RT-PCR was employed to amplify the P gene (Qiagen) utilizing the same reagents and thermocycling conditions described above. PCR products from both H and P genes were extracted and purified from agarose gels (QIAquick PCR purification kit, Qiagen) and submitted to the Georgia Genomic Facility (Athens, GA) for Sanger DNA sequencing. The amplicon identities were confirmed with a BLAST search (http://www.ncbi.nlm.nih.gov/BLAST). The nucleotide sequences obtained in our study were aligned with previously accessioned GenBank sequences of H and P protein from CDV (Fig. 1).
Figure 1.
Phylogenetic relationships among canine distemper virus (CDV) strains based on nucleotide sequences of the hemagglutinin (H) and phosphoprotein (P) genes. A. Phylogenetic tree containing the H gene nucleotide sequences from the Arctic foxes (cases 2, 3, 5–7) investigated in our study and the H gene sequences downloaded from GenBank. Phocine distemper virus was included as the outgroup. B. Phylogenetic relationships among the P gene sequences. Human parainfluenza virus 3 was included as the outgroup. The animal host of each CDV is indicated following the country of origin and the GenBank accessions (in parentheses). Analyses were conducted in MEGA7, and the neighbor-joining method was used to generate the tree. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches.
Phylogenetic trees were inferred for the H and P genes using the neighbor-joining method. Alignment of nucleotide sequences was performed using ClustalW (MEGA7, http://www.megasoftware.net/). Evolutionary distances were computed using the maximum composite likelihood method. The percentage of replicate trees in which the associated taxa clustered using bootstrapping (1,000 iterations) are shown next to the branches. A total of 37 H gene nucleotide sequences and 25 P gene sequences retrieved from GenBank were included in our study. Human parainfluenza virus 3 (family Paramyxoviridae, species Human respirovirus 3) was included as an outgroup in the P gene analysis, and phocine distemper virus (genus Morbillivirus, species Phocine morbillivirus) was included as an outgroup in the H gene analysis to anchor each tree.
The 24 foxes in our study included 4 adult females, 10 adult males, 2 adults of unknown sex, 2 juvenile females and 2 juvenile males, 3 male kits, and 1 female kit. On gross examination, no significant findings were observed. Histopathologic findings included lipid pneumonia (5 of 24; Fig. 2A) and lymphoid depletion (3 of 24). Lymphoid depletion was observed in 3 of 5 foxes with lipid pneumonia (Table 1). Affected lungs had lipid-laden macrophages within ~10–20% of alveolar spaces, with a few lymphocytes and plasma cells. Interstitial changes were mild and were limited to thickening of the alveolar wall and type II pneumocyte hyperplasia. Mild lymphoid depletion with occasional lymphocytolysis was observed in thymus, spleen, and lymph nodes. Syncytial cells were observed in the lymph node of 2 foxes. GMS, ZN, and Gram stains revealed no fungi or bacteria in lung or lymph node. CDV IHC demonstrated antigen in lung (Fig. 2B), mesenteric lymph nodes, spleen, abdominal adipose tissue, and renal pelvic urothelial cells in 5 cases (Table 1). The 5 positive cases were all from individuals culled in 2012 and included 4 kits (3 male, 1 female) and 1 adult female. A similar age predilection was observed during a CDV epizootic in juvenile red foxes in Italy.13
Figure 2.
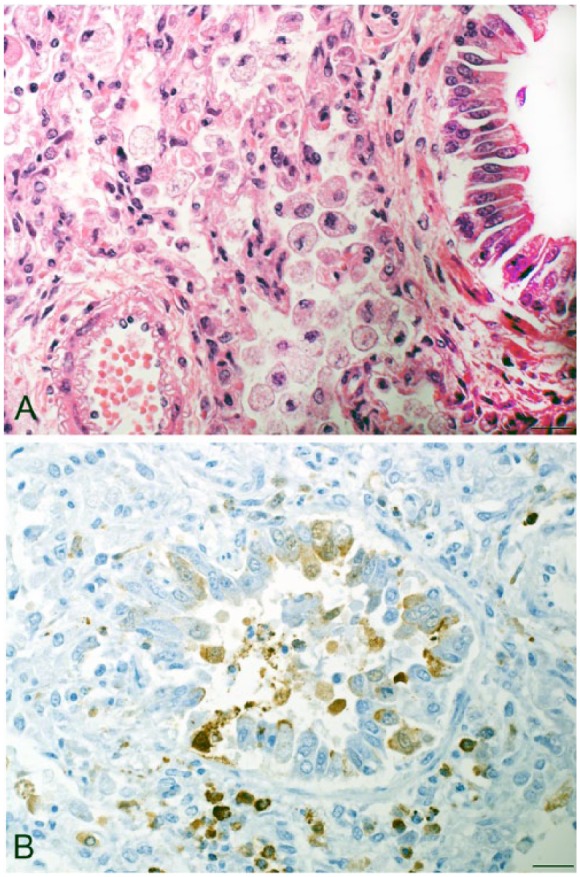
A. Alveolar spaces are filled with foamy macrophages and a few neutrophils in an Arctic fox lung. H&E. Bar = 20 µm. B. Canine distemper virus detected in Arctic fox lung tissue by immunohistochemistry. Brown granules indicate morbillivirus antigen. Bar = 20 µm.
The finding of morbillivirus-positive adipocytes is unusual. No positive staining was observed in the IHC negative controls or in the Arctic fox lacking lipid pneumonia. Although not a natural route of infection, morbillivirus nucleocapsids were present within adipocytes at a site of live measles vaccine administration in humans.5 Interestingly, adipose tissue has been postulated to be an underappreciated viral niche for chronic human immunodeficiency virus infection.6,15 Although not confirmed, viral tropism could exist for metabolically active adipocytes (e.g., brown adipose tissue in young Arctic mammals).
Lipid pneumonia is observed in terrestrial species including domesticated cats and foxes.9 In the foxes that we examined, lipid pneumonia was not severe and could have been an incidental finding. However, the presence of rare syncytial cells and lymphoid depletion were not expected findings. There are rare reports of CDV-associated lipid pneumonia, with a single report in a genet (Genetta genetta).12 Based on our results, evaluation of the clinical significance of lipid pneumonia in terrestrial wildlife may need to be coupled with findings in lymphoid organs and with findings of syncytial cells.
Molecular detection and sequencing of the H and P genes confirmed the presence of CDV in both liver and bone marrow samples. The 5 amplified sequences were 99% homologous to the CDV H and P gene sequences available in GenBank and 100% identical to each other. The H gene sequences identified from the Alaskan foxes in our study clearly fell within the Arctic lineage. These sequences were more closely associated with CDV strains isolated from a dog and a fox from China, a dog in Greenland, and from Baikal seals (Pusa sibirica) in Russia. The Arctic CDV lineage causes clinical disease most commonly in domesticated carnivores; only 2 reports document outbreaks among wild wolves in Italy and wild foxes in China.4,7,20 Phylogenetic analysis of the P-gene fragment from our viral samples identifies a very distinct clade that was not closely related to any previous sequences retrieved from GenBank.
Our results indicate that the CDV strains from the Arctic foxes are different from other strains identified in animal hosts in different parts of the world. The origin of the strain of CDV infecting these foxes remains unclear. Future studies of CDV in Arctic fox populations are needed to improve our understanding of the Arctic marine–terrestrial cycle of morbillivirus, tissue tropism, and the role that CDV Arctic lineage infection plays in natural morbidity and mortality of Arctic foxes.
Acknowledgments
We thank Cyd Hanns, Greta Krafsur, Katie Kokx, Robert Sarren, and Billy Adams for technical assistance, and the personnel of the U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by National Petroleum Reserve-Alaska funds (10-NPRA-14) made available through the State of Alaska Department of Commerce, Community, and Economic Development.
References
- 1. Akerstedt J, et al. Serosurvey for canine distemper virus, canine adenovirus, Leptospira interrogans, and Toxoplasma gondii in free-ranging canids in Scandinavia and Svalbard. J Wildl Dis 2010;46:474–480. [DOI] [PubMed] [Google Scholar]
- 2. Ballard WB, et al. Rabies and canine distemper in an arctic fox population in Alaska. J Wildl Dis 2001;37:133–7. [DOI] [PubMed] [Google Scholar]
- 3. Beineke A, et al. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet Immunol Immunopathol 2009;127:1–18. [DOI] [PubMed] [Google Scholar]
- 4. Beineke A, et al. Cross-species transmission of canine distemper virus—an update. One Health 2015;1:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buck BE, et al. Measles vaccine panniculitis subsequent to vaccine administration. J Ped 1982;101:366–373. [DOI] [PubMed] [Google Scholar]
- 6. Deamouche A, et al. Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog 2015;11:e1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Sabatino D, et al. Arctic lineage-canine distemper virus as a cause of death in Apennine wolves (Canis lupus) in Italy. PLoS One 2014;9:e82356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elton C. Epidemics among sledge dogs in the Canadian arctic and their relation to disease in the arctic fox. Can J Res 1931;5:673–692. [Google Scholar]
- 9. Guarda F, et al. Lipid pneumonia in the red fox (Vulpes vulpes). IBEX 1997;4:13–16. [Google Scholar]
- 10. Guo L, et al. Phylogenetic analysis of the haemagglutinin gene of canine distemper virus strains detected from giant panda and raccoon dogs in China. Virol J 2013;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirk CM, et al. Morbillivirus and Toxoplasma exposure and association with hematological parameters for southern Beaufort Sea polar bears: potential response to infectious agents in a sentinel species. Ecohealth 2010;7:321–331. [DOI] [PubMed] [Google Scholar]
- 12. Lopez-Peña M, et al. Canine distemper in a genet (Gennetta gennetta) associated with endogenous lipid pneumonia. J Comp Pathol 2010;124:207–211. [DOI] [PubMed] [Google Scholar]
- 13. Martella V, et al. Canine distemper epizootic among red foxes, Italy, 2009. Emerg Infect Dis 2010;16:2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez-Gutierrez M, Ruiz-Saenz J. Diversity of susceptible hosts in canine distemper virus infection: a systematic review and data synthesis. BMC Vet Res 2016;12:12–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pallikkuth S, Mohan M. Adipose tissue: sanctuary for HIV/SIV persistence and replication. Trends Microbiol 2016;23:748–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rausch R. On the status of some arctic mammals. Arctic 1953;6:91–147. [Google Scholar]
- 17. Sierra E, et al. Retrospective study of etiologic agents associated with nonsuppurative meningoencephalitis in stranded cetaceans in the Canary Islands. J Clin Microbiol 2014;52:2390–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stone BM, et al. Fatal cetacean morbillivirus infection in an Australian offshore bottlenose dolphin (Tursiops truncatus). Aust Vet J 2011;89:452–457. [DOI] [PubMed] [Google Scholar]
- 19. Zarnke RL, et al. Serologic survey for selected disease agents in wolves (Canis lupus) from Alaska and the Yukon territory, 1984–2000. J Wildl Dis 2004;40:632–638. [DOI] [PubMed] [Google Scholar]
- 20. Zhao JJ, et al. Phylogenetic analysis of the haemagglutinin gene of canine distemper virus strains detected from breeding foxes, raccoon dogs and minks in China. Vet Microbiol 2010;140: 34–42. [DOI] [PubMed] [Google Scholar]