Abstract
Bovine actinobacillosis is typically characterized by pyogranulomatous glossitis (wooden tongue). The involvement of other tissues, generally the skin or lymph nodes, has been regarded as atypical or cutaneous. We describe herein 2 outbreaks of actinobacillosis affecting primarily the lymph nodes of the head and neck. The disease affected 40 of 540 lactating cows in a dairy herd, and 5 of 335 two-y-old steers in a beef herd. Multiple or single, occasionally ulcerated nodules were observed in the region of the mandible, neck, and shoulder, including the parotid, submandibular, retropharyngeal, and prescapular lymph nodes. The histologic lesions were multifocal pyogranulomatous lymphadenitis, dermatitis, and cellulitis with Splendore–Hoeppli material. One steer had an exophytic pyogranuloma in the gingiva and another died because of ruminal tympany secondary to oropharyngeal and esophageal obstruction by a pyogranulomatous mass. Actinobacillus lignieresii was isolated from the lesions and identified by amplification, sequencing, and analysis of the 16S ribosomal (r)DNA gene. Seven of 8 cows recovered after treatment with sodium iodide. Lymphatic actinobacillosis is a frequent disease in Uruguay, southern Brazil, and Argentina. Morbidity is 1–50%; mortality is <1%. A. lignieresii apparently penetrates the intact oral and pharyngeal mucosa, infecting primarily the regional lymph nodes. Later, lesions may extend to the subcutaneous tissue and the skin, causing ulceration. Affected cattle with draining pyogranulomas contaminate the environment, favoring disease transmission, and should be treated with sodium iodide or antibiotics and isolated from the herd in order to control the disease.
Keywords: Actinobacillus lignieresii, bovine, draining abscesses, lymphadenitis, pyogranulomas
Introduction
Actinobacillosis, a disease with worldwide distribution, is caused by Actinobacillus lignieresii, a natural inhabitant of the upper respiratory and digestive tracts of ruminants.23,26 The bacterium, first recognized as a pathogen in South America in the early 20th century,15 causes pyogranulomas in various soft tissues, including the tongue, mouth, pharynx, forestomachs, lymph nodes, lungs, skin, and subcutaneous tissue.15,21,26,29 Actinobacillosis in cattle typically involves the tongue, causing an indurative pyogranulomatous glossitis referred to as “wooden tongue.”23,29 The involvement of other organs, generally the skin or lymph nodes, has been regarded as atypical or cutaneous actinobacillosis.2,5,11,12,21,24 A common presentation of the disease, resulting in draining nodular pyogranulomas in the region of the mandible and neck, has been reported in beef and dairy cattle.1,5,9,10,15,20,25 We describe 2 outbreaks of actinobacillosis affecting primarily the lymph nodes of the mandible and neck in a dairy and a beef herd.
Materials and methods
Between October 2015 and July 2016, outbreaks of actinobacillosis were studied in a dairy herd in Florida and in a beef herd in Colonia, Uruguay. In the dairy herd of 540 lactating Holstein cows, 12 cows and 1 bull were examined clinically. Core needle biopsies of the lesions were used for direct microscopic observation of the exudate and for microbiologic and histologic studies. In one case, the affected prescapular lymph node and the surrounding subcutaneous tissue were excised surgically and processed for bacteriologic and histologic examination.
The beef cattle herd included 335 two-y-old Hereford, Aberdeen Angus, and crossbred steers. Five steers with nodular lesions in the lymph nodes (3), gingiva (1) and oropharynx, and the lung (1) were examined. Samples of the lymph nodes were obtained by core needle biopsies, and samples of the gingiva were obtained postmortem at the slaughterhouse 3 d after the first observation of the lesion. A steer with oropharyngeal and pulmonary lesions died spontaneously of ruminal tympany secondary to pharyngeal and esophageal obstruction and was autopsied. Samples of the lesions in the pharynx and the lungs were collected for bacteriologic and histologic studies. Samples of other organs were also collected for histologic examination.
Tissue samples were fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 4–5 µm, and stained with hematoxylin and eosin for histologic examination. Selected sections were also stained with Gram and Ziehl–Neelsen stains and processed with periodic acid–Schiff (PAS) reaction for the identification of intralesional microorganisms. Additionally, immunohistochemistry (IHC) was performed on selected sections of lymph node and gingival pyogranulomas using a rabbit polyclonal antibody against bacille Calmette–Guerin (BCG; Dako, Carpinteria, CA), an attenuated strain of Mycobacterium bovis that cross-reacts with various other microorganisms, including bacteria and fungi.3 Briefly, the slides were placed into a decloaking chamber for 10 min at 121°C in citrate buffer for epitope unmasking, an anti-rabbit horseradish peroxidase (HRP)-labeled polymer (Envision+ System, Dako) was used as a secondary antibody, and antibody binding was visualized using 3-amino-9-ethylcarbazole (AEC) substrate chromogen (AEC, Dako).
Exudates from lymph nodes, gingiva, pharynx, and lung were observed microscopically for the presence of club-shaped clusters, which are characteristic of bovine actinobacillosis. The samples were inoculated onto blood (Oxoid, Basingstoke, Hampshire, England) and MacConkey (Oxoid) agars and incubated microaerophilically at 37°C for 48 h. Additionally, the samples were inoculated into brain-heart infusion broth (Oxoid) and incubated under the same conditions for 24 h. Aliquots of grown culture were then seeded onto blood and MacConkey agars and incubated microaerophilically at 37°C for 48 h. Bacterial colonies obtained from the microaerophilic cultures that were morphologically similar to those of Actinobacillus spp.22 were selected and subjected to standard biochemical tests for phenotypic characterization. Afterward, DNA from selected colonies was extracted using the GenElute bacterial genomic DNA kit (Sigma-Aldrich, St. Louis, MO), and molecular identification was performed by amplification and sequencing of the almost complete 16S ribosomal (r)DNA gene using a commercial sequencing service (Macrogen, Seoul, South Korea). The sequences were assembled and edited using BioEdit (Ibis Biosciences, Carlsbad, CA) and compared to sequences deposited in the Ribosomal Database Project (RDP), as described previously.30 Maximum likelihood phylogenetic trees were generated based on the Tamura–Nei model28 using the sequences generated in this work and sequences deposited in the RDP with commercial software.14
Eight affected cows from the dairy farm were treated with a single intravenous dose of 60% sodium iodide solution (Windhoek, Munro, Buenos Aires, Argentina; 0.06 g/kg body weight). One animal was treated simultaneously with a single intramuscular dose of ceftiofur (Calier, Buenos Aires, Argentina; 2 mg/kg body weight). Six of the 8 treated cattle were pregnant cows with gestational ages of 90–160 d. All affected steers in the beef herd were sent to a slaughterhouse.
Results
From February to October, because of a forage shortage during a dry season, the dairy herd was maintained in stables and fed tall fescue (Festuca arundinacea, syn. Lolium arundinaceum) hay, alfalfa (Medicago sativa), annual ryegrass (Lolium multiflorum) haylage, and Sorghum sp. silage. The herd also grazed in a tall fescue pasture for 3 h daily. The first affected cows had cutaneous nodules in the region of the mandible and neck in July 2015, nearly 3 mo before the initial veterinary consultation. At the time of the veterinary consultation in October 2015, the practitioner mentioned that 40 (7.4%) animals showed similar lesions. Of the 13 cattle examined during a visit to the farm (12 lactating cows and 1 bull), 7 had multiple nodules in the region of the parotid, submandibular, and retropharyngeal lymph nodes (Fig. 1A), 5 had single lesions in the same region, and one had lesions in the left prescapular lymph node. The nodules, 1–20 cm diameter, were firm, occasionally with a fluctuant surface. Most of the nodules were ulcerated, with granulation tissue around the ulcers, or were covered by alopecic skin (Fig. 1A). The ulcers did not heal and occasionally had yellow exudate overlying the surface. In 3 cases, small nodules, up to 3 cm diameter, were arranged linearly along the lymphatic vessels of the neck. Core needle biopsies yielded purulent exudate and/or white or yellow tissue with an irregular surface and a granular texture. The excisional biopsy of the prescapular nodule in one cow had a lymph node in the center of the nodule, surrounded by a white fibrous capsule. The lymph node had lost its normal structure, and its cut surface was yellow and contained numerous 1–2-mm granules (Fig. 1B). Small, yellow, round, pyogranulomas with irregular surfaces were also observed in the lymph node capsule and the subcutaneous tissue (Fig. 1B).
Figure 1.
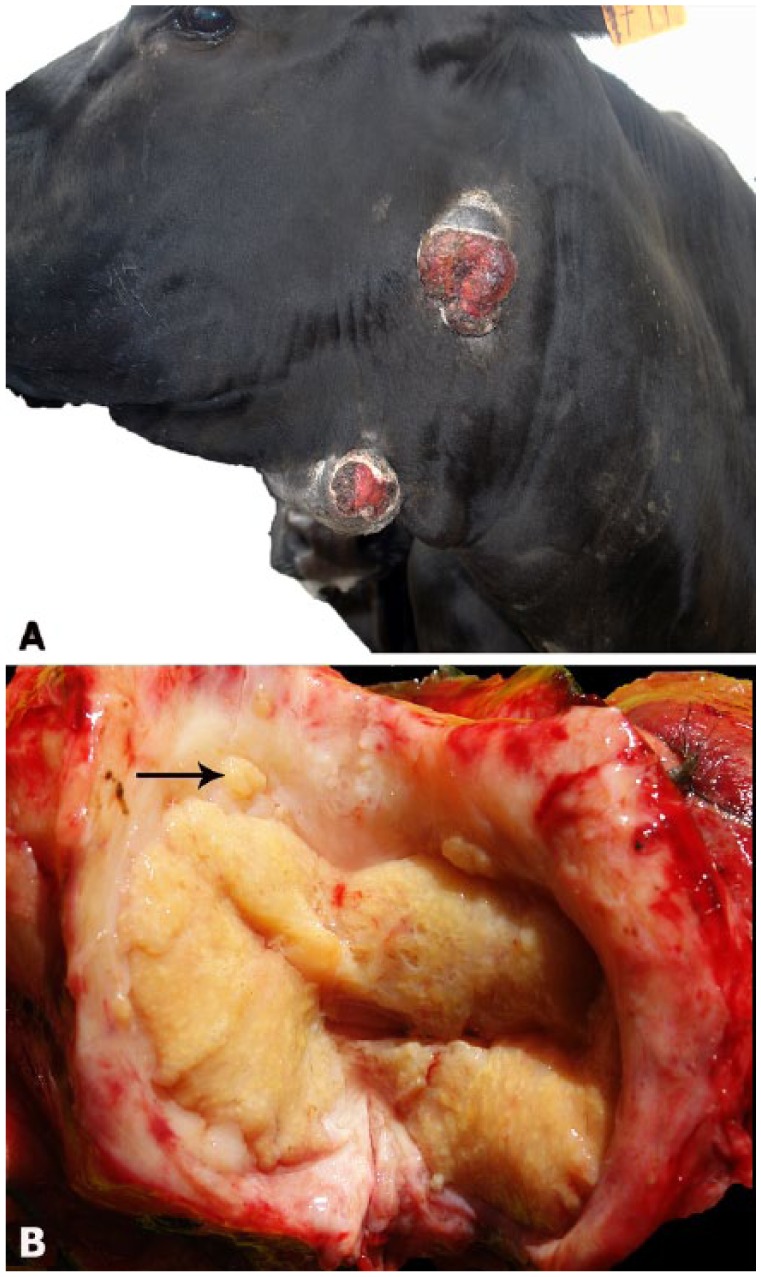
A. Cow with ulcerated, nodular lesions in the lower jaw and neck. B. Excisional biopsy of the prescapular nodule from a cow. The lymph node in the center has a yellow cut surface with numerous 1–2-mm granules and is surrounded by dense fibrous connective tissue. Small, yellow, round, pyogranulomas with irregular surfaces are observed in the lymph node capsule (arrow), suggesting that these lesions extend from the lymph nodes to the neighboring tissues.
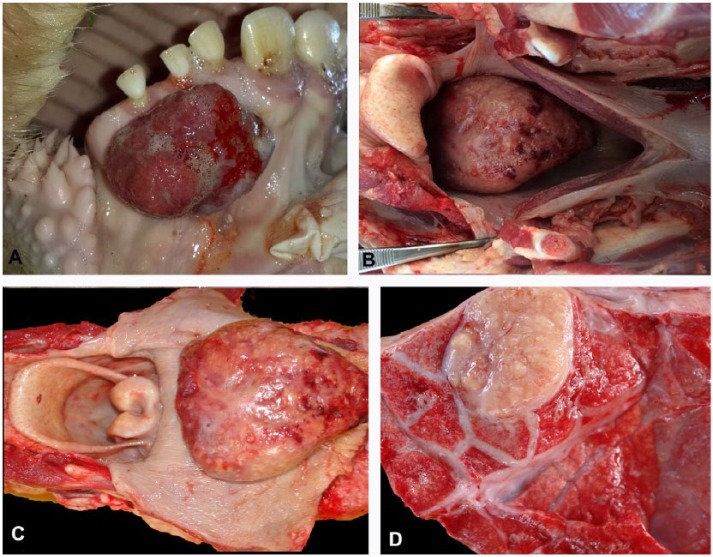
The beef herd grazed in 2 alternating pastures: one of alfalfa, tall fescue, and orchard grass (Dactylis glomerata), and another of brome grass (Bromus catharticus) and red clover (Trifolium repens). The farmer reported that the disease was endemic in the farm, affecting nearly 3% of the herd each year. Three steers had multiple nodular lesions, similar to those observed in the dairy herd, in the region of the parotid, submandibular, and retropharyngeal lymph nodes. One steer had an exophytic ulcerated and bleeding nodular lesion, 4 × 3 × 2 cm, in the gingiva below the right incisors (Fig. 2A). This lesion, examined at the slaughterhouse, was firm and white with yellow granules distributed on the cut surface. A few days after the first visit to the farm, one steer was drooling, with foaming at the mouth, tachypnea, and severe abdominal distention mainly in the left paralumbar fossa, along with severe pain and an abdominal tympanic sound. The animal remained recumbent and unresponsive and died acutely. At autopsy, the mucous membranes were severely cyanotic. An 11 × 7 × 3 cm mass was observed in the dorsal portion of the oropharynx (Fig. 2B, 2C). The mass obstructed the lumen of the oropharynx and the proximal esophagus (Fig. 2B) and had an irregular white surface with hemorrhages and yellow nodules (Fig. 2C). The cut surface was firm and multilobular. The tracheal and bronchial lumens were occupied by a large amount of white stable foam, and diffuse pulmonary congestion and edema were observed. Two ovoid nodules of ~3 cm diameter, with irregular yellow cut surfaces surrounded by a white fibrous capsule, were observed in the left and right caudal pulmonary lobes (Fig. 2D). In the esophagus, at the level of the thoracic inlet, there was a well-demarcated line that separated the congested cervical esophageal mucosa from the pale thoracic esophageal mucosa (bloat line).
Figure 2.
A. Ulcerated exophytic nodular pyogranuloma in the gingiva of a steer. B, C. A nodular pyogranuloma on the dorsal portion of the oropharynx obstructs the lumen of the esophagus of a steer (B). The 11 × 7 × 3 cm nodule expands the oropharynx (C). D. A pulmonary pyogranuloma in the steer with the lesion in the oropharynx.
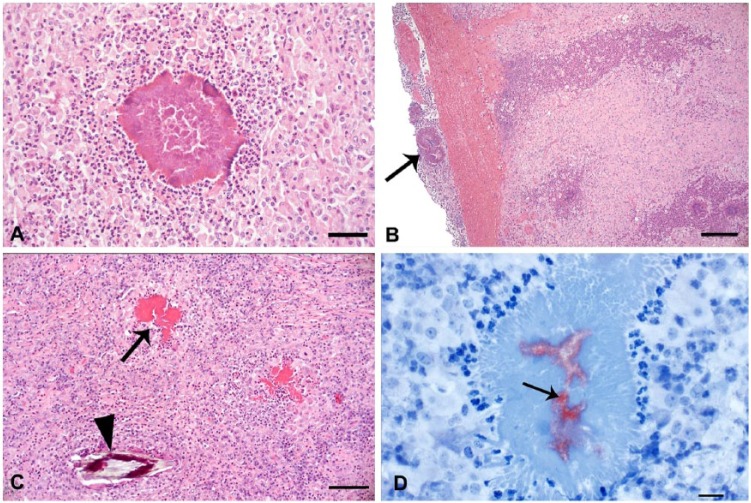
Direct microscopic examination of the exudate obtained by core needle biopsies and autopsy showed the distinctive club-shaped clusters observed in actinobacillosis. The histologic lesions were multifocal-to-coalescing pyogranulomatous lymphadenitis, dermatitis, and cellulitis with dermal ulceration. Pyogranulomas had a necrotic center with cellular debris that occasionally contained gram-negative coccobacilli surrounded by acellular, club-shaped, deeply eosinophilic palisading material, typical of the Splendore–Hoeppli reaction, which was further surrounded by an inflammatory infiltrate of neutrophils, macrophages, and occasional multinucleate giant cells (Fig. 3A). Fibrous connective tissue separated the pyogranulomas. No microorganisms were observed with Ziehl–Neelsen stain or PAS reaction. Inflammatory lesions in the prescapular lymph node extended perinodally to involve the adjacent adipose tissue and skin, and showed pyogranulomas with Splendore–Hoeppli material and diffuse fibrosis and granulation tissue (Fig. 3B). In the gingival lesion, as well as the pyogranulomas typical of actinobacillosis, a foreign body granuloma was observed with palisading macrophages surrounding birefringent vegetal material (Fig. 3C). Typical Actinobacillus-induced pyogranulomas were observed in the oropharyngeal and pulmonary nodules in the steer that died acutely. In the 2 cases processed by IHC, strong, finely granular immunoreactivity restricted to the necrotic centers of the pyogranulomas was observed (Fig. 3D). No immunoreactivity was observed in the areas occupied by Splendore–Hoeppli material, inflammatory infiltrate, fibrosis, or granulation tissues.
Figure 3.
A. Necrotic center of the prescapular lymph node of a dairy cow, with club-shaped clusters and Splendore–Hoeppli material surrounded by neutrophils and macrophages. H&E. Bar = 50 µm. B. The presence of pyogranulomas in the parenchyma and in the outer part of the capsule (arrow) of a prescapular lymph node suggests that lesions in the subcutaneous tissue are an extension from the lymph node lesion. H&E. Bar = 50 µm. C. In addition to the Splendore–Hoeppli material in the pyogranuloma (arrow) in the gingiva of a beef steer, plant material is present within the lesion (arrowhead), suggesting that this lesion originated from a contaminated wound. H&E. Bar = 50 µm. D. Strong, finely granular immunoreactivity (arrow) in an extracellular location within the necrotic center of the pyogranuloma in the gingiva of a beef steer. Anti-BCG immunohistochemistry with hematoxylin counterstain. Bar = 20 µm.
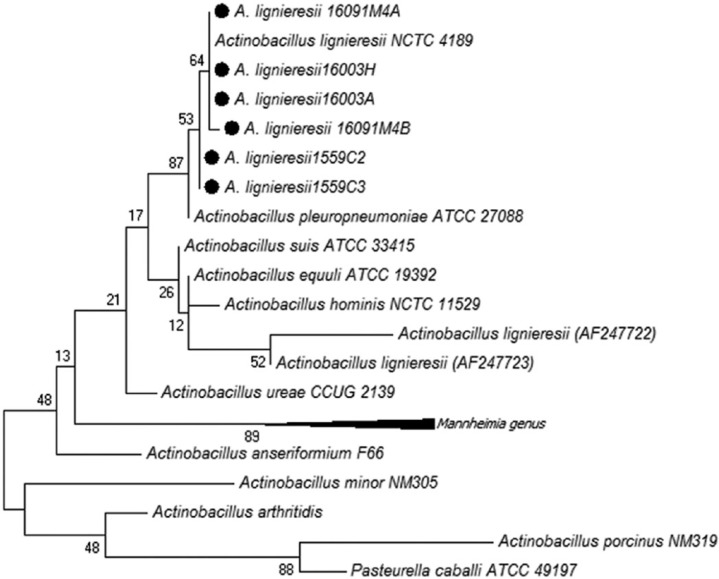
Small sticky colonies of gram-negative rods were isolated on MacConkey and blood agar after 48 h of incubation. Colonies were non-hemolytic; positive for oxidase, urease, and lysine decarboxylase; fermented glucose, lactose, xylose, and mannose; and did not produce hydrogen sulfide or indole, a biochemical profile compatible with Actinobacillus spp. The phylogenetic analysis allowed for classification of the isolates as A. lignieresii (Fig. 4). Sequences were deposited in GenBank under accessions KX196156–KX196159, KY492332, and KY492333.
Figure 4.
A phylogenetic tree constructed using sequences of different species type strains by the maximum likelihood method. The Actinobacillus lignieresii sequences corresponding to isolates obtained in our work are marked with black dots.
Seven of the 8 cattle in the dairy farm recovered within 90 d of treatment. The nodules regressed, and the ulcers healed. Five days after treatment, one of the cows aborted a fetus with a gestational age of 160 d. One cow with severe ulcerated cutaneous lesions showed transitory improvement after treatment, but later relapsed and was therefore sent to slaughter. Other affected cattle were treated by the practitioner with sodium iodine and recovered.
Discussion
The outbreaks reported herein and in previous reports from Uruguay,1,9,10 Brazil,25 and Argentina15 suggest that lymphatic actinobacillosis is significant in the southern regions of South America. In previous outbreaks in Uruguay1,9,10 and in other countries,6,7,20 the disease was associated with the feeding of dry fibrous forage that may injure the mucous membranes of the oral cavity, favoring the colonization of deeper tissues by A. lignieresii, which is a commensal of the upper digestive tract. In contrast, in the beef cattle outbreaks reported herein, the disease occurred in a herd that grazed in green, lush pastures, and the affected dairy herd ingested a typical diet for lactating cows. In southern Brazil, the frequency of actinobacillosis lesions in the lymph nodes of the head and neck in slaughterhouses was similar in cattle from different production systems.19 This information, associated with the absence of lesions in the mouth, suggests that feeding with fibrous foods that may cause trauma to the oral mucosa represents a risk factor for lymphatic actinobacillosis, but it does not occur in all outbreaks and other factors should be considered. In numerous outbreaks in Argentina in 1900–1902,15 a relationship was not found between the disease and the type of forage that the animals were being fed. These authors suggested that the high frequency of the disease may have been associated with previous outbreaks of foot-and-mouth disease, probably because the virus-induced oral lesions favored the bacterial infection, or as a result of the stress and immunosuppression caused by the viral disease. In an outbreak in suckling calves in Uruguay, actinobacillosis was associated with the presence of mud on the cows’ udders as a consequence of high environmental humidity.10 In all of our cases, only a single lesion in the gingiva, in a steer without lesions in the lymph nodes, was associated with intralesional plant material in the pyogranuloma.
Because some of the previously reported outbreaks involved young cattle,1,9,10 incomplete permanent dentition was suggested as a risk factor for this form of the disease.10 However, other authors reported outbreaks in cattle of different ages, including adults,11,20,25 or exclusively in cows.7 Of the outbreaks in our report, one outbreak affected adult cattle in a semi-intensive system and the other affected 2-y-old steers in grazing pastures, indicating that the disease occurs in cattle of different ages, including adults with complete dentition, in different production systems. The affected animals in our report did not show significant weight loss, despite the presence of open, draining cutaneous pyogranulomas for several months. The continuous contamination of the environment by affected animals may have contributed to the transmission of the disease, highlighting the importance of isolating and treating the affected animals as soon as possible after initial diagnosis. Additionally, in other outbreaks, the long clinical course was associated with the occurrence of newly affected animals for periods of 1–6 mo.6,7,10,11
Data from slaughterhouses showed that actinobacillosis affecting the parotid, submandibular, and retropharyngeal lymph nodes is one of the main causes of granulomatous lesions in southern South America. In southern Brazil, 1.15% of the slaughtered cattle showed gross lesions compatible with actinobacillosis, which was confirmed histologically in 82.4% of the cases.19 In Argentina, 14% of the lesions diagnosed macroscopically as tuberculosis by meat inspectors at the slaughterhouses were actually actinobacillosis or actinomycosis.8 This indicates the significance of actinobacillosis as a differential in the diagnosis of tuberculosis and the importance of pursuing histologic and microbiologic examinations of compatible gross lesions for pathologic and etiologic confirmation. In Uruguay, no statistics are publicly available, but an estimated 1–2% of slaughtered cattle have actinobacillosis-like gross lesions in the parotid, submandibular, and retropharyngeal lymph nodes (F. Riet-Correa, unpublished data). The relatively high frequency of lesions at slaughterhouses suggests that actinobacillosis is widespread in the region, causing numerous subclinical cases and, occasionally, outbreaks with a variable incidence of clinically affected animals.
As suggested by the location of the lesions and as observed in the prescapular lymph node obtained surgically, the primary lesion is most likely located in the lymph nodes and later extends to the subcutaneous tissue and skin to cause ulceration. This form of actinobacillosis has been referred to as atypical,4,11,13 unusual,6 or cutaneous.1,15 Because the primary lesion seems to occur in the lymphatic system, the correct designation for this form of the disease should be lymphatic actinobacillosis. A similar localization of the lesions in the parotid, submandibular, and retropharyngeal lymph nodes is observed at slaughterhouses19 and has been reported in other outbreaks of the disease.1,4,6,9–11,13,15 The designation of cutaneous actinobacillosis should be used preferentially in cases in which the lesion is associated with infection of skin wounds by A. lignieresii and may occur in different anatomic locations.2,5,12,16,18 Numerous reports and information from slaughterhouses suggest that lymphatic actinobacillosis is much more frequent than other forms of the disease and may occur with high incidence. The clinical signs and lesions and the absence of concurrent lesions in the oral mucosa in most affected animals suggest that A. lignieresii penetrates through the intact mucosa of the mouth or the oropharynx and localizes primarily in the regional lymph nodes. Occasionally, the agent lodged in the lymph nodes of the head and neck disseminates through the lymphatic vessels, causing lymphangitis and reaching other lymph nodes, as observed in our report in one animal with a lesion in the prescapular lymph node and in some animals with small nodules arranged linearly along the lymphatic vessels of the neck.
In various reports of lymphatic actinobacillosis, the incidence of the disease is variable, ranging from 1–10%,10,11,25 10–20%,1,9,20 20–50%,7,15 and even up to 73%.6 In the outbreaks reported herein, the incidence was 7.4% in the dairy herd and 1.5% in the beef herd, with only 1 death (0.3%) that was attributed to ruminal bloating secondary to esophageal and oropharyngeal obstruction by an extensive compressive pyogranuloma. In general, deaths do not occur or are rare, up to 0.5%.10
In the dairy herd, the response to treatment with sodium iodide was very good. The majority of the animals recovered, and no new cases were observed. These results suggest that the treatment, especially when applied early in the course of the disease, is effective. Similar responses to treatment with iodides or antibiotics have been reported previously.6,7,10,11 Five days after treatment, one cow aborted. Treatment with sodium iodide has been associated with abortion rarely;17 although, the experimental administration of sodium iodide to 10 pregnant cows at double the recommended dose did not induce abortion.17 In our case, because the fetus was not submitted to the laboratory for diagnostic investigation, the cause of the abortion remained undetermined. None of the other 5 pregnant cows that received a therapeutic dose of sodium iodine aborted. If iatrogenic abortion is a concern, cows may be treated with antibiotic formulations that are indicated for pregnant animals.
In the outbreak in beef cattle, in addition to the lymphatic form of the disease, 2 cases did not have obvious lymphatic or cutaneous involvement. One was a case of gingival actinobacillosis associated with the presence of penetrating plant material that was observed histologically. The other was a case of oropharyngeal and pulmonary actinobacillosis. In these 2 outbreaks and in other outbreaks of lymphatic actinobacillosis, no cases involved the tongue,1,11,20,25 suggesting that this latter form of the disease is sporadic and possibly associated with lesions in the mucosa of the tongue that facilitate the invasion of the agent into the submucosa.
The IHC technique of using a polyclonal primary antibody against BCG has been used previously for the intralesional detection of several microorganisms in animal tissues based on its cross-reactivity with various bacterial, fungal, and some protozoal agents.3,27 We used this technique to successfully detect A. lignieresii in the necrotic foci of the pyogranulomas. This antibody has been used to identify other species within the genus Actinobacillus, including A. pleuropneumoniae, A. suis, and A. equuli.3,27
With the aid of molecular and classical microbiological approaches, it was possible to affiliate the isolates obtained from the cases in our report with the species A. lignieresii. All of the isolates clustered with A. lignieresii type strain NCTC 4189. This species is closely related to A. pleuropneumoniae, the etiologic agent of contagious porcine pleuropneumonia, as displayed in the phylogenetic tree. Phenotypically, these 2 species are very similar, and A. pleuropneumoniae differs from A. lignieresii in its ability to induce hemolysis,22 somewhat limiting the biochemical differentiation of these 2 species. Although A. lignieresii has been recognized for more than a century, only 3 A. lignieresii 16S ribosomal (r)DNA gene sequences that had good quality and were more than 1,200 base pairs in length were available in the RDP database at the time that the sequences generated in our report were released. One of the available sequences corresponded to A. lignieresii type strain NCTC 4189; however, the other 2 previously available sequences (AF247722 and AF247723) did not cluster with the type strain or with the sequences generated in our work, suggesting that they probably do not belong to the species A. lignieresii. This demonstrates the importance of conducting phylogenetic analyses of Actinobacillus spp. isolates, particularly those that are difficult to classify phenotypically. The 16S rDNA sequences generated in our work have been released and are publicly available in GenBank, broadening the spectrum of available sequences for this agent.
Acknowledgments
We thank Yisell Perdomo (INIA La Estanzuela) and Karen Sverlow (California Animal Health and Food Safety Laboratory, University of California, Davis) for technical assistance with the histologic and immunohistochemical techniques.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Albornoz L, Sali G. Reporte de un caso de actinobacilosis enzoótica en vaquillonas Holando en sistema pastoril [Case report of enzootic cutaneous actinobacillosis in dairy heifers in a pasture-based production system]. Veterinaria (Montevideo) 2012;48:29–31. Spanish. [Google Scholar]
- 2. Aslani MR, et al. An atypical case of actinobacillosis in a cow. Zentralbl Veterinarmed A 1995;42:485–488. [DOI] [PubMed] [Google Scholar]
- 3. Bonenberger TE, et al. Rapid identification of tissue micro-organisms in skin biopsy specimens from domestic animals using polyclonal BCG antibody. Vet Dermatol 2001;12:41–47. [DOI] [PubMed] [Google Scholar]
- 4. Borsberry S. Atypical actinobacillosis in a dairy replacement herd. Vet Rec 2002;151:308. [PubMed] [Google Scholar]
- 5. Cahalan SD, et al. Atypical cutaneous actinobacillosis in young beef cattle. Vet Rec 2012;171:375. [DOI] [PubMed] [Google Scholar]
- 6. Campbell SG, et al. An unusual epizootic of actinobacillosis in dairy heifers. J Am Vet Med Assoc 1975;66:604–606. [PubMed] [Google Scholar]
- 7. Dhand NK, et al. Outbreak of actinobacillosis in dairy cows. Vet Rec 2003;153:280. [PubMed] [Google Scholar]
- 8. Dubarry J, et al. Actinomicosis y Actinobacilosis: una causa frecuente de lesiones granulomatosas en los bovinos del Departamento Maracó de la provincia de La Pampa - República Argentina [Actinomycosis and actinobacillosis: a frequent cause of granulomatous lesions in cattle in La Pampa province, Argentina]. Ciencia Veterinaria Argentina 2003;6:34–41. Spanish. [Google Scholar]
- 9. Dutra F. Actinobacilosis [Actinobacillosis]. Archivos Veterinarios del Este 2004;2:9. Spanish. [Google Scholar]
- 10. Dutra F. Actinobacilosis ganglionar en terneros [Actinobacillosis of the lymph nodes in calves]. Archivos Veterinarios del Este 2010;6:10–11. Spanish. [Google Scholar]
- 11. Hebeler HF, et al. Atypical actinobacillosis in a dairy herd. Vet Rec 1961;73:517–521. [Google Scholar]
- 12. Holzhauer M, Roumen TPHM. Atypical actinobacillosis in a dairy replacement herd. Vet Rec 2002;151:276. [PubMed] [Google Scholar]
- 13. Kish FG, et al. Atypical actinobacillosis in a dairy cow. J Anim Poultry Sci 2014;1:1–7. [Google Scholar]
- 14. Kumar S, et al. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 2016;33:1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lignierès J, Spitz G. L’actinobacillose [Actinobacillosis]. Bull Soc Centr Med Vet 1902;20:487–536. French. [Google Scholar]
- 16. Margineda CA, et al. Atypical actinobacillosis in bulls in Argentina: granulomatous dermatitis and lymphadenitis. Pesq Vet Bras 2013;33:1–4. [Google Scholar]
- 17. Miller HV, Drost M. Failure to cause abortion in cows with intravenous sodium iodide treatment. J Am Vet Med Assoc 1978;172:466. [PubMed] [Google Scholar]
- 18. Milne MH, et al. Clinical recognition and treatment of bovine cutaneous actinobacillosis. Vet Rec 2001;148:273–274. [DOI] [PubMed] [Google Scholar]
- 19. Mondadori AJ, et al. Actinobacilose em bovinos no Rio Grande do Sul [Actinobacillosis in bovines in Rio Grande do Sul]. Ciência Rural 1994;24:571–577. Portuguese. [Google Scholar]
- 20. Nakazawa M, et al. Collective outbreaks of bovine actinobacillosis. Jap J Vet Sci 1977;39:549–557. [DOI] [PubMed] [Google Scholar]
- 21. Peli A, et al. An atypical case of respiratory actinobacillosis in a cow. J Vet Sci 2009;10:265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phillips JE. The genus Actinobacillus. In: Balows A, et al., eds. The Prokaryotes. New York: Springer, 1992:3342–3351. [Google Scholar]
- 23. Radostits OM, et al. Diseases associated with bacteria—IV. In: Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. 10th ed. Philadelphia, PA: Saunders Elsevier, 2007:1046–1048. [Google Scholar]
- 24. Rebhun WC, et al. Atypical actinobacillosis granulomas in cattle. Cornell Vet 1988;78:125–130. [PubMed] [Google Scholar]
- 25. Riet-Correa F, et al. Actinobacilosis em bovinos [Actinobacillosis in bovines]. Boletím do Laboratório Regional de Diagnóstico, Universidade Federal de Pelotas 1983;2:3l–32. Spanish. [Google Scholar]
- 26. Rycroft AN, Garside LH. Actinobacillus species and their role in animal disease. Vet J 2000;159:18–36. [DOI] [PubMed] [Google Scholar]
- 27. Szeredi L, et al. Application of anti-BCG antibody for rapid immunohistochemical detection of bacteria, fungi and protozoa in formalin-fixed paraffin-embedded tissue samples. Acta Vet Hung 2008;56:89–99. [DOI] [PubMed] [Google Scholar]
- 28. Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 1993;10:512–526. [DOI] [PubMed] [Google Scholar]
- 29. Uzal FA, et al. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 2 St. Louis, MO: Elsevier, 2016:18–19. [Google Scholar]
- 30. Wang Q, et al. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 2007;73:5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]