Abstract
Canine anaplasmosis is a tick-borne disease of dogs that results following infection with Anaplasma phagocytophilum or Anaplasma platys. The SNAP 4Dx Plus test (IDEXX Laboratories) and the VetScan Canine Anaplasma Rapid test (Abaxis) are commercial in-house rapid tests for the detection of antibody to these 2 antigenically related Anaplasma species. We evaluated 2 tests using serum and whole blood samples obtained from reference laboratories and veterinary hospitals. Samples were obtained from regions of the country known to be habitats of the primary tick vectors. The A. phagocytophilum sample set comprised 236 dog sera from the northeastern and midwestern United States; the A. platys sample set comprised 179 sera from dogs living in the southwestern United States. An indirect immunofluorescent antibody (IFA) test and an A. platys species-specific ELISA were used as reference assays for the A. phagocytophilum and A. platys samples, respectively. The SNAP test demonstrated significantly higher sensitivity (84.7% for A. phagocytophilum and 83.1% for A. platys), compared to the VetScan test (39.0% for A. phagocytophilum and 57.6% for A. platys). The specificity of the SNAP test (95.8% for A. phagocytophilum and 99.2% for A. platys) was significantly greater than the VetScan test (85.6% for A. phagocytophilum and 82.5% for A. platys). In a separate clinic study, conducted within an A. phagocytophilum–endemic state (Minnesota) using 154 whole blood samples from client-owned dogs, the VetScan test was negative for 22 of 39 SNAP and IFA seropositive samples.
Keywords: Anaplasma phagocytophilum, dogs, tick-borne diseases
Canine granulocytic anaplasmosis and canine cyclic thrombocytopenia are tick-borne diseases caused by Anaplasma phagocytophilum and Anaplasma platys, respectively. Although these 2 Anaplasma species are related evolutionarily and antigenically, each causes a markedly different clinical presentation, each is transmitted by different tick vectors that are often found in different geographic regions, and each infects different cell types (neutrophils and thrombocytes, respectively).7,11,12,19,20 Both species are capable of infecting humans as well as dogs.1,4
A. phagocytophilum is transmitted by Ixodes spp., which are also competent vectors for Borrelia burgdorferi. Ixodes ticks are found in many parts of the world and, in the United States, are most prevalent in the northeastern, midwestern, and western coastal regions. Within an Ixodes scapularis endemic area of the United States (Minnesota), 29% of dogs were seroreactive only to A. phagocytophilum and 25% were seroreactive to both A. phagocytophilum and B. burgdorferi.2 Typical clinical signs of granulocytic anaplasmosis include lethargy, fever, anorexia, vomiting, diarrhea, and bleeding disorders. A. phagocytophilum can cause hematologic abnormalities, including thrombocytopenia, leukopenia, and anemia.5,7,10,19
A. platys infection produces cyclic thrombocytopenia over a 10–14-d period.12 Although vector competency has not been proven, A. platys is believed to be transmitted by Rhipicephalus sanguineus, a tick found worldwide, which transmits Ehrlichia canis, Babesia spp., and other pathogens. In the United States, canine cyclic thrombocytopenia is more commonly reported in the south. In northeastern Arizona, a region in which only R. sanguineus is found, 10.2–12.4% of dogs were seroreactive to Anaplasma spp.6 In North America, A. platys is considered less pathogenic than A. phagocytophilum; however, reported clinical abnormalities include fever, anorexia, bleeding disorders, and anterior uveitis.8
The value of diagnosing and screening for tick-borne diseases in dogs has gained increasing importance as tick distributions have expanded geographically, morbidity associated with acute or chronic infection and coinfections has been defined, and effective acaricide products have been developed.3,13,15,18,22 Indirect immunofluorescent antibody (IFA) tests are commercially available and have long served as the mainstay for diagnosing anaplasmosis. IFA cannot differentiate A. phagocytophilum and A. platys infections.12,21 Speciation can be achieved using PCR methods using whole blood samples.5,12 In a 2014 study, a species-specific ELISA was reported.18
The SNAP test (SNAP 4Dx Plus test, IDEXX Laboratories, Westbrook, ME) is a rapid in-house ELISA previously validated for concurrent detection of antibodies to A. phagocytophilum, A. platys, E. canis, Ehrlichia ewingii, B. burgdorferi, and heartworm antigen.9,13,16,21 The VetScan test (VetScan Canine Anaplasma Rapid test, Abaxis, Union City, CA) is commercially available for detection of Anaplasma antibodies but its performance has not been reported, to our knowledge. We compared the performance of these 2 tests for the detection of Anaplasma antibodies in naturally exposed dogs. For our study, only the Anaplasma portion of the SNAP test was assessed.
Study samples for A. phagocytophilum were collected from regional IDEXX Reference Laboratories and individual clinics located in northeast and upper midwest United States. Samples were originally submitted to the reference laboratories for A. phagocytophilum IFA testing unrelated to our study. Sera or plasma were collected after requested testing was completed by the reference laboratory; IFA results were used as the reference for these samples (IFA titer cutoff ≥ 1:50). Samples (n = 236) were obtained, with a majority from IDEXX Reference Laboratories and 83 from 2 clinics. One-half of the samples (n = 118) were IFA reactive with a wide range of IFA titers (Fig. 1).
Figure 1.
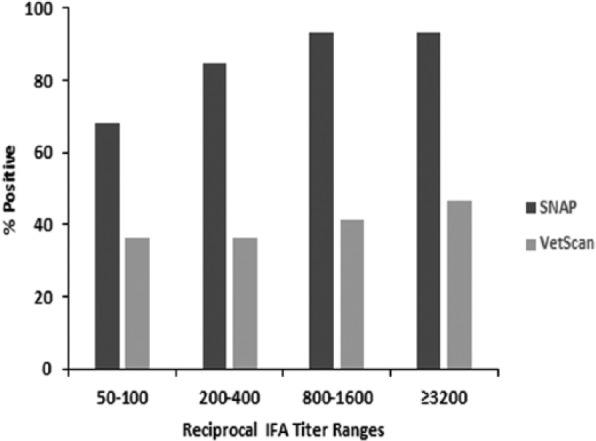
Percent positive results on the SNAP and VetScan tests for samples within different reciprocal indirect immunofluorescent antibody (IFA) test titer ranges. Of 118 IFA-positive samples, the number of samples within each of the reciprocal IFA titer ranges was 22 (50–100), 52 (200–400), 29 (800–1,600), and 15 (≥3,200).
To identify A. platys samples, archived sera originally collected from dogs living in the southwestern United Sates (Hopi Reservation, AZ) were screened at IDEXX Laboratories (Westbrook, ME) using an A. platys–specific ELISA.6,18 The peptide reagent used in this ELISA was distinctly different from the one used in the SNAP test. One hundred seventy-nine samples were identified: 59 positive and 120 negative.
Samples were randomized and blind-labeled before testing with the SNAP and VetScan tests. There were no additional freeze–thaw cycle or sample handling differences in testing events between the 2 tests. Testing and visual interpretation of results were performed following the manufacturers’ instructions supplied with each test kit. Each test result was interpreted independently by 3 laboratory technicians without knowledge of the IFA or ELISA results. Tests were designated positive or negative based on a consensus of 2 or 3 technicians.
Statistical analysis was performed (SAS v.9.4, SAS Institute, Cary, NC). Sensitivity and specificity for rapid tests were determined using the IFA or species-specific ELISA results as the reference method. Statistical significance was determined by McNemar exact tests (α = 0.05). To adjust for multiple comparisons, we used the step-down Holm–Bonferroni method applied to the exact McNemar p values, which allowed control for the family-wise error rate in the strong sense without independence assumption.
Using A. phagocytophilum IFA as the reference method, the SNAP test demonstrated significantly higher sensitivity (84.7% for SNAP and 39.0% for VetScan, p < 0.001) and specificity (95.8% for SNAP and 85.6% for VetScan, p = 0.0118; Table 1). Compared to the A. platys–specific ELISA, sensitivity was 83.1% and specificity was 99.2% for the SNAP test, and 57.6% and 82.5% for the VetScan test, respectively. These differences between SNAP and VetScan were also statistically significant (p = 0.0026 for sensitivity and p < 0.001 for specificity).
Table 1.
SNAP and VetScan test results for canine samples from Anaplasma phagocytophilum– and Anaplasma platys–endemic regions of the United States.
| Samples | SNAP test | VetScan test |
|---|---|---|
| A. phagocytophilum | ||
| Positive (n = 118) | ||
| No. positive (% sensitivity) | 100 (84.7) | 46 (39.0) |
| 95% CI | 77.0–90.2 | 30.7–48.0 |
| Negative (n = 118) | ||
| No. negative (% specificity) | 113 (95.8) | 101 (85.6) |
| 95% CI | 90.1–98.4 | 78.0–90.9 |
| A. platys | ||
| Positive (n = 59) | ||
| No. positive (% sensitivity) | 49 (83.1) | 34 (57.6) |
| 95% CI | 71.3–90.6 | 44.9–69.4 |
| Negative (n = 120) | ||
| No. negative (% specificity) | 119 (99.2) | 99 (82.5) |
| 95% CI | 94.9–100 | 74.6–88.3 |
CI = confidence interval. A. phagocytophilum and A. platys samples were collected from endemic regions in the northeastern/upper midwestern and southern United States, respectively. Samples were assigned based on results of reference tests. The A. phagocytophilum reference test was the IFA; the A. platys reference test was the species-specific ELISA.
Test sensitivity was plotted for samples within different titer level groups for all IFA seropositive samples (Fig. 1). Among the 118 A. phagocytophilum IFA seropositive samples, there were 72 VetScan-negative results and 18 SNAP-negative results. VetScan was false negative for more than half of the samples in every titer range, including the highest titer range (≥1:3,200). SNAP sensitivity was significantly higher for the following titer groups: 1:200–1:400 (p < 0.0001), 1:800–1:1,600 (p < 0.0001), and ≥3,200 (p = 0.0156).
To further compare performance of the 2 test kits in a clinic setting, a study was conducted in a veterinary hospital in Minnesota, an area endemic for A. phagocytophilum. Dogs were tested at the sole discretion of the attending veterinarians with fresh whole blood samples. The SNAP and VetScan tests were run side-by-side for comparison according to the manufacturers’ instructions. Certified veterinary technicians performed tests and recorded results. Different kit lots were used in the clinic study than those used in the study described above for both SNAP and VetScan tests. A total of 154 dogs were tested in a period of over 4 months, of which 42 (27.3%) were SNAP test positive and 20 (13.0%) VetScan test positive. Samples with positive results on either test were submitted to IDEXX Reference Laboratories for confirmation by A. phagocytophilum IFA (2 SNAP-positive, VetScan-negative samples had insufficient volume for testing). The IFA result was negative for 1 of the 40 SNAP-positive samples and 3 of the 20 VetScan-positive samples. The single IFA-negative, SNAP-positive sample was VetScan positive, and 2 of the 3 VetScan-positive, IFA-negative samples were SNAP negative. Consistent with findings from the first study, more than half of SNAP and IFA seropositive dogs were negative by VetScan. Among the 39 samples positive in both SNAP and IFA, the percentage of positive VetScan results within the IFA titer groups 1:50–1:100, 1:200–1:400, and 1:800–1:1,600 was 0% (0 of 7), 56% (14 of 25), and 43% (3 of 7), respectively.
Discrepant results between IFA and rapid tests are more commonly found within low titer samples, which may be related to enhanced IFA sensitivity or, potentially, a lack of IFA specificity associated with cross-reactivity to other species of bacteria.17 Reduced sensitivity found in the VetScan test for high titer samples may indicate an issue in assay design or failure of the test to detect antibodies to all A. phagocytophilum variants, which could be a result of the antigen used in the test.12,14
Acknowledgments
We thank Sean Hardy for the statistical analysis.
Footnotes
Declaration of conflicting interests: J Liu, H Bewsey, TP O’Connor, and R Chandrashekar are employees of IDEXX Laboratories.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Arraga-Alvarado CM, et al. Case report: molecular evidence of Anaplasma platys infection in two women from Venezuela. Am J Trop Med Hyg 2014;91:1161–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beall MJ, et al. Serological and molecular prevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Ehrlichia species in dogs from Minnesota. Vector Borne Zoonotic Dis 2008;8:455–464. [DOI] [PubMed] [Google Scholar]
- 3. Bowman D, et al. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: results of a national clinic-based serologic survey. Vet Parasitol 2009;160:138–148. [DOI] [PubMed] [Google Scholar]
- 4. Breitschwerdt EB, et al. Intravascular persistence of Anaplasma platys, Ehrlichia chaffeensis, and Ehrlichia ewingii DNA in the blood of a dog and two family members. Parasit Vectors 2014;7:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diniz PP, Breitschwerdt EB. Anaplasma phagocytophilum infection (canine granulocytotropic anaplasmosis). In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 4th ed. St. Louis, MO: Elsevier, 2012: 244–253. [Google Scholar]
- 6. Diniz PP, et al. High prevalence of tick-borne pathogens in dogs from an Indian reservation in northeastern Arizona. Vector Borne Zoonotic Dis 2010;10:117–123. [DOI] [PubMed] [Google Scholar]
- 7. Eberts MD, et al. Typical and atypical manifestations of Anaplasma phagocytophilum infection in dogs. J Am Anim Hosp Assoc 2011;47:e86–e94. [DOI] [PubMed] [Google Scholar]
- 8. Gaunt S, et al. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasit Vectors 2010;3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldstein RE, et al. Performance comparison of SNAP® 4Dx® plus and AccuPlex®4 for the detection of antibodies to Borrelia burgdorferi and Anaplasma phagocytophilum. Intern J Appl Res Vet Med 2014;12:141–147. [Google Scholar]
- 10. Granick JL, et al. Anaplasma phagocytophilum infection in dogs: 34 cases (2000–2007). J Am Vet Med Assoc 2009;234:1559–1565. [DOI] [PubMed] [Google Scholar]
- 11. Hoskins JD, et al. Antibodies to Ehrlichia canis, Ehrlichia platys, and spotted fever group rickettsiae in Louisiana dogs. J Vet Intern Med 1988;2:55–59. [DOI] [PubMed] [Google Scholar]
- 12. Little SE. Ehrlichiosis and anaplasmosis in dogs and cats. Vet Clin North Am Small Anim Pract 2010;40:1121–1140. [DOI] [PubMed] [Google Scholar]
- 13. Little SE, et al. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2010–2012. Parasit Vectors 2014;7:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McMahan CS, et al. Factors associated with Anaplasma spp. seroprevalence among dogs in the United States. Parasit Vectors 2016;9:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicholson WL, et al. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol 2010;26:205–212. [DOI] [PubMed] [Google Scholar]
- 16. O’Connor TP. SNAP assay technology. Topics Compan An Med 2015;30:132–138. [DOI] [PubMed] [Google Scholar]
- 17. O’Connor TP, et al. Comparison of an indirect immunofluorescence assay, western blot analysis, and a commercially available ELISA for detection of Ehrlichia canis antibodies in canine sera. Am J Vet Res 2006;67:206–210. [DOI] [PubMed] [Google Scholar]
- 18. Qurollo BA, et al. A serological survey of tick-borne pathogens in dogs in North America and the Caribbean as assessed by Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeensis, E. ewingii, and Borrelia burgdorferi species-specific peptides. Infect Ecol Epidemiol 2014;4:24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ravnik U, et al. Anaplasmosis in dogs: the relation of haematological, biochemical and clinical alterations to antibody titre and PCR confirmed infection. Vet Microbiol 2011;149:172–176. [DOI] [PubMed] [Google Scholar]
- 20. Sainz A, et al. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasit Vectors 2015;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stillman BA, et al. Performance of a commercially available in-clinic ELISA for detection of antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii and Dirofilaria immitis antigen in dogs. J Am Vet Med Assoc 2014;245:80–86. [DOI] [PubMed] [Google Scholar]
- 22. Yancey CB, et al. Regional seroreactivity and vector-borne disease co-exposures in dogs in the United States from 2004–2010: utility of canine surveillance. Vector Borne Zoonotic Dis 2014;14:724–732. [DOI] [PubMed] [Google Scholar]


