Abstract
We investigated the occurrence of infectious pathogens during an outbreak of bovine respiratory disease (BRD) in a beef cattle feedlot in southern Brazil that has a high risk of developing BRD. Nasopharyngeal swabs were randomly collected from steers (n = 23) and assessed for the presence of infectious agents of BRD by PCR and/or RT-PCR assays. These included: Histophilus somni, Mannheimia haemolytica, Pasteurella multocida, Mycoplasma bovis, bovine respiratory syncytial virus (BRSV), bovine coronavirus (BCoV), bovine viral diarrhea virus (BVDV), bovine alphaherpesvirus 1 (BoHV-1), and bovine parainfluenza virus 3 (BPIV-3). Pulmonary sections of one steer that died with clinical BRD were submitted for pathology and molecular testing. The frequencies of the pathogens identified from the nasopharyngeal swabs were: H. somni 39% (9 of 23), BRSV 35% (8 of 23), BCoV 22% (5 of 23), and M. haemolytica 13% (3 of 23). PCR or RT-PCR assays did not identify P. multocida, M. bovis, BoHV-1, BVDV, or BPIV-3 from the nasopharyngeal swabs. Single and concomitant associations of infectious agents of BRD were identified. Fibrinous bronchopneumonia was diagnosed in one steer that died; samples were positive for H. somni and M. haemolytica by PCR. H. somni, BRSV, and BCoV are important disease pathogens of BRD in feedlot cattle in Brazil, but H. somni and BCoV are probably under-reported.
Keywords: Bovine coronavirus, bovine respiratory syncytial virus, Brazil, Histophilus somni, molecular testing.
The bovine respiratory disease (BRD) complex is an entity that is associated with several infectious agents9,14 coupled with management systems,14 as well as environmental factors.13,14 BRD is the most important cause of livestock mortality in feedlots in the United States and is responsible for 16% of the non-predator losses to beef cattle (USDA. Cattle and calves predator death loss in the United States, 2010. USDA-APHIS-VS-CEAH 643.0312, https://goo.gl/8pTRbp). In Brazil, we have previously investigated the economic effects of BRD on beef cattle (n = 188,862) and demonstrated that 86.9% (11,577 of 13,315) of the sick animals on feed developed BRD.1 In that study, BRD-related mortality losses were estimated at $14,334 per 10,000 head of cattle; morbidity caused by BRD resulted in a loss of $16,315 per 10,000 head of cattle.1
Pathogens frequently associated with BRD include bacteria such as Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis.8,9 Viral agents commonly identified in cattle with BRD are bovine viral diarrhea virus (BVDV), bovine respiratory syncytial virus (BRSV), bovine alphaherpesvirus 1 (BoHV-1), bovine parainfluenza virus 3 (BPIV-3), and bovine coronavirus (BCoV).8
Most studies that have investigated the occurrence of disease pathogens associated with BRD have been done predominantly in North America8,9 and Australia.4 There is little information about the occurrence of infectious agents associated with BRD in beef cattle feedlots in Brazil relative to the number of cattle reared under this fattening system. We evaluated the possible participation of 9 pathogens associated with respiratory disease during an outbreak of BRD in a beef cattle feedlot in southern Brazil during August 2015.
The feedlot is in the rural part of the city of Apucarana (latitude: 23º 33’ 03” S; longitude: 51º 27’ 39” W), in the northern region of the state of Paraná, in southern Brazil. The owner indicated that 100 head of 2-y-old Nelore cattle were recently acquired from a feed yard in the city of Ponta Grossa, ~249 km distant. This feedlot had a suggested elevated risk for BRD given the following: 1) the animals were transported over a long distance; 2) there was a short period of adaptation before being placed in the feedlot; and 3) immunization and/or metaphylaxis were not done.
Fifty of these animals demonstrated clinical signs of acute pulmonary distress (dyspnea, extended head and neck, and audible noise when breathing) within the first week on feed; 10 of these died within 15 d of arrival at the feedlot. Three steers were autopsied on location by the consulting veterinarian, but pulmonary tissue from only one of these was submitted for pathology and molecular testing. The affected steers were medicated with a single dose of enrofloxacin (intramuscular, 1 mL/40 kg). Prior to treatment, nasopharyngeal swabs were collected from steers (n = 23) with clinical manifestations of BRD, placed on ice, and submitted for molecular testing. All molecular testing was done within 1 wk of the onset of clinical disease.
Nucleic acids were extracted from the nasopharyngeal swabs and the pulmonary tissue, and used in PCR or reverse transcription (RT)-PCR assays for bacterial pathogens such as H. somni, M. haemolytica, P. multocida, and M. bovis. Viral agents were investigated by targeting the glycoprotein C gene of BoHV-1, the 5’-UTR region of BVDV, HN gene of BPIV-3, the nucleocapsid protein (N) gene of BCoV, and glycoprotein G gene of BRSV. A list of the primers used to target the specific genes of infectious agents associated with BRD is provided (Supplementary Table 1).
Positive controls included DNA/RNA from cell culture–adapted Los Angeles strain of BoHV-1 and the NADL strain of BVDV; nucleic acid of H. somni, BPIV-3, BCoV, BRSV, M. bovis, M. haemolytica, and P. multocida were obtained from previous studies.10,11 Nuclease-free water (Invitrogen, Carlsbad, CA) was used as the negative control in all PCR or RT-PCR assays.
The products of all assays were separated by electrophoresis in 2% agarose gels, stained with ethidium bromide, and examined under ultraviolet light. The amplified PCR or RT-PCR products were then purified (PureLink quick gel extraction and PCR purification combo kit, Life Technologies, Carlsbad, CA) and submitted for direct sequencing (BigDye Terminator v3.1 cycle sequencing kit, 3500 genetic analyzer sequencer, Applied Biosystems, Carlsbad, CA).
In the one animal for which tissue was available for gross examination, there was marked expansion of interlobular septa by edema and exudate with areas of consolidation of the lung. Histologic examination revealed severe multifocal fibrinous bronchopneumonia characterized by accumulations of a neutrophilic exudate admixed with tissue debris and fibrin within the lumens of the bronchi, bronchioles, and alveoli. In addition, there were areas of pulmonary edema, thrombosis, and congestion with marked distension of interlobular septa by fibrin and edema.
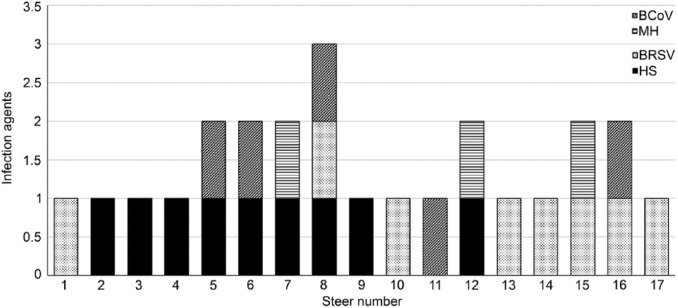
Most (74%; 17 of 23) of the steers evaluated during this outbreak contained the nuclei acid of at least 1 of the following 4 pathogens: H. somni, M. haemolytica, BRSV, and BCoV; direct sequencing confirmed the PCR and RT-PCR results. The frequency of the infectious agents identified in steers either singly or in association were 39% (9 of 23) for H. somni, 35% (8 of 23) for BRSV, 22% (5 of 23) for BCoV, and 13% (3 of 23) for M. haemolytica (Fig. 1). All PCR and RT-PCR assays to detect P. multocida, M. bovis, BoHV-1, BVDV, and BPIV-3 were negative.
Figure 1.
Single and mixed associations of infectious agents in steers with clinical manifestations of bovine respiratory disease. BCoV = bovine coronavirus; BRSV = bovine respiratory syncytial virus; HS = Histophilus somni; MH = Mannheimia haemolytica.
The most frequent single infectious agents of BRD at this feedlot were BRSV (22%; 5 of 23) and H. somni (17%; 4 of 23). The most common simultaneous occurrences identified during our investigation were of H. somni and BCoV (17%; 4 of 23) and H. somni with M. haemolytica (9%; 2 of 23). Mixed infection of H. somni, BRSV, and BCoV was identified in one steer. The nucleic acids of H. somni and M. haemolytica were amplified from the pneumonic lung of the steer submitted for testing, and the histologic lesions noted were consistent with this coinfection.
The identification of BRSV in steers from this feedlot might suggest that this pathogen is endemic within the geographic regions of our study. Studies done in the state of Rio Grande do Sul, in southern Brazil, have demonstrated that BRSV was associated with BRD in several herds.3,5 In addition, a retrospective study identified antigens of BRSV in cattle with bronchointerstitial pneumonia from southern and southeastern Brazil.6 In a previous study, H. somni and BRSV were the only disease pathogens associated with BRD in a beef cattle feedlot from São Paulo, Brazil.11 However, a longitudinal study only identified P. multocida and M. haemolytica as infectious agents in a beef cattle feedlot from the state of Minas Gerais, southeastern Brazil.1
BVDV was not identified during our investigation; similar results were noted in earlier investigations.1,11 Collectively, these results contrast with the viral-associated causes of BRD in beef cattle from North America,8 Europe,12 and Australia,4 where BVDV is frequently incriminated in BRD. Studies are required from other geographic regions in Brazil to fully understand the participation of BVDV in BRD in beef cattle in Brazil.
The identification of BCoV in steers with clinical BRD suggests that this pathogen contributed to the development of the pulmonary disease at this feedlot. It must be highlighted that respiratory disease associated with BCoV seems to be more pronounced in animals that have recently entered a feedlot,7 as was the case during the outbreak herein described. Although BCoV is an established pneumoenteric pathogen associated with several clinical syndromes in cattle worldwide, including BRD,7 descriptions of the occurrence of this pathogen as a cause of BRD in cattle in Brazil are lacking. Previously, we described the dual tropism of a strain of BCoV isolated from an outbreak of BRD in feedlot cattle from southern Brazil, during which 67% (8 of 12) of the steers evaluated were positive for BCoV by RT-PCR.2 Consequently, our findings suggest that BCoV is a cause of BRD in cattle in Brazil. Given that there is no vaccine commercially available that includes BCoV to immunize cattle before entering feedlots, outbreaks of BRD related to this pathogen are likely to increase as our ability to detect this pathogen is improved at local laboratories.
Supplementary Material
Acknowledgments
We thank Dr. Antônio Sversuti for referring these outbreaks for laboratory investigation. We thank Marcos V. Vieira and João V. Veronez, undergraduates in Veterinary Medicine, Universidade Norte do Paraná, for their participation during the collection of samples. SA Headley, TES Oliveira, AF Alfieri, JPE Saut, and AA Alfieri are recipients of National Council for Scientific and Technological Development (CNPq; Brazil) fellowships and/or grants.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Baptista AL, et al. Bovine respiratory disease complex associated mortality and morbidity rates in feedlot cattle from southeastern Brazil. J Infect Dev Ctries 2017; Accepted. [DOI] [PubMed] [Google Scholar]
- 2. Beuttemmuller E, et al. Brazilian strain of bovine respiratory coronavirus is derived from dual enteric and respiratory tropism. Genet Mol Res 2017;16:gmr16029580. [DOI] [PubMed] [Google Scholar]
- 3. Brasil NDA, et al. Doenças respiratórias em bezerros na região sul do Rio Grande do Sul: estudo retrospectivo de 33 surtos [Respiratory diseases in calves in southern Rio Grande do Sul: study of 33 outbreaks]. Pesq Vet Bras 2013;33:745–751. Portuguese. [Google Scholar]
- 4. Cusack PM, et al. The medicine and epidemiology of bovine respiratory disease in feedlots. Aust Vet J 2003;81:480–487. [DOI] [PubMed] [Google Scholar]
- 5. Driemeier D, et al. Manifestação clínico-patológica de infecção natural pelo Vírus Respiratório Sincicial Bovino (BRSV) em bovinos de criação extensiva no Rio Grande do Sul, Brasil [Clinic-pathological aspects in the natural infection of bovine respiratory syncytial virus (BRSV) in extensive management of cattle in Rio Grande do Sul, Brazil]. Pesq Vet Bras 1997;17:77–81. Portuguese. [Google Scholar]
- 6. Flores EF, et al. A retrospective search for bovine respiratory syncytial virus (BRSV) antigens in histological specimens by immunofluorescence and immunohistochemistry. Pesq Vet Bras 2000;20:139–143. [Google Scholar]
- 7. Fulton RW, et al. Bovine coronavirus (BCV) infections in transported commingled beef cattle and sole-source ranch calves. Can J Vet Res 2011;75:191–199. [PMC free article] [PubMed] [Google Scholar]
- 8. Gagea MI, et al. Diseases and pathogens associated with mortality in Ontario beef feedlots. J Vet Diagn Invest 2006;18:18–28. [DOI] [PubMed] [Google Scholar]
- 9. Griffin D, et al. Bacterial pathogens of the bovine respiratory disease complex. Vet Clin North Am Food Anim Pract 2010;26:381–394. [DOI] [PubMed] [Google Scholar]
- 10. Headley SA, et al. Histophilus somni is a potential threat to beef cattle feedlots in Brazil. Vet Rec 2014;175:249. [DOI] [PubMed] [Google Scholar]
- 11. Headley SA, et al. Bovine respiratory disease associated with Histophilus somni and bovine respiratory syncytial virus in a beef cattle feedlot from Southeastern Brazil. Semin Cienc Agrar 2017;38:283–294. [Google Scholar]
- 12. O’Neill R, et al. Patterns of detection of respiratory viruses in nasal swabs from calves in Ireland: a retrospective study. Vet Rec 2014;175:351. [DOI] [PubMed] [Google Scholar]
- 13. Snowder GD, et al. Bovine respiratory disease in feedlot cattle: environmental, genetic, and economic factors. J Anim Sci 2006;84:1999–2008. [DOI] [PubMed] [Google Scholar]
- 14. Taylor JD, et al. The epidemiology of bovine respiratory disease: what is the evidence for predisposing factors? Can Vet J 2010;51:1095–1102. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.