Abstract
A 7-y-old Black Russian Terrier dog was evaluated for chronic lameness of the right forelimb and concurrent weight loss. Clinical examination revealed a pigmented mass arising from digit 4 of the right forelimb; the digit was amputated and submitted for histologic evaluation. Histologically, the neoplasm was composed of ill-defined streams, sheets, and clusters of melanocytes admixed with a distinct population of malignant epithelial cells forming glands and nests. The diagnosis was a biphasic malignant melanoma adenocarcinoma, a rarely reported neoplasm in human medicine that has not been described in veterinary medicine, to our knowledge.
Keywords: Adenocarcinoma, biphasic neoplasms, dogs, melanoma
A 7-y-old, male, Black Russian Terrier dog was evaluated for a wound on the right forelimb. The dog had a prior history of 2 mo of lameness and recent licking of the paw of that limb. A mass was noted on digit 4 of the right forelimb resulting in bulbous expansion of P3 and of the digital pad, with erythema and fissures of the pad. The digit was amputated and submitted for histopathology to the New Hampshire Veterinary Diagnostic Laboratory (Durham, NH). The digit was fixed in 10% neutral-buffered formalin, and portions were demineralized using 10% formic acid. Sections of the mass and demineralized digit were processed routinely and stained with hematoxylin and eosin. Expanding the dermis and obliterating the native tissues was a variably well-demarcated, non-encapsulated, infiltrative biphasic neoplasm consisting of an outer portion of fusiform neoplastic melanocytes arranged in bundles and streams amidst a fine fibrous stroma, and an inner portion composed of stellate-to-polygonal neoplastic melanocytes admixed with neoplastic epithelial cells arranged in nests and tubules in a fine-to-moderate fibrous stroma (Figs. 1, 2). Approximately 75% of the fusiform melanocytes contained fine light-brown cytoplasmic granules, and >95% of those in the mixed portion were pigmented. Both the melanocytic and epithelial populations had moderate anisokaryosis. There were 4 mitoses within neoplastic melanocytes, and 3 mitoses in neoplastic epithelial cells in ten 400× fields. The neoplasm did not extend to the examined margin sections.
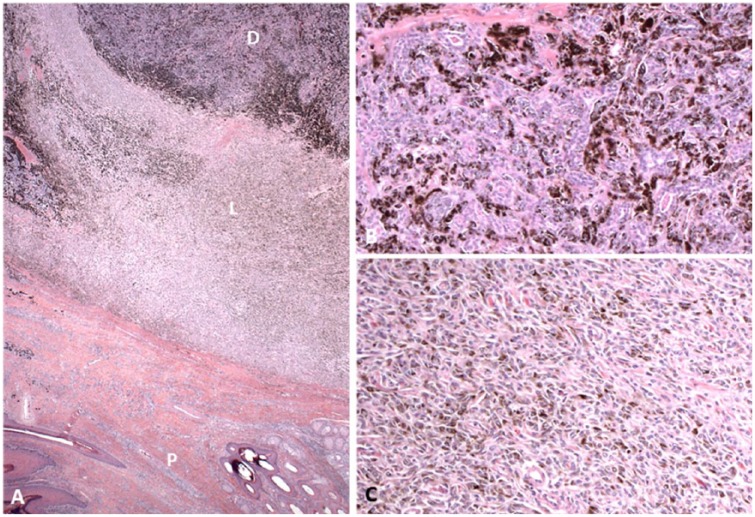
Figure 1.
Biphasic melanoma–adenocarcinoma from digit 4 of the right forelimb of a Black Russian Terrier dog. A. The lesion is oriented with the palmar surface (P) at the bottom of the image. The 2 patterns of the neoplastic melanocytes can be appreciated, with the lighter staining portion (L) composed of fusiform cells palmar to the darker staining portion (D) composed of more plump melanocytes and glandular structures more dorsal. H&E. 20×. B. Higher magnification of the darker staining area, wherein the neoplastic melanocytes are arranged in small clusters admixed with neoplastic glandular structures. H&E. 100×. C. Higher magnification of the lighter staining area, wherein the neoplastic cells are arranged in interwoven streams and bundles. H&E. 100×.
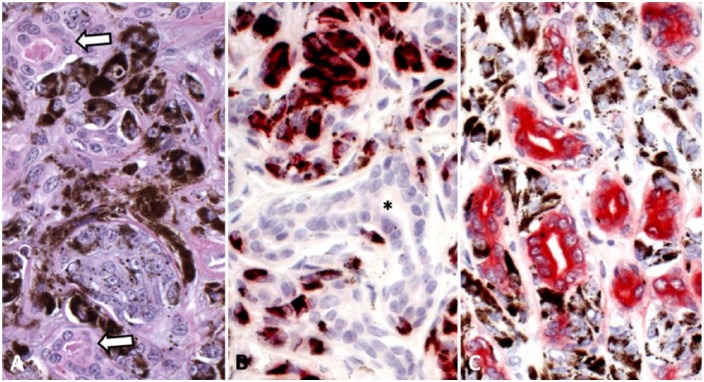
Figure 2.
Biphasic melanoma–adenocarcinoma from digit 4 of the right forelimb of a Black Russian Terrier dog. A. High magnification of the darker staining area with plump, heavily pigmented neoplastic melanocytes admixed with neoplastic epithelial structures (arrows) that occasionally contain lumina with amorphous eosinophilic granular contents. H&E. 400×. B. High magnification of anti–melan A immunohistochemistry with labeling only of melanin-laden neoplastic cells. The staining appears red-brown because of superimposition of melanin and the red chromogen. Note that the neoplastic glandular structure in the center of the image does not express melan A (*). Leica bond polymer red detection. 400×. C. High magnification of anti–cytokeratin AE1/3 immunohistochemistry. All neoplastic glandular structures are labeled, and the melanin-laden neoplastic cells do not label. Leica bond polymer red detection. 400×.
Immunohistochemistry (IHC) was performed at the New York State Animal Health Diagnostic Center at Cornell University College of Veterinary Medicine (Ithaca, NY). All immunohistochemical stains were performed according to standardized laboratory procedures on the Leica automated staining platform (Leica Biosystems, Buffalo Grove, IL). The following antibodies were used: melan A (PA0233, Bond ready-to-use primary antibody, Leica), tyrosinase-related protein 2 (TRP2; ab103463, Abcam, Cambridge, MA; 1:100), PNL2 (ab12502, Abcam; 1:200), cytokeratin (CK)AE1/AE3 (M3515, Dako North America, Carpinteria, CA; 1:200), and CK7 (M7018, Dako; 1:50). The melanocytic population had strong cytoplasmic immunoreactivity for melan A and lacked immunoreactivity for TRP2, PNL2, CKAE1/3, and CK7 (Fig. 2). The epithelial population had strong cytoplasmic immunoreactivity for CKAE1/3, but lacked immunoreactivity for CK7, melan A, TRP2, and PNL2 (Fig. 2). The diagnosis was a biphasic malignant neoplasm composed of malignant melanoma and adenocarcinoma. Six months following surgery, the dog remained free of local recurrence and metastatic disease.
In a retrospective study encompassing 428 amputated digits from dogs submitted to 3 veterinary diagnostic laboratories, malignant neoplasms were diagnosed in 229 digits (53.5%).16 Of these neoplasms, malignant melanoma was the second most common, accounting for 22.6% of the malignant neoplasms.16 Malignant melanoma was also the second most common malignant neoplasm of the digit in 2 other studies encompassing 117 and 64 cases, accounting for 31.6% and 17.8%, respectively.3,7 In these same studies of 428, 117, and 64 cases, adenocarcinoma was reported only in the former, and only 3 cases were described.3,7,16 CK7 IHC was used to attempt to demonstrate apocrine glandular origin of the neoplastic epithelial cells, with the lack of expression interpreted as indicating a non-apocrine origin of these neoplastic cells.9
Digital malignant melanomas in dogs generally have a shorter survival time than those elsewhere in the skin, with reported 2-y survival rates of 11–56%.3,6,11,15,16 Hematogenous metastasis of digital malignant melanoma is relatively common, including a case report of suspected uveal metastasis of a digital malignant melanoma.1 In our case, the neoplastic melanocytes expressed melan A, but did not label with TRP2 or PNL2. This is intriguing because labeling for these 3 proteins seems fairly equivalent in terms of sensitivity, with 1 study of oral amelanotic melanoma indicating that PNL2 was the most sensitive of the 3.14 Our finding offers support to the utility of wider application of a melanoma IHC cocktail as described for oral melanocytic tumors.14
Most unique in our case is the presence of 2 histologically and immunohistochemically distinct populations of malignant neoplastic cells in the digit. These 2 cellular populations are of different ontogeny—melanocytes are of neural crest origin and the adnexal glandular component is of ectodermal origin. Such an occurrence, wherein the 2 neoplastic populations are intimately and inexorably entwined in a single mass, is referred to as a biphasic tumor. In contrast, a collision tumor is a neoplasm wherein 2 populations are closely apposed, but clearly delineated, as if they had collided.8,12 A third type of mixed phenotype neoplasm is a combined tumor, in which 2 histologically distinct neoplastic populations arising from a common progenitor cell commingle; one well known example is a combined hepatocellular cholangiocarcinoma described in dogs.13
There is a report of a single collision tumor excised from the lip of a dog and found to contain tightly apposed, but distinct, malignant melanoma and anaplastic sarcoma.4 A biphasic tumor is a rarity in veterinary medicine, especially when one component is melanocytic. The first mention of a biphasic tumor including malignant melanoma in an animal was a rabbit with a biphasic malignant melanoma and malignant trichoblastoma.2 The first reports of biphasic tumors including a melanocytic component in dogs were in 2016, describing oral lesions containing squamous cell carcinoma and malignant melanoma.8,10 In these dogs, the neoplasm was referred to as a squamomelanocytic tumor and thought to be biphasic based on the intimate mixing of the 2 neoplastic phenotypes, and the lack of perceived concurrent expression of melanocytic proteins and cytokeratin by individual neoplastic cells.8,10
It is possible that the oral squamomelanocytic tumors reported by others and the neoplasm we present may actually be a combined tumor.8,10 In the case of a melanocytic neoplasm and an epithelial neoplasm, it seems unlikely that a common progenitor cell could lead to both cell types. A neoplasm is described in an aged woman composed of a mixture of a melan-A–expressing melanocytic component and a CKAE1/AE3-expressing adenocarcinomatous component.5 Using molecular analysis of both histopathologic phenotypes, the authors demonstrated that 1) they shared chromosomal aberrations that are commonly described in melanomas; 2) they shared an uncommon unique chromosomal deletion; and 3) they shared an identical single nucleotide substitution in NRAS. Further analysis showed some genetic variability between the 2 populations, as should be expected from 2 phenotypically divergent related cell lines. Finally, using immunofluorescence, cells at the interface of the 2 populations expressed S100 and keratin.5
It is tempting to consider the possibility that the lesion we describe, as well as other collision or biphasic tumors, may actually represent combined tumors. Determining this would necessitate laser capture microdissection and advanced molecular analysis. In our case, a digital tumor from a dog that was demonstrated to contain intimately intertwined malignant neoplastic cells displayed melanocytic and adenocarcinomatous differentiation. IHC labeling revealed that the populations expressed distinct protein profiles (melan A or pan-cytokeratin, respectively). Although there were no aggregates of cells labeled with pan-cytokeratin and containing melanin pigment, and no glandular neoplastic cells expressing melan A, a combined tumor cannot be entirely ruled out without molecular analysis. Whether this was a biphasic tumor or a combined tumor, our case indicates the potential for melanocytic tumors of the digit in dogs to contain additional cell line phenotypes, as has been demonstrated with oral squamomelanocytic tumors.8,10
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Esson D, et al. Suspected uveal metastasis of a nail bed melanoma in a dog. Vet Ophthalmol 2007;10:262–266. [DOI] [PubMed] [Google Scholar]
- 2. Golbar HM, et al. A collision tumour consisting of malignant trichoblastoma and melanosarcoma in a rabbit. J Comp Pathol 2014;151:63–66. [DOI] [PubMed] [Google Scholar]
- 3. Henry CJ, et al. Canine digital tumors: a Veterinary Cooperative Oncology Group retrospective study of 64 dogs. J Vet Intern Med 2005;19:720–724. [DOI] [PubMed] [Google Scholar]
- 4. Jakab C, Balka G. First report of a malignant collision skin tumour with malignant melanoma and anaplastic sarcoma components in a dog. Acta Vet Hung 2012;60:245–255. [DOI] [PubMed] [Google Scholar]
- 5. Jalas JR, et al. Metastatic melanoma with striking adenocarcinomatous differentiation illustrating phenotypic plasticity in melanoma. Am J Surg Pathol 2011;35:1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laprie C, et al. MIB-1 immunoreactivity correlates with biologic behaviour in canine cutaneous melanoma. Vet Dermatol 2001;12:139–147. [DOI] [PubMed] [Google Scholar]
- 7. Marino DJ, et al. Evaluation of dogs with digit masses: 117 cases (1981–1991). J Am Vet Med Assoc 1995;207:726–728. [PubMed] [Google Scholar]
- 8. Muscatello LV, et al. Oral squamomelanocytic tumour in a dog: a unique biphasic cancer. J Comp Pathol 2016;154:211–214. [DOI] [PubMed] [Google Scholar]
- 9. Pieper JB, et al. Coordinate expression of cytokeratins 7 and 14, vimentin, and Bcl-2 in canine cutaneous epithelial tumors and cysts. J Vet Diagn Invest 2015;27:497–503. [DOI] [PubMed] [Google Scholar]
- 10. Rodríguez F, et al. Collision tumour of squamous cell carcinoma and malignant melanoma in the oral cavity of a dog. J Comp Pathol 2016;154:314–318. [DOI] [PubMed] [Google Scholar]
- 11. Schultheiss PC. Histologic features and clinical outcomes of melanomas of lip, haired skin, and nail bed locations of dogs. J Vet Diagn Invest 2006;18:422–425. [DOI] [PubMed] [Google Scholar]
- 12. Scruggs JM, et al. Cutaneous collision cancers: a report of two squamomelanocytic malignancies and review of the literature. Dermatol Surg 2011;37:1679–1683. [DOI] [PubMed] [Google Scholar]
- 13. Shiga A, et al. Combined hepatocellular and cholangiocellular carcinoma in a dog. J Vet Med Sci 2001;63:483–486. [DOI] [PubMed] [Google Scholar]
- 14. Smedley RC, et al. Immunohistochemical diagnosis of canine oral amelanotic melanocytic neoplasms. Vet Pathol 2011;48:32–40. [DOI] [PubMed] [Google Scholar]
- 15. Smedley RC, et al. Prognostic markers for canine melanocytic neoplasms: a comparative review of the literature and goals for future investigation. Vet Pathol 2011;48:54–72. [DOI] [PubMed] [Google Scholar]
- 16. Wobeser BK, et al. Diagnoses and clinical outcomes associated with surgically amputated canine digits submitted to multiple veterinary diagnostic laboratories. Vet Pathol 2007;44:355–361. [DOI] [PubMed] [Google Scholar]