Abstract
We compared 3 major cross-match (XM) tests to identify dog erythrocyte antigen (DEA) 7 blood incompatibilities in dogs as a result of anti–DEA 7 antibodies: gel (GEL), standard tube (TUBE) agglutination, and immunochromatography strips (STRIP). Blood samples from 42 dogs were typed for DEA 7; 2 tested DEA 7–positive (DEA 7+). The 40 DEA 7–negative (DEA 7–) plasma samples were cross-matched against the 2 DEA 7+ and 3 DEA 7– red blood cell (RBC) samples by GEL to identify samples with anti–DEA 7 antibodies. Twenty DEA 7– plasma samples without and with anti–DEA 7 antibodies were cross-matched with samples of the 2 DEA 7+ RBCs in a double-blind fashion using the TUBE and STRIP XM methods. GEL results were used as the reference method for comparison. To determine relationships between results, 2 × 2 tables were used. Cohen kappa coefficient (κ) was calculated between results of GEL and the other 2 methods. With GEL, 21 of 40 XM tests were positive and 19 of 40 negative for anti–DEA 7 antibodies. The same results were obtained by TUBE, whereas only 1 of 40 XM tests was positive by STRIP. There was a statistically significant relationship between results of GEL and TUBE (p < 0.000) with perfect agreement (κ = 1.000), but not between GEL and STRIP results (p = 1.000) in which agreement was equivalent to chance (κ = 0.0453). The GEL and TUBE XM tests, but not STRIP, are useful methods for identification of DEA 7 incompatibilities caused by anti–DEA 7 antibodies.
Keywords: Anti–DEA 7 antibodies, blood typing, canine, cross-matching, gel, immunochromatography, tube
Introduction
Blood typing and cross-matching are serologic tests designed to determine compatibility between blood donor and recipient. The standard tube method (TUBE) is the most widely reported technique for cross-match (XM) testing in veterinary patients in both past and recent studies.1,5,10 However, its specificity, sensitivity, accuracy, and positive and negative predictive values are not known, to our knowledge. In addition, this method has some limitations, including how to interpret weak agglutination reactions and subjectivity in performing and reading tests. In human medicine, gel column technology (GEL)8 appears be more sensitive than the TUBE assay.20 GEL testing provides greater standardization of laboratory techniques, reading of the final reaction is more objective, and GEL testing is considered superior to TUBE methods in human transfusion medicine, especially for identifying antibodies to red blood cells (RBCs).11 Many previous studies in veterinary medicine have also used this method instead of TUBE XM techniques.2–4,9,15 A novel point-of-care immunochromatographic strip (STRIP) kit (Canine LabTest crossmatch XM, Alvedia, Limonest, France), based on an immunochromatographic technology, has been introduced for cross-matching in dogs, and may detect canine XM incompatibilities as accurately as neutral GEL technology when enhanced with the use of canine antiglobulin.4
Naturally occurring canine antibodies against dog erythrocyte antigen (DEA) 3, 5, and 7 have been identified.5 The prevalence of naturally occurring antibodies to DEA 7 in DEA 7–negative (DEA 7–) dogs has been reported in up to 50% of dogs tested;5,15,16 however, the clinical importance of such antibodies is undetermined because a transfusion reaction or reduced survival of incompatible RBCs has not been demonstrated. Given the paucity of information on the clinical importance of anti–DEA 7 antibodies and the inability to determine the DEA 7 status of blood recipients given the lack of a commercial test, it would be useful to know if the XM tests available in dogs can identify blood incompatibility as a result of anti–DEA 7 antibodies in a DEA 7– blood recipient. This could also be useful for future research to identify and study anti–DEA 7 antibodies.
We compared the ability of neutral GEL column, standard TUBE agglutination, and immunochromatographic STRIP XM tests to identify DEA 7 blood incompatibilities caused by anti–DEA 7 antibodies in canine blood samples. Our hypothesis was that all 3 XM methods are equally valid for identification of DEA 7 blood incompatibility caused by anti–DEA 7 antibodies.
Our prospective observational in vitro study was performed using canine whole blood samples collected from 42 Spanish Greyhounds (Galgos) living in a shelter in South Madrid, Spain in April 2017. No information was known about health status and transfusion history in any of these dogs. All blood samples were originally collected as part of a health program evaluation of Galgos available for adoption with signed informed consent from the director of the shelter. Surplus blood from the health program samples was utilized for our study. Based on the University of Milan animal use regulations, formal ethical approval was not needed because dogs were sampled with the informed consent of the director of the shelter as part of a health program for adoption.
Jugular venipuncture was performed using a 20-gauge needle. Four mL of blood was collected into an EDTA tube (Vacutainer K2 EDTA, Becton, Dickinson, Franklin Lakes, NJ) and 2.7 mL into a sodium citrate anticoagulant solution (Vacutainer CITRATE, Becton, Dickinson). Blood typing was performed within 48 h on EDTA-anticoagulated whole blood stored at 4–6°C. EDTA whole blood stored for 48 h at 4–6°C was then centrifuged at 2,183 × g (ALC 4222 centrifuge, A.L.C. International, Milan, Italy) to obtain EDTA plasma and RBCs to use in testing for anti–DEA 7 antibodies using GEL. Samples anticoagulated with citrate were centrifuged at 2,183 × g (ALC 4222 centrifuge) and stored at 4–6°C for up to 48 h. Citrated plasma and RBCs were used to detect anti–DEA 7 antibodies using TUBE and STRIP XM tests.
DEA 1 blood type was determined on EDTA whole blood with STRIP using a monoclonal antibody (Canine LabTest DEA 1 blood typing, Alvedia) following the manufacturer’s guidelines and as described previously.13 DEA 4 and 7 blood types was determined on EDTA RBCs by agglutination in a neutral GEL column as described previously7,15 using polyclonal anti–DEA 4 and 7 antisera (Canine DEA 4 and DEA 7 antisera, Animal Blood Resources International, Stockbridge, MI) imported for use in Italy with the authorization of the Italian Health Minister (protocol authorization 0024135-23/09/2015-DGSAF-COD_UO-P). All samples tested DEA 4+ with strong agglutination reactions (4+). Two of 42 (5%) dogs tested DEA 7+, and 40 of 42 (95%) dogs were DEA 7– (Table 1).
Table 1.
Blood types of EDTA whole blood samples in 42 dogs. Dog erythrocyte antigen (DEA) 1 was evaluated with an immunochromatographic STRIP technique using monoclonal antibodies. DEA 4 and 7 was determined with neutral GEL column agglutination using polyclonal antisera. All samples tested DEA 4+ with 4+ agglutination.
| DEA 1 | DEA 7+ |
DEA 7– |
Total (n) | |
|---|---|---|---|---|
| AS ⩾2+ | AS = 0 | AS = 1+ | ||
| DEA 1+ (n = 18) | 2 | 16 | 0 | 18 (43) |
| DEA 1– (n = 24) | 0 | 22 | 2 | 24 (57) |
| Total (n) | 2 (5) | 38 (90) | 2 (5) | 42 (100) |
– = negative; + = positive; AS = agglutination strength. Numbers in parentheses are percentages.
To identify dogs with anti–DEA 7 antibodies, EDTA plasma from all 40 DEA 7– dogs was cross-matched using the GEL XM test against the 2 EDTA RBC samples of DEA 7+ dogs (80 XM tests total). Samples with positive XM tests were then cross-matched against 3 DEA 7– RBC samples. The GEL XM test was considered the standard reference XM method to identify antibodies because it has been the accepted methodology for canine XM assessments.2–4 The GEL XM test was performed as described previously.2,4,14–16 Briefly, 0.8% RBC-LISS (low-ionic strength solution, Bio-Rad ID-Diluent 2, DiaMed Microtyping System, Cressier, Switzerland) suspensions were prepared from the 2 DEA 7+ EDTA RBC samples and from 3 DEA 7– EDTA RBC samples. Fifty μL of this 0.8% RBC-LISS suspension and 25 μL of EDTA plasma from each DEA 7– sample were mixed in the reaction chamber of the neutral gel column (Bio-Rad ID-Cards, NaCl, Enzyme test and cold agglutinins, DiaMed) and incubated at 37°C for 15 min in a special incubator (ID-Incubator 37S I, DiaMed). Gel columns were centrifuged (ID-Centrifuge 24S II, DiaMed) using a preset cycle (80 × g for 10 min) and macroscopically examined for agglutination strength. Positive agglutination reactions were graded from 1+ to 4+ according to the manufacturer’s instructions. Reactions ⩾1+ were considered positive.2,14,16
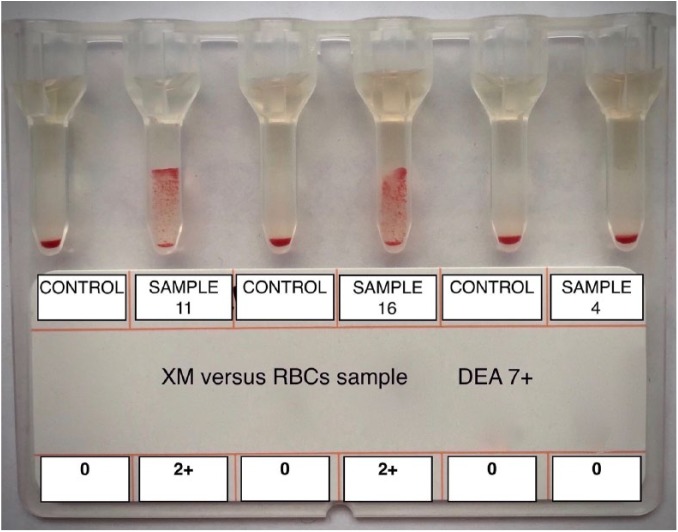
Samples that had ⩾1+ agglutination with 2 DEA 7+ RBCs samples, but not with the 3 DEA 7– RBCs samples, were classified as containing anti–DEA 7 antibodies6 and therefore were identified as samples with incompatible, or positive, cross-matching with the GEL test. Samples that had no agglutination with DEA 7+ RBCs were classified as containing no anti–DEA 7 antibodies (GEL XM negative or compatible). Auto-controls of DEA 7– plasma incubated with its own RBC suspension were also performed with each GEL XM test to exclude autoagglutination (Fig. 1).
Figure 1.
Example of canine cross-match with GEL column agglutination technology. Plasma samples 11 and 16 tested positive, or incompatible, versus dog erythrocyte antigen (DEA) 7+ red blood cells (RBCs) with 2+ agglutination. Plasma sample 4 tested negative, or compatible, given that no agglutination was evident in gel column. All auto-controls (DEA 7– plasma vs own RBCs) tested negative (absence of autoagglutination).
Of 80 XM tests performed with GEL, 21 were positive and 59 negative. All auto-controls were negative for autoagglutination.
All positive GEL XM tests (n = 21) and a similar number of randomly chosen negative GEL XM tests (n = 19) were repeated in a double-blind fashion using both TUBE and STRIP methods for comparison. The major TUBE XM method was performed as described previously.19 The major cross-matching was performed by washing DEA 7+ erythrocytes from the citrate tubes 4 times with physiologic saline. After washing, cells were resuspended in saline to a final concentration of 4%. In 12 × 75 mm glass tubes (PYREX tube borosilicate glass, Corning Inc., Corning, NY), 2 drops of DEA 7+ RBC suspension were combined with 2 drops of DEA 7– plasma. All tubes were incubated at 37°C for 15 min, and after standard centrifugation at 1,100 × g for 15 s (ALC 4222 centrifuge), the tubes were examined for signs of hemolysis and for macroscopic and microscopic agglutination. Any degree of hemolysis and/or macro- or microscopic agglutination was considered a positive result and therefore an incompatible cross-match.
A regular non–antiglobulin-enhanced XM test using the STRIP method was performed according to the manufacturer’s instructions as described previously.4 Three drops of suspension buffer 1, ~10 μL of packed RBCs from the DEA 7+ sample collected with a strip, and 3 drops of plasma of a DEA 7– sample were placed into a 3-mL tube. The suspension was gently mixed, and, after incubation at room temperature for 10 min, the RBCs were washed twice with washing suspension solution and centrifuged at 1,000 × g for 2 min. Then, 2 drops of migration buffer 2 were added to the pellet, and the suspension was gently mixed. The tip of the chromatographic strip was placed into the RBC suspension for 2–5 min to allow the RBC suspension to diffuse to the top of the strip. After incubation, the strip was removed, and the banding pattern was read immediately. The strip was impregnated with different antibodies at 2 levels 1 cm apart to form the following bands: a general positive control anti-glycophorin antibody (labeled “C”) that binds to all canine RBCs, and the anti-canine antiglobulin at the testing site (labeled “XM”) binding only RBCs coated with immunoglobulin (Ig)G, IgM, complement C3, or some combination of these. A positive or incompatible cross-match between RBCs and the plasma tested was identified when a red band of any intensity, other than the control band, was identified on the strip.
Separate investigators performed the GEL, TUBE, and STRIP XM tests in order to limit bias. Each investigator was blinded to the results of the other methods until the results were completed and interpreted.
To determine the relationship between results obtained with various methods, 2 × 2 tables were used. A p value ⩽0.05 was considered statistically significant. A Cohen kappa coefficient (κ) was calculated using a clinical research calculator (MedCalc Software v.16.4.3, https://www.medcalc.org/) with 95% confidence interval (95% CI), to evaluate the agreement beyond chance between results of GEL and other methods.
GEL test results were the same as the TUBE XM test results, with positive or incompatible results observed in 21 of 40 (52%) tests, moderate agglutination reaction (2+) on microscopic evaluation in 6 of 40 tests, hemolysis in 11 of 40, agglutination (2+) and hemolysis in 4 of 20 tests (Table 2). A negative or compatible cross-match was present in 19 of 40 (48%) tests. Only 1 of 40 tests had a weak-positive reaction using the STRIP test (Table 2).
Table 2.
Results of GEL, TUBE, and immunochromatographic STRIP cross-matching (XM) techniques using DEA 7– canine plasma samples against two DEA 7+ canine erythrocyte samples (A and B). Agglutination in GEL and agglutination and/or hemolysis reaction using the TUBE test indicate the positive, or incompatible, results.
| DEA 7– plasma sample | XM technique | |||||||
|---|---|---|---|---|---|---|---|---|
| GEL |
TUBE |
STRIP |
||||||
| DEA 7+ RBC sample A | DEA 7+ RBC sample B | DEA 7+ RBC sample A | DEA 7+ RBC sample B | DEA 7+ RBC sample A | DEA 7+ RBC sample B | |||
| Macro | Micro | Macro | Micro | |||||
| 1–9 | – | – | – | – | – | – | – | – |
| 10 | 1+ | 1+ | Hemolysis | – | Hemolysis | – | – | – |
| 11 | 1+ | 2+ | Hemolysis | – | Hemolysis | – | – | – |
| 12 | 2+ | 2+ | Hemolysis | – | Hemolysis | – | – | – |
| 13 | – | 1+ | – | – | Hemolysis | – | – | – |
| 14 | 1+ | 1+ | – | Agglutination | – | Agglutination | – | – |
| 15 | 1+ | 1+ | Hemolysis | – | Hemolysis | Agglutination | – | + |
| 16 | 1+ | 1+ | – | Agglutination | – | Agglutination | – | – |
| 17 | 1+ | 1+ | Hemolysis | Agglutination | Hemolysis | – | – | – |
| 18 | 1+ | 1+ | Hemolysis | Agglutination | Hemolysis | – | – | – |
| 19 | 1+ | 1+ | Hemolysis | Agglutination | Hemolysis | – | – | – |
| 20 | 1+ | 1+ | – | Agglutination | – | Agglutination | – | – |
– = negative; + = positive; DEA = dog erythrocyte antigen; macro = macroscopic evaluation; micro = microscopic evaluation; RBC = red blood cells; XM = cross-matching. All agglutination reactions using the TUBE XM technique were 2+. Agglutination using the GEL XM technique: 1+ = very few RBC agglutinates were dispersed in the lower part of the gel, with most RBCs at the bottom of the tube; 2+ = all RBCs were agglutinated and dispersed in the gel.
There was a statistically significant relationship between the results of GEL and TUBE tests (p < 0.000), but not between GEL and STRIP results (p = 1.000). Agreement quantified by Cohen kappa had perfect agreement (κ = 1.000, 95% CI: 1.000–1.000) for comparison of TUBE to GEL, but agreement equivalent to chance (κ = 0.0453; 95% CI: –0.0427 to 0.133) between GEL and STRIP tests.
Our data indicate that the GEL XM is equivalent to the conventional TUBE XM for detection of anti–DEA 7 antibodies, as previously demonstrated for other antibodies in human medicine20 and to identify compatibility in equine medicine.9 GEL was simple to perform and required no technical skill, thereby overcoming the practical difficulties of performing TUBE XM. In fact, unlike agglutination in the traditional TUBE method, the GEL test reactions are stable, allowing observation or review for up to 3 days. The average time required for a single compatibility test by the conventional TUBE method was ~60 min, whereas the time required for the GEL and STRIP tests used in our study was ~30 min, which is an advantage in cases of emergency blood transfusion. Other advantages include the decreased sample volume needed for testing. The major disadvantage of the GEL technology is the need to purchase the special centrifuge to accommodate the microtube cards used for testing.
In line with many previous studies of typing blood transfusion recipients,3,4,7 we considered a 1+ agglutination reaction a negative result for blood typing, whereas the same grade of reaction was considered positive for antibody screening. We believe this interpretation protects the blood recipient, given that 1+ agglutination is a weak reaction that in some instances might be nonspecific. If the blood recipient is considered negative for the blood type that produced a 1+ reaction, the recipient will never receive blood containing that RBC antigen and therefore should not become sensitized to that antigen. Conversely, when antibody screening donors, a weak reaction could lead to partial destruction of the incompatible-transfused RBCs; therefore a weak 1+ agglutination result should be considered a positive result. The opposite logic is required for blood donor screening. Results with GEL XM in our study were never higher than 2+ agglutination, and the majority of reactions had 1+ agglutination, but these were simple to interpret despite weak agglutination.
The majority of TUBE XM positive results in our study were concordant with GEL XM results only if results of microscopic evaluation of the TUBE method were considered. The standard major TUBE XM method was performed as described previously,19 and samples were evaluated macro- and microscopically for agglutination. Any degree of microscopic agglutination was considered positive, as reported in previous studies.1,18 However, some authors and laboratories (both for horses and for small animals) have chosen to forgo the microscopic cross-match evaluation.9,12 This decision was based on the high accuracy of microscopic tube agglutination compatibility scores when compared to standard tube macroscopic agglutination, indicating that gross or macroscopic evaluation is adequate for determining compatibility and that microscopic evaluation may not be necessary.9 However, when using TUBE agglutination, weak macroscopic reactions are difficult to interpret, and results are subjective. We believe that it is acceptable to forgo microscopic evaluation of the TUBE agglutination method when testing for DEA 1, DEA 4, or Dal (Dalmatian) blood type–incompatible, transfusion-induced antibodies, which have been demonstrated to be high agglutinating and reacting antibodies responsible for acute hemolytic transfusion reactions.1,10 In addition, veterinary textbooks19 report the absence of macro- or microscopic agglutination as negative reactions. As anti–DEA 7 antibodies are weak agglutinating antibodies,14 omitting microscopic evaluation could lead to false-negative results, as demonstrated in our study.
In addition, several positive cross-matches detected by the TUBE technique were based on the presence of hemolysis rather than agglutination. This result was in contrast with a previous study on characterization of anti–DEA 7 antibodies using the GEL column technique in which anti–DEA 7 antibodies did not have any hemolytic activity.14 However, the GEL method was not designed for detection of hemolysins in any species.9
The STRIP technique is an innovative and entirely new approach to immunohematology, which has recently proven valuable for dogs13 and for canine cross-matching when antiglobulin-enhanced.4 In a previous report,4 and in our experience, the STRIP test was simple to perform for cross-matching, but the positive bands were weak and interpretation was difficult.
A 2017 study demonstrated that a canine-specific, antiglobulin-enhanced XM test utilizing a neutral GEL column technique and the immunochromatographic STRIP technique, as used in our study, gave entirely concordant results in identifying incompatible and compatible cross-matching in post-transfusion alloimmunized canine blood recipients.4 However, a direct comparison with the regular non–antiglobulin-enhanced XM tests was not performed in that study. To our knowledge, a formal validation study has never been performed for the immunochromatographic STRIP XM method except by the manufacturer. The same study4 detected no naturally occurring anti–DEA 7 alloantibodies in DEA 7– dogs using the immunochromatographic STRIP method and an antiglobulin-enhanced GEL cross-match. This result was different in our study in which 52% of DEA 7– tested plasma samples had anti–DEA 7 antibodies identified only by GEL and TUBE XM tests with weak reactions. This may be explained by the low sensitivity of the STRIP XM technique for detection of weak reactive alloantibodies. In addition, the prevalence of naturally occurring anti–DEA 7 antibodies could be geographical or breed related, with Galgo and Italian Corso dogs having a relatively high prevalence of naturally occurring anti–DEA 7 antibodies.15,16 We had no knowledge of any prior transfusion history in the dogs in our study, hence it is possible that some DEA 7– dogs had been transfused with DEA 7+ blood, resulting in the production of anti–DEA 7 antibodies that could not be considered “naturally occurring.”
Our study has a number of limitations. First, we included only a small number of samples, and there were only 2 DEA 7+ samples, which undermines the confidence in our conclusions; larger studies are needed to confirm the findings. As a consequence of using surplus whole blood samples collected for other purposes, we used plasma samples with different anticoagulants to perform cross-matching on GEL (EDTA plasma samples) compared to TUBE and STRIP (citrate plasma samples). However, the anticoagulants are both commonly used in transfusion medicine and hematology, and both are considered acceptable for both standard TUBE and GEL tube XM methods.17 No information was available about prior transfusion or health status in any of the dogs from which blood was collected. Some of these dogs might have received an incompatible DEA 1 blood transfusion in their past, and this could have been responsible for generation of alloantibodies different from anti–DEA 7 antibodies (e.g., anti–DEA 1 antibodies). This could explain why one plasma sample in our study reacted against one DEA 7+ RBC sample but not against the other, leaving doubt that this sample contained true anti–DEA 7 antibodies. We did not type for DEA 5 and DEA 3 because corresponding antisera were not available, and naturally occurring antibodies do exist against these DEAs.5 Finally, we did not evaluate inter-observer variation in the reading of the results obtained with different XM methods.
The GEL and TUBE XM tests, but not STRIP, are useful methods for identification of DEA 7 incompatibilities caused by anti–DEA 7 antibodies. Additional studies are needed to clarify whether STRIP XM testing enhanced with canine antiglobulin could improve the ability of this technique to identify reactions caused by anti–DEA 7 antibodies.
Acknowledgments
This study was presented as a poster at the 60th AAVLD/121st USAHA Annual Meeting, San Diego, CA, October 12–18, 2017.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by Piano di Sostegno alla Ricerca 2015–2017, Linea 2, University of Milan, Milan, Italy. The immunochromatographic cross-match tests were provided courtesy of Alvedia.
ORCID iD: Eva Spada  https://orcid.org/0000-0003-3898-6955
https://orcid.org/0000-0003-3898-6955
References
- 1. Blais MC, et al. Canine Dal blood type: a red cell antigen lacking in some Dalmatians. J Vet Intern Med 2007;21:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Euler CC, et al. Survey of two new (Kai 1 and Kai 2) and other blood groups in dogs of North America. J Vet Intern Med 2016;30:1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goulet S, Blais MC. Characterization of anti-Dal alloantibodies following sensitization of two dal-negative dogs. Vet Pathol 2018;55:108–115. [DOI] [PubMed] [Google Scholar]
- 4. Goy-Thollot I, et al. Pre- and post-transfusion alloimmunization in dogs characterized by 2 antiglobulin-enhanced cross-match tests. J Vet Intern Med 2017;31:1420–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hale AS, Wefelmann J. Incidence of canine serum antibody to known dog erythrocyte antigens in potential donor population. J Vet Intern Med 2006;20:768–769. [Google Scholar]
- 6. Harris R, Hochman H. Revised P values in testing blood group antibodies. Fisher’s exact test revisited. Transfusion 1986;26:494–496. [DOI] [PubMed] [Google Scholar]
- 7. Kessler RJ, et al. Dog erythrocyte antigens 1.1, 1.2, 3, 4, 7, and Dal blood typing and cross-matching by gel column technique. Vet Clin Pathol 2010;39:306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lapierre Y, et al. The gel test: a new way to detect red cell antigen-antibody reactions. Transfusion 1990;30:109–113. [DOI] [PubMed] [Google Scholar]
- 9. Luethy D, et al. Comparison of tube, gel, and immunochromatographic strip methods for evaluation of blood transfusion compatibility in horses. J Vet Intern Med 2016;30:1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Melzer KJ, et al. A hemolytic transfusion reaction due to DEA 4 alloantibodies in a dog. J Vet Intern Med 2003;17:931–933. [PubMed] [Google Scholar]
- 11. Novaretti MCZ, et al. Comparison of conventional tube test with diamed gel microcolumn assay for anti-D titration. Clin Lab Haematol 2003;25:311–315. [DOI] [PubMed] [Google Scholar]
- 12. Odunayo A, et al. Incidence of incompatible crossmatch results in dogs admitted to a veterinary teaching hospital with no history of prior red blood cell transfusion. J Am Vet Med Assoc 2017;250:303–308. [DOI] [PubMed] [Google Scholar]
- 13. Seth M, et al. Comparison of gel column, card, and cartridge techniques for dog erythrocyte antigen 1.1 blood typing. Am J Vet Res 2012;73:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spada E, et al. Activity, specificity, and titer of naturally occurring canine anti–DEA 7 antibodies. J Vet Diagn Invest 2016;28:705–708. [DOI] [PubMed] [Google Scholar]
- 15. Spada E, et al. Dog erythrocyte antigens (DEA) 1, 4, 7 and suspected naturally occurring anti-DEA 7 antibodies in Italian Corso dogs. Vet J 2017;222:17–21. [DOI] [PubMed] [Google Scholar]
- 16. Spada E, et al. Prevalence of naturally occurring antibodies against dog erythrocyte antigen 7 in a population of dog erythrocyte antigen 7–negative dogs from Spain and Italy. Am J Vet Res 2016;77:877–881. [DOI] [PubMed] [Google Scholar]
- 17. Vap LM, et al. ASVCP quality assurance guidelines: control of preanalytical and analytical factors for hematology for mammalian and nonmammalian species, hemostasis, and crossmatching in veterinary laboratories. Vet Clin Pathol 2012;41:8–17. [DOI] [PubMed] [Google Scholar]
- 18. Villarnovo D, et al. Preliminary evaluation of a gel tube agglutination major cross-match method in dogs. Vet Clin Pathol 2016;45:411–416. [DOI] [PubMed] [Google Scholar]
- 19. Wardrop K. Clinical blood typing and crossmatching. In: Weiss D, Wardrop K, eds. Schalm’s Veterinary Hematology. Ames, IA: Wiley Blackwell, 2010:1101–1105. [Google Scholar]
- 20. Weisbach V, et al. Comparison of the performance of four microtube column agglutination systems in the detection of red cell alloantibodies. Transfusion 1999;39:1045–1050. [DOI] [PubMed] [Google Scholar]