Abstract
Streptococcus equi subspecies zooepidemicus septicemia of alpacas and llamas, also called alpaca fever, is characterized clinically by fever, depression, recumbency, and death, and pathologically by polyserositis. Although a few natural and experimental cases of the disease have been reported, very little information about the pathology of spontaneous cases has been published. We present a detailed gross and microscopic description of 3 spontaneous cases of alpaca fever and review the literature on this condition. Typical of spontaneous and experimental infections with S. equi ssp. zooepidemicus, the 3 animals had disseminated fibrinosuppurative polyserositis with vascular thrombosis and intralesional gram-positive cocci. In addition, 2 of the animals had severe fibrinosuppurative pneumonia, endocarditis, and myocardial necrosis; the third animal had transmural pleocellular enteritis with prominent lymphangitis. The enteric lymphangitis observed in the latter suggests that dissemination of S. equi ssp. zooepidemicus occurred through lymphatic circulation and that, at least in this animal, the portal of entry of infection was the alimentary system.
Keywords: Alpaca fever, alpacas, lymphangitis, polyserositis, septicemia, Streptococcus equi subspecies zooepidemicus
Streptococcus equi subspecies zooepidemicus septicemia of alpacas and llamas, also called alpaca fever, is characterized clinically by elevated body temperature, depression, digestive tract alterations, recumbency, and death. Pathologically, alpaca fever is characterized mainly by polyserositis.10,13 In Peru, where this disease was first described, the morbidity in some alpaca herds has been estimated to be as high as 10%. It is hypothesized that stressors, including transport, may result in subclinical carriers developing clinical systemic disease.10,13
Although alpaca fever has been reported previously in South American camelids, only limited information on the pathology of the spontaneous disease has been published. We present a detailed gross and microscopic description of 3 spontaneous cases of alpaca fever occurring in North America, and we also review the literature on this condition.
Three alpacas from different farms were submitted to the California Animal Health & Food Safety (CAHFS) Laboratory (San Bernardino and Davis branches) for autopsy and diagnostic workup, between May 2015 and May 2016. Animal 1 was a 7-d-old, 8.6 kg female; animal 2 was a 10-y-old, 73.0 kg male; and animal 3 was a 4-y-old, 51.5 kg male. These 3 cases were individual cases, and no additional animals were sick on any of the properties. Animals 1 and 2 were found dead without clinical signs being observed prior to death; animal 3 had a 5-d history of anorexia followed by death. In addition, animal 1 belonged to a herd of alpacas that was kept on a Thoroughbred horse farm, although direct contact with the horses was not reported.
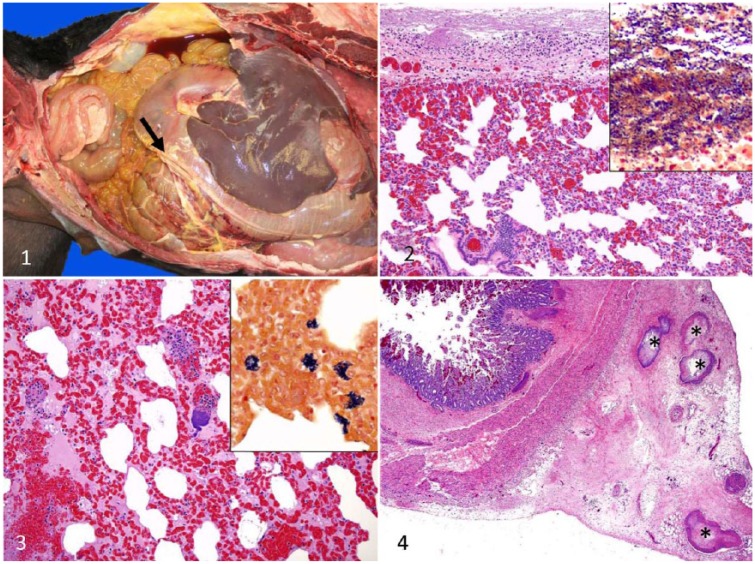
Autopsy was performed on each of the 3 animals. The carcass of animal 1 was in good nutritional condition, and the carcasses of animals 2 and 3 were in fair nutritional condition, with a small fat deposits and mild, generalized muscle atrophy. The most striking gross anatomic lesion in the 3 animals consisted of multiple strands of fibrin attached to the parietal and visceral pleura. The lungs were congested, edematous, and collapsed, and there was a moderate amount of froth in the trachea and lower airways. In addition, animal 1 had ~1 L of serofibrinous exudate free in the thoracic cavity. In animal 2, there were endocardial and epicardial ecchymoses, petechial and ecchymotic hemorrhages of the congested abdominal serosas, and a few intra-abdominal fibrin strands (Fig. 1). The liver of this animal had a prominent acinar pattern, and the whole carcass was mildly icteric. No other significant gross anatomic lesions were observed in the 3 examined carcasses.
Figures 1–4.
Lesions caused by spontaneous infection of alpacas by Streptococcus equi subspecies zooepidemicus.
Figure 1. Fibrinous peritonitis (arrow) in mildly icteric carcass of case 2.
Figure 2. Fibrinosuppurative pleuritis in case 2. H&E. Insert: myriad gram-positive cocci in the pleura. Gram stain.
Figure 3. Diffuse congestion, alveolar edema, hemorrhage, and colonies of cocci in the lung in case 2. H&E. Insert: colonies of gram-positive cocci in alveolar space and interstitium. Gram stain.
Figure 4. Small intestinal transmural pleocellular enteritis, including submucosal and serosal edema, hemorrhages, and mesenteric thrombi with cocci in blood and lymphatic vessels (asterisks) in case 1.
Ancillary tests were performed on samples from the 3 animals according to standard operating procedures of CAHFS, unless otherwise specified. Samples of trachea, lungs, heart, liver, spleen, kidneys, adrenal glands, tongue, esophagus, gastric compartments, small intestine, cecum, colon, and brain from the 3 animals were collected and fixed in 10% buffered (pH 7.2) formalin for 48 h and processed routinely for the production of 4 µm-thick H&E-stained sections. Selected sections were also stained with Gram, PAS, Giemsa, phosphotungstic acid hematoxylin (PTAH), or processed by immunohistochemistry for factor VIII.
Samples of lung, liver, peritoneal exudate, small intestine, and colon from the 3 animals were collected aseptically and subjected to aerobic or microaerophilic bacterial culture. Briefly, these specimens were inoculated onto 5% sheep blood Columbia agar plates (Hardy Diagnostics, Santa María, CA) and incubated aerobically or in 5–10% CO2 at 37°C for 48 h. A real-time PCR (rtPCR) to detect a fragment of the Salmonella-specific invA gene was performed on intestinal content as described previously.8 Salmonella culture was performed on bile, colon pool, and/or intestinal content, using tetrathionate or selenite enrichment broth and selective plate media. Frozen sections of spleen were processed by rtPCR for bovine viral diarrhea, bluetongue, and epizootic hemorrhagic disease viruses. Feces were examined for parasite eggs by a flotation method. A heavy-metal screen including lead, manganese, iron, mercury, arsenic, molybdenum, zinc, copper, and cadmium was performed on liver samples by inductively coupled argon plasma–emission spectrometry. Selenium concentration in the liver was determined by inductively coupled plasma spectrometry using hydride generation. Stomach content from animal 3 was examined grossly by a botanist for toxic plant identification.
Microscopically, fibrinocellular polyserositis was observed affecting peritoneum, pericardial sac, and pleura of the 3 animals, and it was characterized by multifocal deposits of fibrin admixed with macrophages, lymphocytes, plasma cells, neutrophils, and myriad gram-positive cocci. This lesion was most severe in the pleura (Fig. 2). The pleural blood vessels were diffusely congested and had multifocal perivascular hemorrhages; pleural lymphatic vessels were focally cuffed by macrophages and lymphocytes. In addition, the pulmonary parenchyma, including alveoli and interstitium of the 3 animals, was diffusely congested and edematous with areas of atelectasis and hemorrhage (Fig. 3). Alveolar spaces also contained fibrin, neutrophils, macrophages, lymphocytes, plasma cells, and gram-positive cocci (Fig. 3).
Animal 1 had marked transmural congestion of the small intestine, and the intestinal submucosa was diffusely expanded by edema and hemorrhage, with multifocal thrombosis of submucosal and mesenteric blood and lymphatic vessels (Fig. 4), which also contained myriad intraluminal gram-positive cocci. Other microscopic lesions included fibrinous endocarditis and myocardial necrosis (animals 2 and 3). Individual gram-positive cocci or colonies of these microorganisms were observed in lungs of the 3 animals, in intestine of animal 1, and in heart, serosas, spleen, liver, kidney, and adrenal cortex of animals 2 and 3. The cocci were seen in the parenchyma and in the lumen of blood vessels in these organs.
In all cases, S. equi ssp. zooepidemicus was isolated from liver, lung, and peritoneal exudate. No other aerobic bacterial pathogens were isolated from any of the samples of the 3 animals cultured. PCR for Salmonella was negative in all 3 animals.
Animal 3 had marginally low levels of copper in the liver (19 ppm; reference interval [RI]: 25–100 ppm). The remaining heavy metals in this animal, and all heavy metals in the other 2 animals, were within RIs.
S. equi ssp. zooepidemicus is a gram-positive, beta-hemolytic, Lancefield group C organism, and is the bacterium most frequently isolated from the respiratory tract of clinically healthy horses, and horses with pneumonia.4,21 This microorganism has been associated with multiple syndromes in several animal species. S. equi ssp. zooepidemicus causes suppurative respiratory infections in young horses and uterine infections in elderly mares.21 In dogs, this microorganism causes a disease characterized by sudden onset of pyrexia, dyspnea, hemorrhagic nasal discharge, pulmonary hemorrhage, pleural effusion, and death.16 A case of polyserositis associated with S. equi ssp. zooepidemicus has been described in a camel.19 In ruminants, this microorganism has been associated with sporadic cases of mastitis.15,17 In pigs and non-human primates, S. equi subsp. zooepidemicus causes polyarthritis, bronchopneumonia, pleuritis, epicarditis, endocarditis, and meningitis.18 In humans, S. equi ssp. zooepidemicus has been rarely isolated in association with consumption of contaminated food,3 or after contact with affected animals, which confirms its zoonotic character.9
S. equi ssp. zooepidemicus is recognized as the cause of so-called alpaca fever, one of the most significant diseases of alpacas and llamas in South America.6,7,10,12 In some countries, the morbidity of spontaneous alpaca fever in alpacas is 5–10%, with lethality of 50–100%.6 Outbreaks are typically associated with stress.14
In alpacas, this disease may occur in acute, subacute, or chronic forms.2,10 Acute and subacute forms are usually characterized by anorexia, depression, and high fever.2,10 The chronic forms are characterized by local infections, including abscesses in multiple locations, and orchitis, with death occurring 4–8 d following the onset of clinical signs.2,10
Systemic and localized forms of alpaca fever have been described.13 Young animals may be naturally predisposed to the systemic manifestation of alpaca fever, whereas this form seems to be seen only rarely in adult animals.13 However, 2 of the animals in our study were adults and they suffered from the systemic form of the disease, suggesting that this form of alpaca fever may be more common in adult animals than previously thought. Animal 3 was mildly copper deficient. Although we cannot rule out that copper deficiency predisposed this alpaca to the infection, it would seem unlikely, as the hepatic copper level was only marginally low.
Lungs and abdominal and thoracic serosas are usually affected in acute, subacute, and chronic cases, with abscesses in multiple organs being observed in some chronic cases.10 No abscesses were seen in any of the animals of our study, despite the fact that one of the cases was characterized as subacute to chronic. In an experimental study performed in llamas, no internal abscesses were observed, despite the fact that one animal survived up to 13 d after inoculation.6
The pathogenesis of alpaca fever has not been completely elucidated. S. equi ssp. zooepidemicus is considered a mucous membrane commensal in alpacas, and it has been suggested that transmission occurs orally via contaminated objects or direct contact with infected animals.10
Although it is assumed that, in natural cases, the infection becomes systemic following ingestion of the organism,2,10 experimental intratracheal inoculation of S. equi ssp. zooepidemicus in llamas has led to systemic disease and lesions typical of alpaca fever.6 It is therefore possible that aerogenous transmission also occurs in natural cases of the disease.
In cases of aerogenous pulmonary infection complicated with pleuritis, the most likely explanation for pleuritis is extension of infection from the pulmonary parenchyma into the pleura, as has been observed in horses.4 The 3 alpacas described in our report displayed various degrees of pneumonia, which supports this theory. However, in the llamas experimentally inoculated intratracheally with S. equi ssp. zooepidemicus mentioned above,6 5 of 6 animals developed pleuritis, but only 1 of them had pneumonia. The reason for the different prevalence of pneumonia between the spontaneous cases described herein and the experimental cases described previously6 is unknown.
In healthy carriers of S. equi ssp. zooepidemicus, onset of clinical disease can be predisposed by viral infections, trauma, high temperatures, or other stressors such as transportation, inclement weather, or malnutrition.1,10,20 In both spontaneous and experimental infections, systemic disease is characterized by polyserositis involving the serosas of the thoracic and abdominal cavities, and occasionally meningitis.7,13 The clinical signs in animals with polyserositis include dyspnea, colic, tense and tender abdomen, and constipation.10
In the case of the neonatal cria in our study, the same personnel worked at both the equine and the alpaca facilities of the ranch. The personnel or their clothing or equipment may have acted as mechanical vectors conveying S. equi ssp. zooepidemicus from the horses to the alpaca herd, given that this bacterium is ubiquitous in equine populations.10 The potential contribution to the dissemination of alpaca fever of keeping alpaca and horses on the same premises has been proposed previously (Tavela A, et al. Outbreak of S. equi ssp. zooepidemicus polyserositis in two llamas housed with horses in South Tyrol, Italy. Proc XXVI World Buiat Cong; November 2010; Santiago, Chile. Available at: https://goo.gl/FqRbBP).13 Although there were no records of previous cases of S. equi ssp. zooepidemicus infections in horses on this farm, this is a commensal organism for horses and it is therefore likely that it was present in horses on the farm. The possible transmission from horses to an alpaca remains, nevertheless, speculative. No other cases of alpaca fever had occurred on this ranch for at least 12 mo prior to the case reported herein.
Management of South American camelids in South and North America differs significantly, which results in animals exposed to different pathogens. In particular, South American camelids tend to be more frequently intermingled with several other livestock species. In South America, S. equi ssp. zooepidemicus is considered a commensal organism of the oral cavity of alpacas and llamas, and 2 main diseases are recognized: 1) local and superficial, associated with wound infection, and 2) systemic, secondary to ingestion, or possibly inhalation of the organism (alpaca fever).10 In studies carried out in North America, S. equi ssp. zooepidemicus is a rare contributor to oral infections and does not appear to be a common oral or respiratory tract commensal,7,11 thus in cases of exposure, animals will likely trigger a strong immune response. In addition, in North America, alpacas and llamas are usually exposed less frequently to horses, pigs, or other common hosts of the organism.10
Natural cases of S. equi ssp. zooepidemicus infection in alpacas revealed similar serosal findings to llamas inoculated with the same agent, which displayed fibrinosuppurative polyserositis with intralesional bacteria.5,7,13 In the 3 cases presented here, there was pneumonia. In addition, 2 of the 3 alpacas had focal myocardial necrosis and the presence of bacterial emboli in several organs, including liver, spleen, adrenal glands, and kidneys. It has been proposed that only young animals are predisposed to the systemic manifestation of the disease,13 which is not in agreement with our findings.
Although all 3 animals in our study had pulmonary lesions, the neonate also had severe lesions in the intestinal tract, including enteric lymphatic vessels. These lesions may be associated with a different route of bacterial infection and dissemination; however, the number of animals in our study is not large enough to draw definitive conclusions.
Acknowledgments
We thank Dr. William Talbot for providing clinical and autopsy data.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by California Animal Health and Food Safety Laboratories. Juan M. Corpa received financial support for researcher mobility from Program “Ayudas a la movilidad investigadora CEU-Banco de Santander 2015/16” of Universidad CEU Cardenal Herrera and Generalitat Valenciana (BEST/2016/285).
References
- 1. Andrews AH, Cox A. Suspected nutritional deficiency causing anemia in llamas (Lama glama). Vet Rec 1997;140:153–154. [DOI] [PubMed] [Google Scholar]
- 2. Aubry P, et al. Septic orchitis in an alpaca. Can Vet J 2000;41:704–706. [PMC free article] [PubMed] [Google Scholar]
- 3. Bordes-Benítez A, et al. Outbreak of Streptococcus equi subsp. zooepidemicus infections on the island of Gran Canaria associated with the consumption of inadequately pasteurized cheese. Eur J Clin Microbiol Infect Dis 2006;25:242–246. [DOI] [PubMed] [Google Scholar]
- 4. Carvallo F, et al. Retrospective study of fatal pneumonia in racehorses. J Vet Diagn Invest 2017;29:450–456. [DOI] [PubMed] [Google Scholar]
- 5. Cebra CK, et al. Acute gastrointestinal disease in 27 new world camelids: clinical and surgical findings. Vet Surg 1998;27:112–121. [DOI] [PubMed] [Google Scholar]
- 6. Cebra CK, et al. Pathogenesis of Streptococcus zooepidemicus infection after intratracheal inoculation in llamas. Am J Vet Res 2000;61:1525–1529. [DOI] [PubMed] [Google Scholar]
- 7. Cebra ML, et al. Tooth root abscesses in new world camelids: 23 cases (1972–1994). J Am Vet Med Assoc 1996;209:819–822. [PubMed] [Google Scholar]
- 8. Cheng CM, et al. Rapid detection of Salmonella in foods using real-time PCR. J Food Prot 2008;71:2436–2441. [DOI] [PubMed] [Google Scholar]
- 9. Eyre DW, et al. Streptococcus equi subspecies zooepidemicus meningitis—a case report and review of the literature. Eur J Clin Microbiol Infect Dis 2010;29:1459–1463. [DOI] [PubMed] [Google Scholar]
- 10. Fowler ME, Bravo PW. Infectious diseases. In: Fowler ME, Bravo PW, eds. Medicine and Surgery of Camelids. 3rd ed. Ames, IA: Blackwell, 2010:173–230. [Google Scholar]
- 11. Gerros TC, Andreasen CB. Analysis of pleural fluid and transtracheal aspirates in clinically healthy llamas (Llama glama). Vet Clin Pathol 1999;28:29–32. [DOI] [PubMed] [Google Scholar]
- 12. Hewson J, Cebra CK. Peritonitis in a llama caused by Streptococcus equi subsp. zooepidemicus. Can Vet J 2001;42:465–467. [PMC free article] [PubMed] [Google Scholar]
- 13. Jones M, et al. Outbreak of Streptococcus equi ssp. zooepidemicus polyserositis in an alpaca herd. J Vet Intern Med 2009;23:220–223. [DOI] [PubMed] [Google Scholar]
- 14. Moro M, Guerrero G. La alpaca: Enfermedades infecciosas y parasitarias [The alpaca: infectious and parasitic diseases]. (Series 8, Dissemination Bulletin.) Lima, Peru: Universidad Nacional Mayor de San Marcos, Instituto Veterinario de Investigaciones Tropicales y de Altura, Centro de Investigación, 1971:17–19. Spanish. [Google Scholar]
- 15. Obied AI, et al. Mastitis in Camelus dromedarius and the somatic cells content of camel’s milk. Res Vet Sci 1996;61:55–58. [DOI] [PubMed] [Google Scholar]
- 16. Priestnall S, Erles K. Streptococcus zooepidemicus: an emerging canine pathogen. Vet J 2011;188:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharp MW, et al. Streptococcus zooepidemicus infection and bovine mastitis. Vet Rec 1995;137:128. [DOI] [PubMed] [Google Scholar]
- 18. Soedarmanto I, et al. Identification and molecular characterization of serological group C streptococci isolated from diseased pigs and monkeys in Indonesia. J Clin Microbiol 1996;34:2201–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stoughton WB, Gold J. Streptococcus equi subsp zooepidemicus pleuropneumonia and peritonitis in a dromedary camel (Camelus dromedarius) calf in North America. J Am Vet Med Assoc 2015;247:300–303. [DOI] [PubMed] [Google Scholar]
- 20. Timoney JF. The pathogenic equine streptococci. Vet Res 2004;35:397–409. [DOI] [PubMed] [Google Scholar]
- 21. Timoney JF, et al. Cloning and sequence analysis of a protective M-like protein gene from Streptococcus equi subsp. zooepidemicus. Infect Immun 1995;63:1440–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]