Abstract
We evaluated 134 cutaneous plasmacytomas in 125 dogs submitted to the University of Tennessee surgical biopsy service between 2009 and 2012 to determine whether the presence of intravascular neoplastic cells had prognostic significance. Tumors occurred in middle-aged to geriatric dogs (range: 5–16 y, mean: 9.6 y) and most frequently involved the skin of the head and distal limbs. Diagnoses were made based on light microscopy, and in some cases confirmed by immunoreactivity of neoplastic cells for MUM1. Tumors were categorized as having or not having intravascular neoplastic cells within sections examined. The intravascular location of tumor cells was confirmed by immunoreactivity of endothelial cells for factor VIII–related antigen in 3 cases. Neoplastic cells within vessel lumens were identified in 20 of 125 dogs (16%). Submitting veterinary practices were contacted for follow-up data on patients including local recurrence and cutaneous plasmacytomas in other locations. Follow-up information was acquired on 99 dogs (79%). Recurrence was documented in one dog with cutaneous plasmacytomas; both masses had incomplete margins and intravascular neoplastic cells. Additional distant cutaneous plasmacytomas were later diagnosed in 3 patients; none of these dogs had intravascular neoplastic cells. In no cases were cutaneous plasmacytomas suspected to be a cause of death or reason for euthanasia. Intravascular neoplastic cells were more common in tumors of the distal limbs (36%) compared to other locations (11%; p = 0.0007). The presence of intravascular neoplastic cells did not affect prognosis in cutaneous plasmacytomas.
Keywords: Cutaneous plasma cell tumor, dogs, intravascular, myeloma, neoplasia, plasmacytoma, skin
Canine cutaneous plasmacytoma (one form of extramedullary plasmacytoma) is a solitary round-cell neoplasm of the skin most commonly identified in middle-aged and geriatric dogs. Cutaneous plasmacytoma, osseous plasmacytoma, and multiple myeloma are neoplastic entities of plasma cell origin. Plasmablastic and lymphoplasmacytic lymphoma are entities of B-lymphocyte origin found in lymph nodes and non-cutaneous organs. Additional plasmacytoid entities are reported in humans.1,12 Plasmacytomas are more common in cutaneous than non-cutaneous locations and typically occur in the skin of the head, trunk, and legs. Subcutaneous plasmacytomas are rare but reported.8 Dogs with cutaneous plasmacytomas are most commonly 6–13 y of age, with an average age of 10 y. Reported breed predilections include the American and English Cocker Spaniels, Airedale Terrier, Kelly Blue Terrier, Scottish Terrier, and Yorkshire Terrier. A weak sex predilection towards male dogs has been reported.3,7
Several immunohistochemical labels for plasmacytomas are utilized in veterinary medicine, including multiple myeloma oncogene 1/interferon regulatory factor 4 (MUM1/IRF4), CD79a, CD20, and CD45RA. One study of 108 canine patients revealed a sensitivity of 93.5% for MUM1/IRF4 labeling versus 56.2% and 19.4% for CD79a and CD20, respectively.10 Although cutaneous plasmacytoma cells differ morphologically from non-neoplastic plasma cells, they frequently express monoclonal immunoglobulins and vimentin.2,6
A typical cutaneous plasmacytoma is a solitary, firm, poorly haired to alopecic, and variably ulcerated nodular mass that is most commonly diagnosed based on routine surgical biopsy, sometimes with the assistance of immunophenotyping. Such plasmacytomas are usually well-circumscribed within the dermis and panniculus and composed of sheets or packets of round cells with minimal-to-moderate plasmacytoid differentiation, supported by fine collagenous stroma.3,4 Binucleate and multinucleate cells are common, and mitotic figures may be few or numerous. A narrow zone of unaffected dermis (grenz zone) commonly separates these neoplasms from overlying epidermis. Secondary amyloid deposition of immunoglobulin origin is occasionally present.11 Systemic paraneoplastic syndromes such as hypercalcemia or hypergammaglobulinemia are not recognized with cutaneous plasmacytomas.4 Histologic categories have been proposed, with the monomorphic blastic cell type most frequently described. In one study, polymorphic blastic cell type was suggestive of malignancy9; other studies have not correlated cell morphology with behavior.
Cutaneous plasmacytomas routinely exhibit benign behavior. Rarely, malignant cutaneous plasmacytomas are reported, characterized by local invasion and tissue destruction.9 In most neoplasms, the presence of intravascular neoplastic cells is a feature of metastatic potential, but we have noted this feature in cutaneous plasmacytomas. We wished to determine if the presence of intravascular tumor cells in cutaneous plasmacytomas affected clinical outcome with regard to clinically relevant metastasis, local recurrence, or the future identification of multiple myeloma within the animal.
Data used in our study were sourced from the University of Tennessee (Knoxville, TN) pathology database. Biopsies accessioned between 2009 and 2012 in which the microscopic diagnosis of “plasma cell tumor” or “plasmacytoma” was reported in one or more slides were reviewed. Samples reported from haired skin and oral mucocutaneous junction were retained for use, whereas oral and gastrointestinal mucosal plasmacytomas were excluded because of proposed differences in biologic behavior within extramedullary plasmacytomas of these sites. These submissions included partially, closely, and widely excised neoplasms. In total, 134 cutaneous plasmacytomas were identified, submitted from the haired skin of 125 dogs.
We reexamined archived hematoxylin and eosin (H&E)-stained slides under light microscopy for the presence of intravascular neoplastic cells. All slides had been routinely prepared by the same histotechnology team. The presence of neoplastic cells within one or more vessels was classified as a positive result for intravascular neoplastic cells, and those without as negative. No quantitation was attempted. Immunohistochemistry for MUM1 was performed in 5 cases in which the primary mass and intravascular neoplastic cells were in close proximity to confirm plasmacytoid lineage both in the primary mass and in intravascular neoplastic cells. Immunohistochemistry for factor VIII–related antigen was performed in 3 independently selected cases to confirm the location of neoplastic cells within endothelium-lined vessels and to determine whether intravascular neoplastic cells were surrounded by endothelium.
The submitting veterinarian was contacted in each case to ascertain whether local recurrence, distant occurrence, or other plasmacytoid disease was diagnosed at any point following gross excision until that patient’s death or January 2015. Standardized survey questions were either asked by telephone or by email. Questions included absence or presence of a mass occurrence at the site of excision, development of masses (with or without histologic diagnosis) at other cutaneous sites, status of the animal as alive or deceased, and for deceased animals the cause of, and age at, death. In all cases, digital or hard-copy medical records at the primary practices were consulted to determine whether either undiagnosed cutaneous masses or diagnosed cutaneous plasmacytomas occurred or recurred at any point in later life. No lymph nodes were sampled, nor were dogs examined by diagnostic imaging modalities or postmortem examination specifically for the purposes of our study. In rare cases, postmortem reports were available; cutaneous plasmacytomas were not diagnosed in these cases at the time of death.
Statistical analysis was performed using SAS v.9.4 (SAS Institute, Cary, NC). Chi-square and Fisher exact tests were performed to establish the presence of relationships between age at diagnosis, body location, marginal excision status, presence of intravascular neoplastic cells, local recurrence, distant post-excisional occurrence, and post-excisional survival time. Sex of patients was not evaluated. Neoplasms were categorized by location of origin into 3 groups, chosen by relative frequency: head and ears, distal limbs below the level of the carpus/tarsus including digits, and other cutaneous locations.
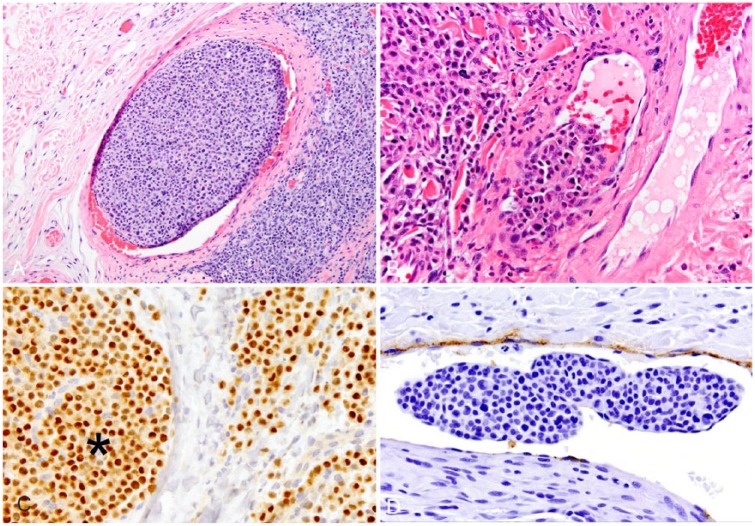
Twenty dogs (16%) were found to have neoplastic cells within thin-walled vessels in one or more submitted tissues during the study period (Fig. 1A, 1B), consistently identified as aggregates of plasmacytoid cells morphologically consistent with adjacent cells of the primary mass. Although red blood cells were sometimes present within the vessel lumens, we did not determine whether the vessels were veins or lymphatics. Neoplastic cells both adjacent to and within vessels in all 5 samples demonstrated strong nuclear labeling for MUM1 (Fig. 1C), consistent with plasma cell differentiation. In all 3 samples immunohistochemically labeled for factor VIII–related antigen, superficial cells within intraluminal round-cell aggregates were not immunoreactive, indicating that the cells were free within intraluminal spaces (Fig. 1D). Within the endothelium of these vessels, there was strong cytoplasmic labeling for factor VIII–related antigen in all cases.
Figure 1.
Sections of haired skin from 4 dogs contain intravascular aggregates of neoplastic plasma cells adjacent to cutaneous plasmacytomas. A, B. Hematoxylin and eosin. C. Multiple myeloma oncogene 1/interferon regulatory factor 4 (MUM1/IRF4) immunohistochemistry (IHC). D. Factor VIII–related antigen IHC. Intraluminal cells (*) demonstrate strong nuclear labeling for MUM1. Attenuated superficial cells within the intraluminal mass do not label for factor VIII–related antigen.
Of the 99 dogs with follow-up information, local recurrence was documented in 1 animal, within 1 y of biopsy submission; 2 cutaneous plasmacytomas were diagnosed on surgical pathology in this dog during the study period. Intravascular neoplastic cells were identified within both specimens, and surgical margins were not achieved at either location. Fine-needle aspirate cytology of a local lymph node revealed probable metastatic neoplastic plasma cells. Extramedullary multiple myeloma was strongly suspected but never conclusively established by bone marrow cytology. The patient was normoglobulinemic and systemically asymptomatic, and was euthanized 3 y after initial biopsy submission for metastatic anal sac apocrine gland adenocarcinoma.
Three dogs had a cutaneous plasmacytoma develop later at a distant site; in none of these 3 cases were intravascular neoplastic cells identified within examined sections.
Analysis of neoplasms by location was performed based on initial descriptions stated within the surgical biopsy submission form. Of the 134 tumors, 9 were solely described as arising from “haired skin.” Of the remaining 125 tumors, the most common locations were the head and ears (36 tumors, 29%), followed by distal limb below the level of the carpus/tarsus and digits (28 tumors, 22%) and the trunk and tail (26 tumors, 21%). Less common sites were the proximal limbs (16 tumors, 13%) and the inguinal region (5 tumors, 4%). Fourteen tumors (11%) were reported from mucocutaneous margins. Intravascular neoplastic cells occurred in significantly more specimens of the distal limbs, feet, and digits than in other locations: 10 of 28 (36%) tumors of the distal limbs compared to 10 of 89 (11%) tumors of other locations (p = 0.0007). Three of 35 (9%) neoplasms of the head and ears demonstrated intravascular neoplastic cells, whereas 7 of 54 (13%) neoplasms of other cutaneous locations had this feature.
The ages of study dogs at diagnosis of cutaneous plasmacytoma was 5–16 y with a mean of 9.6 y. Twenty-six of the 125 dogs were lost to follow-up because of relocation of the client or lapse in the veterinarian–client–patient relationship. Forty-three dogs were presumed alive at time of study based on last report of life status within 6 mo of data collection. The remaining 56 dogs were deceased by the end of the study period; 37 dogs had been euthanized for various reasons. Complications of multiple myeloma were identified as the cause of death in one dog; no intravascular neoplastic cells were identified within examined sections in this case. Other neoplastic causes for euthanasia included mast cell tumors, mediastinal carcinoma, soft tissue sarcoma, splenic hemangiosarcoma, and anal sac apocrine gland adenocarcinoma. One dog was euthanized because of an undiagnosed soft tissue mass of the mid-abdominal cavity identified by ultrasonography. Non-neoplastic disease processes that led to euthanasia included congestive heart failure, arthritis, renal failure, liver failure, anorexia/enteritis, gastric dilation and volvulus, endocrinopathies, intervertebral disc disease, urinary incontinence, blindness, and other complications of advanced age that decrease quality of life. One dog was euthanized after failing to recover from abdominal surgery. A reason for euthanasia could not be established in 15 subjects. Nine dogs died naturally for reasons including lymphosarcoma, enteritis, renal failure, congestive heart failure, neurologic abnormalities, respiratory signs, and bleeding splenic mass. A further 10 dogs died of unknown cause. In no cases were cutaneous plasmacytomas suspected as a direct or indirect cause of death or euthanasia. No correlation was seen between the age of animal from which tissue was submitted and likelihood of intravascular neoplastic cells.
In 56 dogs, the neoplastic cells were >5 mm from examined margins within specimens. Neoplastic cells extended to within 5 mm of examined margins within 1 or more cutaneous plasmacytomas from 11 dogs, and extended to the margins in an additional 23 cases. Complete excision could not be accurately evaluated in 35 dogs. Outside of the previously described case, no study dogs demonstrated local recurrence.
Findings indicate that the presence of intravascular neoplastic cells in cases of cutaneous plasmacytoma are both relatively frequent and do not statistically correlate with local recurrence, clinically relevant metastasis, or multiple myeloma. The incidence of intravascular neoplastic cells in these cases only considers findings within a standard veterinary surgical biopsy of a cutaneous mass; likewise, prognosis only accounts for documented clinical findings in animals not lost to follow-up. Study animals were not completely staged, leading to the potential for undiagnosed instances of recurrence or metastasis that did not result in reported clinical disease.
The presence of intravascular neoplastic cells within a tumor demands consideration of hematogenous or lymphatic invasion by the neoplastic population. Tumor emboli are individual or aggregated neoplastic cells that have entered a vascular lumen without endothelialization and persist by evading the host immune system.5 Examined cases of intravascular neoplastic cells adjacent to cutaneous plasmacytomas demonstrated moderate-to-large aggregates of cells. This finding is unusual in a cutaneous round-cell neoplasm, given the lack of adhesion molecules between plasma cells. Two alternative hypotheses were considered. First, cutaneous plasmacytomas may be prone to intravascular displacement of neoplastic cells and supporting stroma by pressure or trauma. Such cases might be expected with greater frequency within tumors of the distal limbs, as was found in our study population. Alternatively, cutaneous plasmacytoma cells may be predisposed to bulging into vasculature, as is characteristic in some neuroendocrine tumors, and are surrounded by attenuated endothelium, which was not identified on routine biopsy or through immunohistochemical labeling in our study.
Three animals in our study group were suspected to have multiple myeloma underlying cutaneous plasmacytoid lesions diagnosed as cutaneous plasmacytoma. The first dog (discussed above) had multiple cutaneous masses, intravascular neoplastic cells, and possible lymph node metastasis; the dog was euthanized because of metastatic anal sac apocrine gland adenocarcinoma. The other 2 dogs did not have intravascular neoplastic cells identified with their cutaneous plasmacytomas. One dog had 2 masses diagnosed as cutaneous plasmacytoma, and the pathologist suggested the possibility of extramedullary multiple myeloma based on surgical pathology. That dog was later diagnosed with and died of complications of multiple myeloma (mentioned above). A third patient was diagnosed with frequently recurring cutaneous and non-cutaneous plasmacytomas prior to, during, and following the study period. This dog was negative for Bence Jones proteins and monoclonal gammopathy, but repeated surgical debulking and medical treatment for multiple myeloma was instituted. The patient died following intraabdominal surgery for gastric dilation.
Although these 3 dogs may not belong in a set of true cutaneous plasmacytomas, they were retained in our study group based on study criteria and to establish whether intravascular neoplastic cells should increase the concern for underlying multiple myeloma. Although intravascular neoplastic cells were identified adjacent to the cutaneous plasmacytoma in one unconfirmed case of multiple myeloma, the other 2 cases with confirmed multiple myeloma did not have intravascular neoplastic cells.
Intravascular neoplastic cells are not an uncommon finding in cutaneous plasmacytomas, especially in those of the distal limbs. Although the mechanism is unknown, their presence does not indicate an increased potential for metastasis or a worse prognosis.
Acknowledgments
We thank referring veterinarians and animal owners for participation in this study, and Research Computing Support at University of Tennessee for statistical analysis.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Arber DA, et al. The 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–2405. [DOI] [PubMed] [Google Scholar]
- 2. Breuer W, et al. Immunoglobulin-producing tumours in dogs and cats. J Comp Pathol 1993;109:203–216. [DOI] [PubMed] [Google Scholar]
- 3. Cangul IT, et al. Clinico-pathological aspects of canine cutaneous and mucocutaneous plasmacytomas. J Vet Med A Physiol Pathol Clin Med 2002;49:307–312. [DOI] [PubMed] [Google Scholar]
- 4. Clark GN, et al. Extramedullary plasmacytomas in dogs—results of surgical excision in 131 Cases. J Am Anim Hosp Assoc 1992;28:105–111. [Google Scholar]
- 5. Cullen JM, et al. An overview of molecular cancer pathogenesis, prognosis, and diagnosis. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: Wiley, 2017:1–26. [Google Scholar]
- 6. Day MJ. Immunophenotypic characterization of cutaneous lymphoid neoplasia in the dog and cat. J Comp Pathol 1995;112:79–96. [DOI] [PubMed] [Google Scholar]
- 7. Gross TE, et al. Lymphocytic tumors: cutaneous plasmacytoma. In: Skin Diseases of the Dog and Cat. 2nd ed. Ames, IA: Blackwell, 2005:866–872. [Google Scholar]
- 8. Lester SJ, Mesfin GM. A solitary plasmacytoma in a dog with progression to a disseminated myeloma. Can Vet J 1980;21:284–286. [PMC free article] [PubMed] [Google Scholar]
- 9. Platz SJ, et al. Prognostic value of histopathological grading in canine extramedullary plasmacytomas. Vet Pathol 1999;36:23–27. [DOI] [PubMed] [Google Scholar]
- 10. Ramos-Vara JA, et al. Immunohistochemical detection of multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4) in canine plasmacytoma: comparison with CD79a and CD20. Vet Pathol 2007;44:875–884. [DOI] [PubMed] [Google Scholar]
- 11. Rowland PH, et al. Cutaneous plasmacytomas with amyloid in six dogs. Vet Pathol 1991;28:125–130. [DOI] [PubMed] [Google Scholar]
- 12. Valli VE, et al. Tumors of the hemolymphatic system. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: Wiley, 2017:203–321. [Google Scholar]