Abstract
A 14-y-old bay Quarter Horse gelding was presented with progressive neurologic signs, elevated rectal temperature, and icterus for 3 d prior to death. Postmortem examination revealed icterus, large amounts of serosanguineous fluid in the abdominal cavity, widespread petechiae and ecchymoses in several organs, and a large, pale, and well-demarcated focus of necrosis in the liver. Histologically, there was coagulative necrosis surrounded by a rim of inflammatory cells and large numbers of gram-positive rods, which were identified as Clostridium novyi by immunohistochemistry. Liver samples tested by PCR were positive for C. novyi type B flagellin and alpha toxin genes, but negative for Clostridium haemolyticum and other clostridia. Based on postmortem findings and ancillary tests, a definitive diagnosis of infectious necrotic hepatitis (INH) was made. Mostly a disease of ruminants, also known as black disease, INH has rarely been reported in horses, and a definitive etiologic diagnosis has not been achieved previously; the etiology of all cases reported to date was identified as C. novyi but the type was not determined. Animals are predisposed to clostridial hepatitis when hepatic anaerobiosis is established. Such conditions allow germination and proliferation of bacterial spores, resulting in production and release of toxins. INH, caused by C. novyi type B, and bacillary hemoglobinuria, caused by C. haemolyticum, are mechanistically and pathologically almost indistinguishable. Because these 2 microorganisms are closely related, differentiation requires molecular tools.
Keywords: Black disease, Clostridium novyi type B, horses, infectious necrotic hepatitis
Infectious necrotic hepatitis (INH) is caused by Clostridium novyi type B. The disease typically affects sheep, in which it is also referred to as “black disease” given the dark discoloration of the subcutaneous tissue caused by intense venous congestion seen in the carcasses of animals dying from this disease.6 Although INH is also thought to occur in other species including cattle, goats, and horses, very limited information is available about this disease in animals other than sheep.2,3,19,15 Moreover, because of its strict anaerobic nature, isolation and identification of C. novyi type B is rarely achieved.15 For this reason, reports of INH are commonly limited to presumptive diagnosis based on histopathology, fluorescent antibody test (FAT), and/or immunohistochemistry (IHC). The type of C. novyi is rarely determined. Herein we present a confirmed case of equine INH caused by C. novyi type B, and also review the literature on the disease.
A 14-y-old, 511-kg bay Quarter Horse gelding was submitted to the San Bernardino branch of the California Animal Health and Food Safety Laboratory System for postmortem examination and diagnostic workup. The animal had a 3-d history of progressive neurologic signs, rectal temperature of 38.8–40.5°C, and had been treated unsuccessfully with nonsteroidal anti-inflammatory drugs, prior to death. A full autopsy was performed ~6 h after death.
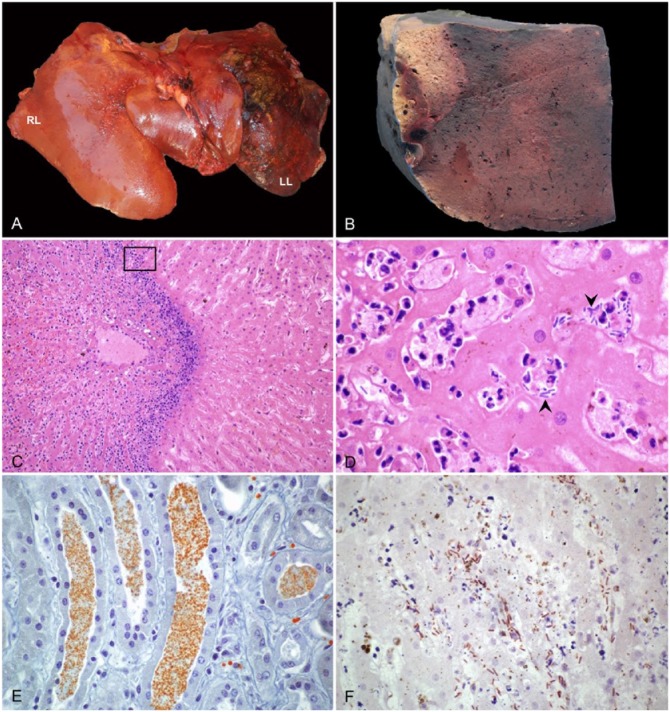
At autopsy, the horse was in good nutritional condition, with adequate amount of fat reserves, and in moderate state of postmortem decomposition. There was yellow discoloration of the sclera, subcutaneous and adipose tissue, and articular cartilages. Multifocal ecchymotic-to-suffusive hemorrhages were present in most muscles, serosa of the intestinal tract, mesentery, spleen, pleura, and in papillary muscles of the heart. Venous blood was deep red-black. The pericardial sac was distended with a large amount of serosanguineous fluid, and there were ~5 L of a similar fluid containing abundant fibrin strands in the abdominal cavity. The liver was markedly enlarged and had rounded edges; the left lobe was firm, dark-red to black, and had numerous subcapsular and deep parenchymal gas bubbles and abundant fibrin over the capsule (Fig. 1A). On cut section of this lobe, there was a ~15 cm diameter, pale area surrounded by a thin hemorrhagic rim (Fig. 1B). The meninges overlying the brain and spinal cord were diffusely congested, and there were petechial hemorrhages throughout the cerebral cortex. No other significant gross lesions were observed in the rest of the carcass.
Figure 1.
Liver of a horse with infectious necrotic hepatitis. A. The parenchyma is dark-red to black with abundant fibrin covering the capsular surface of the left lobe (LL). RL = right lobe. B. On cut section of the left lobe, there is a large, clearly demarcated pale area of necrosis. C. Large random focus of coagulative necrosis, surrounded by a rim of neutrophils. H&E. D. Higher magnification of the area within the box in panel C, showing viable and degenerate neutrophils and large rods (arrowheads) among degenerate and necrotic hepatocytes. H&E. E. Orange-brown granules stained with Okajima stain for hemoglobin, within renal tubules. F. Clostridium novyi indirect immunoperoxidase staining in an area of hepatic necrosis, with numerous positive rods within the affected tissue.
Samples of brain, lung, heart, diaphragm, liver, spleen, stomach, intestine, pancreas, adrenal gland, mesentery, kidney, and urinary bladder were collected and fixed in 10% buffered formalin (pH 7.2) for 48 h and processed routinely for the production of 4 µm-thick hematoxylin and eosin (H&E)-stained sections. Liver and kidney sections were also stained with Gram and Okajima stains, respectively.
Samples of liver were collected aseptically and subjected to aerobic and anaerobic culture on 5% sheep agar plates for 48 h. PCR was carried out for West Nile virus (WNV) on central nervous system pooled samples and for equid herpesvirus 1 (EHV-1) on a nasal swab. A heavy-metal screen including lead, manganese, iron, mercury, arsenic, molybdenum, zinc, copper, and cadmium was performed on liver samples. Cerebrospinal fluid was tested for antibodies against Sarcocystis neurona using an indirect FAT. Liver smears were processed by FAT for Clostridium chauvoei, Clostridium septicum, Clostridium sordelli, and C. novyi using commercial conjugates (VMRD, Pullman, WA). Additionally, formalin-fixed, paraffin-embedded sections of liver were processed by an indirect immunoperoxidase technique for C. novyi, using a commercial kit (RTU VECTASTAIN Elite ABC system, Vector Laboratories, Burlingame, CA). Endogenous peroxidase activity was extinguished with a 3% hydrogen peroxide solution, followed by pepsin treatment for antigen retrieval. Samples were then incubated with Background Punisher (Biocare Medical, Concord, CA) to block nonspecific binding, before incubation with goat anti–C. novyi polyclonal antibody (VMRD) at 37°C for 60 min. Vector NovaRED chromogen was used for visualization (Vector Laboratories). Sections of liver incubated with normal goat serum instead of primary antibodies were used as negative control. Liver from a cow in which C. novyi had been identified by culture, and PCR was used as a positive control.
Liver samples were processed for DNA extraction (QIAamp DNA FFPE tissue kit, Qiagen, Hilden, Germany) following the manufacturer’s instructions. The extracted DNA was used as template for a multiplex PCR assay to amplify segments of the flagellin genes (fliC) of C. septicum, C. novyi type A, C. novyi type B, C. chauvoei, and Clostridium haemolyticum, as described previously.17 Additionally, we developed a conventional PCR to amplify a segment of the C. novyi type B alpha toxin (TcnA) gene (GenBank accession JENV01000127.1), the main virulence factor of this microorganism. The following oligonucleotide sequences were designed: 5’-TGATGTTGACCATCCTTGCTCT-3’ (CNtBaF) and 5’-CCTTATGCAAAGGGATGGCG-3’ (CNtBaR), which amplify a ~342-bp DNA fragment of the target gene. Conventional PCR was performed in a total volume of 25 µL containing 5 µL of extracted DNA, 0.25 µL of each primer (10 µM), 7 µL of nuclease-free water, and 12.5 µL of PCR Master Mix (2X, Promega, Madison, WI), containing Taq DNA polymerase (pH 8.5, 50 U/mL), dNTPs (400 µM), and MgCl2 (3 mM). The thermocycler profiles were as follows: 94°C for 5 min, 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1.5 min, followed by a final extension step at 72°C for 7 min. The samples were held at 4°C until the PCR reactions were visualized under ultraviolet light in ethidium bromide (Amresco, Solon, OH)-stained 1% agarose gels (Agarose SFR, Amresco). DNA extracted from C. novyi type B (ATCC 25758), C. novyi type A, C. septicum, C. chauvoei, and C. haemolyticum cultures were used as positive controls. Water instead of DNA was used in negative control reactions.
Microscopically, the liver capsule was covered by abundant fibrin mixed with cell debris, and there were subcapsular and deep parenchymal emphysematous bullae of various sizes. The most striking finding was multifocal to focally extensive coagulative necrosis, surrounded by a rim of large numbers of viable and degenerate neutrophils, hemorrhage, fibrin, cell debris, and large aggregates of gram-positive bacterial rods, some of which had subterminal spores (Fig. 1C, D). In intervening parenchyma and in the less affected liver, there was bile stasis, multifocal hemorrhages, and segmental-to-diffuse fibrinoid necrosis of blood vessels, many of which also contained fibrin thrombi. The renal tubular epithelium had mild cytoplasmic vacuolation, and protein and granular casts were present in the lumen of renal tubules. The casts stained positively with Okajima stain (Fig. 1E). In the brain, there was diffuse congestion, multifocal and randomly scattered perivascular hemorrhages, and moderate-to-marked expansion of Virchow–Robin space with acidophilic edema. The visceral peritoneum was covered by abundant fibrin admixed with large numbers of viable and degenerate neutrophils; most peritoneal blood vessels were congested and/or contained fibrin thrombi. A few small arteries and veins in the cortex and medulla of the adrenal gland also had fibrin thrombi. The spleen had vascular congestion and multifocal parenchymal and capsular hemorrhages. The lungs were diffusely congested and had multifocal hemorrhages and edema, in addition to a few blood vessels containing fibrin thrombi. Multiple subendocardial, subepicardial, and subpleural hemorrhages were seen.
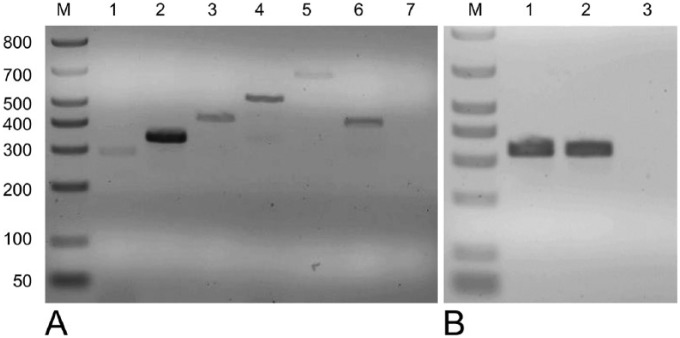
The great majority of intralesional gram-positive rods observed in the liver were positive for C. novyi IHC (Fig. 1F). FAT was positive for C. novyi, but negative for the other clostridial species tested. Liver samples were positive for C. novyi type B fliC and TcnA genes, and negative for the fliC genes of the other clostridia tested (Fig. 2). Samples of cerebrospinal fluid were negative for S. neurona immunofluorescent antibody assay. PCR for WNV and EHV-1 was negative. No significant bacterial growth was achieved by aerobic or anaerobic culture. The heavy-metal screen revealed an elevated concentration of iron in liver samples (1,500 ppm; reference interval: 100–300 ppm).
Figure 2.
A. Electrophoretic analysis of multiplex PCR products targeting fliC gene of histotoxic clostridia. Lane M: molecular size marker; lane 1: Clostridium septicum positive control (294 bp); lane 2: Clostridium novyi type A positive control (343 bp); lane 3: C. novyi type B positive control (427 bp); lane 4: Clostridium chauvoei positive control (535 bp); lane 5: Clostridium haemolyticum positive control (694 bp); lane 6: DNA extracted from liver samples; lane 7: negative control. B. Electrophoretic analysis of 342-bp products obtained by conventional PCR targeting C. novyi type B alpha toxin gene. Lane M: molecular size marker; lane 1: C. novyi type B positive control; lane 2: DNA extracted from liver samples; lane 3 = negative control.
We made a presumptive diagnosis of INH based on gross and microscopic changes, coupled with results of FAT and IHC for C. novyi. The diagnosis of INH produced by C. novyi type B was confirmed by PCR detection of the fliC and TcnA genes of this microorganism. To our knowledge, only 6 cases of suspected IHN have been reported previously in horses. Three cases occurred in Australia,8,9,11 and one each in New Zealand,23 Scotland,19 and the United States (Montana).16 In those cases, a presumptive diagnosis was based on gross and microscopic lesions coupled with detection of C. novyi by FAT,9,16,19 IHC,23 and/or culture.9,19 However, the type of C. novyi involved was not determined in any of those cases.
C. novyi is a gram-positive, spore-forming, anaerobic bacillus, which is commonly found in soil and feces of animals.15 It is classified into types A, B, and C based on the range of toxins produced.4 C. novyi type A produces mainly the lethal, edema-inducing alpha toxin (TcnA), and the non-lethal phospholipase, gamma toxin; this bacterium is associated with gas gangrene of man and animals, usually acting together with other clostridia. C. novyi type B also produces TcnA, in addition to the necrotizing and hemolytic beta toxin, although the former is considered the most important virulence factor for the pathogenesis of INH. C. novyi type C is considered to be non-toxigenic and non-pathogenic.13,15 C. haemolyticum was previously known as C. novyi type D, and it is still referred to by this name by some authors; therefore, we also discuss this microorganism here. C. haemolyticum also produces beta toxin, but in much larger quantities than C. novyi type B; this toxin is considered the main virulence factor of this microorganism, and it is responsible for bacillary hemoglobinuria (BH), a disease affecting mainly cattle that is clinically and pathologically very similar to INH.11,10,14
The spores of C. novyi are highly resistant to environmental conditions and, given that the spores are commonly found in soil, grazing animals may ingest them frequently.6,19 Although not fully proved, the current dogma is that, after ingestion, the spores of C. novyi type B are absorbed from the intestine and reach the liver via the portal circulation, after which they are spread to other organs. The spores are phagocytized and remain latent in Kupffer cells of the liver, and in macrophages of the spleen and bone marrow.2,6 INH has commonly been considered to be the counterpart of BH because both diseases are believed to occur after liver injury, causing necrosis and the associated anaerobic conditions that are required for germination of latent spores and the production of toxins.14 Migration of immature forms of Fasciola hepatica through the liver is considered the most important predisposing factor for both BH and INH in ruminants, and both diseases are more common in areas with high prevalence of fascioliasis.1 INH of ruminants has also been associated with Cysticercus tenuicollis and other parasites, including Fascioloides magna, Dicrocoelium dendriticum, and Thysanosoma actinoides,2,6,15,21 although the role of these parasites in the pathogenesis of the disease has not been demonstrated. However, any hepatic injury leading to generation of anaerobic conditions may be a predisposing factor for INH. These include, but are not limited to, liver abscesses, biopsies, fatty change, telangiectasia, and toxic agents. Once TcnA is produced and released by the vegetative forms of C. novyi type B, its monoglycosyl transferase activity inactivates several guanosine 5’-triphosphate (GTP)-binding proteins in cells of the host, resulting in alteration and redistribution of the actin cytoskeleton.3 TcnA also disrupts the vimentin and tubulin system.7,18 These changes appear to be more dramatic in capillary endothelium, leading to the widespread fluid extravasation seen in INH15 and probably the multisystemic thrombosis observed in the horse of our report. In sheep, the highly acute nature of the disease makes the detection of clinical signs difficult, with animals usually found dead. In cases in which signs are observed, they are nonspecific, and include weakness, lethargy, anorexia, hyperthermia, tachypnea, hyperpnea, and finally recumbency. Clinicopathologic changes may include neutrophilia with a left shift, azotemia, metabolic acidosis, and elevated liver and muscle enzyme activities.15,19
Given that only 6 cases of presumptive INH have been described previously in horses, available epidemiologic, clinical, and pathology information is scant. Based on this limited number of cases, there is no apparent sex or breed predisposition in cases of equine INH, and both young and adult animals may be affected. The clinical course is usually longer in horses than in ruminants, lasting 12–72 h, and the outcome has always been fatal. Clinical signs vary from mild-to-severe depression and reluctance to move, neurologic signs including ataxia and head tilt, hyperemic and/or icteric mucous membranes and ocular sclera, abdominal pain, and recumbency.20,23 In one case in which abdominocentesis was performed, an increased amount of turbid fluid with elevated protein concentration and specific gravity was obtained.9
Clinicopathologic profiles of horses with INH are highly variable, and they may represent different stages of toxemia and liver damage. These alterations may include neutrophilia with a left shift, elevated liver enzyme activity, thrombocytopenia, hyperfibrinogenemia, hyperbilirubinemia, and decreased concentration of electrolytes.9,16,19 In addition, increased activated partial thromboplastin, prothrombin, and thrombin times have been reported.23 These changes, in combination with the capillary microthrombi observed, are suggestive of disseminated intravascular coagulation, a process likely to occur during the course of toxemia and hemolysis in large animals.22,23 The resulting consumption of coagulation factors, their decreased synthesis because of hepatic failure, and the synergistic action of TcnA on vascular endothelium are presumably responsible for the widespread hemorrhages observed in cases of INH.
Although icterus is not described in ruminants, horses may sometimes exhibit this sign associated with INH.19,23 Icterus seems to be related to the severity of hepatic damage and, to some extent, to hemolysis, as in our case, in which hemoglobin was visualized in the renal tubules by Okajima stain. The horse in our study had moderately increased iron concentration in the liver, suggestive of a hemolytic process.5 This level of iron in the liver is, however, a common finding in horses dying from a variety of causes and it is often considered an incidental finding associated with congestion and postmortem autolysis (authors’ unpublished observation).
Although it can be hypothesized that horses are more susceptible than other species to the action of the hemolytic beta toxin produced by C. novyi type B, hemoglobinuric changes have not been described in presumptive cases of INH.16,23 Compared with C. haemolyticum, C. novyi type B produces lower levels of beta toxin;15 therefore, its contribution to the pathogenesis of INH is presumed to be minor, causing some degree of hemolysis in horses, but not enough to induce significant hemoglobinuria-associated changes. In addition, none of the previous cases ruled out the role of C. haemolyticum as a causative agent of disease, so final conclusions cannot be drawn from those cases. C. haemolyticum has been implicated in a case of septic peritonitis in a mare by identification with MALDI-TOF; however, PCR or toxin identification was not attempted in that particular case.11
The predisposing factor for INH in horses has not always been determined, although the hepatic migrations of F. hepatica and related trematodes, as well as members of the Strongylidae family, have been suggested.9,12,19 Additionally, anthelminthic treatment prior to the onset of clinical signs has been suggested as a potential trigger of the disease,9,16,23 presumably by causing some degree of hepatic necrosis associated with the destruction of migrating parasites. However, no scientific evidence is available to support this hypothesis. Unfortunately, no known predisposing factors were determined in our case.
As in our case, most previous reports described a single (multiple in one case19) focus of necrosis affecting the liver, consistently located in the left lobe. This contrasts with INH of sheep in which randomly distributed, small necrotic foci are more common.6,15 The reason for this variation in distribution is unknown. It is possible that the predominance of lesions on the left lobe is related to so-called portal streaming, which is responsible for differential flow of portal blood to particular lobes of the liver. Because C. novyi is presumably absorbed from the small intestine, and the blood from this organ follows portal streaming to the left lobe, this may result in this lobe being predominantly affected.6 The absence of changes associated with hepatic encephalopathy (e.g., Alzheimer type II astrocytosis associated with hyperammonemia)6 may suggest that neurologic signs in this and previous cases were consequence of hemorrhages, congestion, and perivascular edema noted in the brain, related to toxemia and the action of TcnA on the capillary endothelium.
INH should be in the list of differential diagnoses of horses with neurologic clinical signs, toxemia, acute liver failure, and peritonitis. Even though treatment with high doses of penicillin and tetracyclines may be effective against C. novyi, the advanced production of toxins usually results in a poor prognosis.19 In areas in which the disease is clearly associated with liver flukes and/or other parasites, reducing the parasitic burden and limiting the access of animals to poorly drained pastures may be attempted as preventive measures.15
Given that several clostridial diseases are assumed to be associated with accelerated postmortem decomposition, an important consideration for the correct diagnosis of INH is the need for rapid evaluation and preservation of tissues after the death of the animal.6,23 Clinical history, routine autopsy, histopathology, and immunostaining of C. novyi in liver lesions are essential to provide a presumptive diagnosis of INH. Because isolation is not always successful, given the strict anaerobic requirements of the microorganism, molecular identification by PCR is a valuable tool to provide an etiologic diagnosis, and to differentiate C. novyi type B from other clostridial pathogens.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bagadi HO, Sewell MH. An epidemiological survey of infectious necrotic hepatitis (Black Disease) of sheep in Southern Scotland. Res Vet Sci 1973;15:49–53. [PubMed] [Google Scholar]
- 2. Bagadi HO, Sewell MH. Experimental studies on infectious necrotic hepatitis (Black Disease) of sheep. Res Vet Sci 1973;15:53–61. [PubMed] [Google Scholar]
- 3. Belyi Y, Aktories K. Bacterial toxin and effector glycosyltransferases. Biochim Biophys Acta 2010;2:134–143. [DOI] [PubMed] [Google Scholar]
- 4. Borrmann E, Schulze F. Detection of Clostridium novyi type B alpha toxin by cell culture systems. FEMS Immunol Med Microbiol 1999;24:275–280. [DOI] [PubMed] [Google Scholar]
- 5. Bozorgmanesh R, et al. Hemolytic anemia in horses associated with ingestion of Pistacia leaves. J Vet Intern Med 2015;29:410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cullen JM, Stalker ML. Necrotic hepatitis (black disease). In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 6th ed., Vol. 2 Philadelphia, PA: Elsevier, 2016:316. [Google Scholar]
- 7. Donelli G, Fiorentini C. Bacterial protein toxins acting on the cytoskeleton. New Microbiol 1994;17:345–362. [PubMed] [Google Scholar]
- 8. Dumaresq JA. A case of black disease in the horse. Aust Vet J 1939;15:53–57. [Google Scholar]
- 9. Gay CC, et al. Infectious necrotic hepatitis (black disease) in a horse. Equine Vet J 1980;12:26–27. [DOI] [PubMed] [Google Scholar]
- 10. Hauer PJ, et al. Cloning and molecular characterization of the beta toxin (phospholipase C) gene of Clostridium haemolyticum. Anaerobe 2004;10:243–254. [DOI] [PubMed] [Google Scholar]
- 11. Hepworth-Warren KL, et al. Septic peritonitis in a Percheron mare associated with Clostridium haemolyticum. Equine Vet Educ 2017; 29:603-608. doi: 10.1111/eve.12531. [DOI] [Google Scholar]
- 12. Hollingsworth TC, Green VJD. Focal necrotizing hepatitis caused by Clostridium novyi in a horse. Aust Vet J 1978;54:58. [DOI] [PubMed] [Google Scholar]
- 13. Nakamura S, et al. Taxonomic relationships among Clostridium novyi type A and B, Clostridium haemolyticum and Clostridium botulinum type C. J Gen Microbiol 1983;129:1473–1479. [DOI] [PubMed] [Google Scholar]
- 14. Navarro M, et al. Bacillary hemoglobinuria. In: Uzal FA, et al., eds. Clostridial Diseases of Animals. 1st ed Ames, IA: Wiley Blackwell, 2016:265–274. [Google Scholar]
- 15. Navarro M, Uzal FA. Infectious necrotic hepatitis. In: Uzal FA, et al., eds. Clostridial Diseases of Animals. 1st ed Ames, IA: Wiley Blackwell, 2016:275–279. [Google Scholar]
- 16. Oaks JL, et al. Apparent Clostridium haemolyticum/Clostridium novyi infection and exotoxemia in two horses. J Vet Diagn Invest 1997;9:324–325. [DOI] [PubMed] [Google Scholar]
- 17. Sasaki Y, et al. Phylogenetic analysis and PCR detection of Clostridium chauvoei, Clostridium haemolyticum, Clostridium novyi types A and B, and Clostridium septicum based on the flagellin gene. Vet Microbiol 2002;86:257–267. [DOI] [PubMed] [Google Scholar]
- 18. Selzer J, et al. Clostridium novyi α-toxin-catalyzed incorporation of GlcNAc into Rho subfamily proteins. J Biol Chem 1996;41:25173–25177. [DOI] [PubMed] [Google Scholar]
- 19. Smith GW. Black disease. In: Smith BP, ed. Large Animal Internal Medicine. 5th ed. St. Louis, MO: Mosby Elsevier, 2015:849–850. [Google Scholar]
- 20. Sweeney HJ, Greig A. Infectious necrotic hepatitis in a horse. Equine Vet J 1986;18:150–151. [DOI] [PubMed] [Google Scholar]
- 21. Uzal FA, et al. Un caso de hepatitis infecciosa necrosante en oveja sin Fasciola hepatica [A case of infectious necrotic hepatitis in a Fasciola hepatica-free sheep]. Rev Med Vet 1996;77:377–379. Spanish. [Google Scholar]
- 22. Watson JL, Deem Morris D. Disorders of coagulation factors. In: Smith BP, ed. Large Animal Internal Medicine. 5th ed. St. Louis, MO: Mosby Elsevier, 2015:1051–1052. [Google Scholar]
- 23. Whitfield LK, et al. Necrotic hepatitis associated with Clostridium novyi infection (black disease) in a horse in New Zealand. N Z Vet J 2015;63:177–179. [DOI] [PubMed] [Google Scholar]