Abstract
Adiaspiromycosis is a mycotic infection caused by thermally dimorphic fungi classified as Emmonsia parva and E. crescens (formerly Chrysosporium spp.) until recently, when new classifications were proposed. We document the pathologic findings in a severe case of adiaspiromycosis, with lymph node involvement, in a wild European rabbit (Oryctolagus cuniculus). The rabbit exhibited granulomatous pneumonia with tracheobronchial lymph node enlargement. Histopathologically, the lung was expanded by myriad, densely cellular, heterophilic and granulomatous foci, surrounding bi- to trilaminar adiaspores. Adiaspore density was considered to be similar in all lung lobes. In the left caudal lung lobe, 80 adiaspores were counted in a 50-mm2 area using digital image analysis. The mean and median adiaspore diameters were 240 ± 52 μm and 255 μm, respectively. Tracheobronchial lymph nodes exhibited moderate numbers of similar adiaspores. PCR amplification of DNA extracted from microdissected adiaspores failed to identify Emmonsia spp.–specific DNA. These data suggest that adiaspiromycosis may result in severe granulomatous pneumonia in wild European rabbits. Although confirmation of the etiologic agent by PCR using DNA extracted from formalin-fixed tissue is not always successful, digital image analysis can be used to aid accurate assessment of adiaspore density and morphology.
Keywords: Adiaspiromycosis, digital pathology, Emmonsia, lung, lymph node, Oryctolagus cuniculus, rabbits
Adiaspiromycosis is a mycotic infection that typically causes granulomatous pulmonary lesions. The etiologic agents are thermally dimorphic fungi classified as Emmonsia parva and E. crescens (formerly Chrysosporium spp.) until recently, when new classifications were proposed. Disease is typically associated with wild rodents, although it has been sporadically diagnosed in a wide range of wild and domestic animals.5 Rare reports have mentioned adiaspiromycosis infections in laboratory rabbits10 and desert cottontail rabbits (Sylvilagus audubonii),28 but to our knowledge, there exists very limited description of the gross and microscopic lesions of the disease in rabbits. We document herein the pathologic findings in a severe case of adiaspiromycosis, with lymph node involvement, in a wild European rabbit (Oryctolagus cuniculus), and review the literature on reclassification of the etiologic agent and the presentation of this mycosis in wild and domestic animals.
A wild female European rabbit was shot for population control, and the cadaver was one of a group donated to the anatomic pathology service of the Department of Veterinary Medicine, University of Cambridge (Cambridge, UK), for research and teaching purposes. At postmortem examination, the rabbit weighed 1.6 kg, and was therefore considered to be an adult.19 Postmortem examination revealed that the lungs were multifocally beige and pink, were firm, and were expanded by myriad coalescent granulomas that were ~1 mm diameter (Figs. 1, 2). The lungs had a gritty texture when cut. Tracheobronchial lymph nodes were cream, and had dimensions ~5 × 8 × 5 mm. The liver had multifocal, sharply demarcated, cream foci, up to 2 mm diameter, which were visible either flush to the capsular surface, or slightly raised, and extended into the underlying parenchyma. Such foci were distributed randomly throughout all of the hepatic lobes and, based on gross appearance, were attributed to Eimeria stiedae infection. Based on the gross morphology of the uterus and mammary gland, the doe was not considered to be pregnant or lactating.
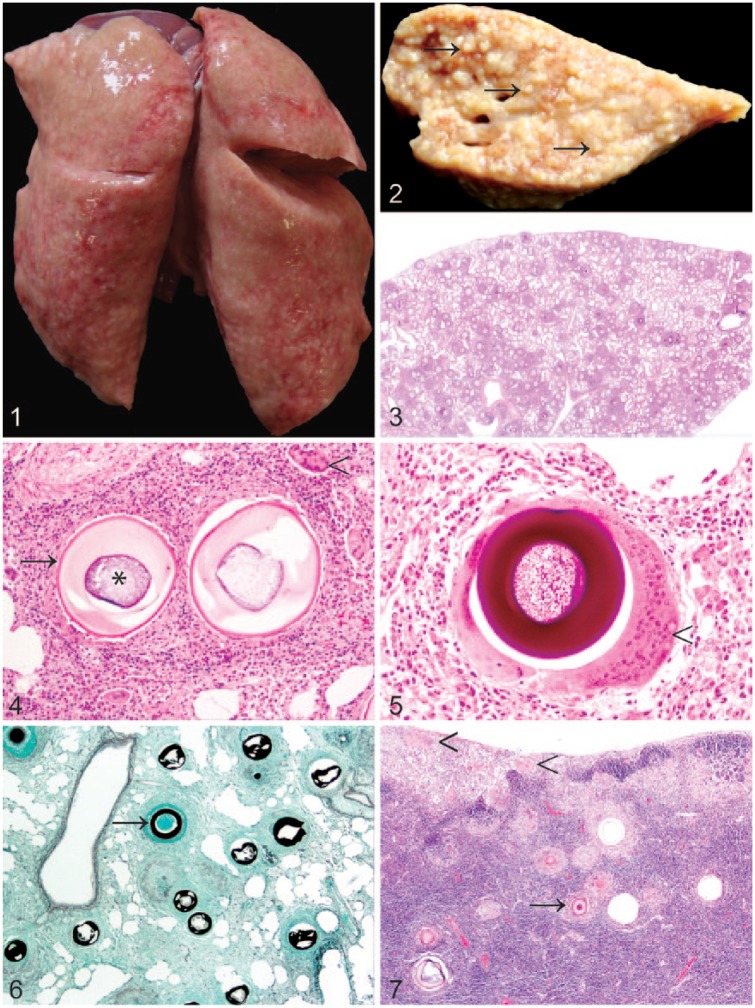
Figures 1–7.
Pulmonary adiaspiromycosis in a wild European rabbit (Oryctolagus cuniculus).
Figure 1. The lungs were firm and expanded by myriad granulomas.
Figure 2. The cut surface of the left caudal lung lobe was expanded by variably sized, coalescent, granulomas (arrows). Formalin-fixed tissue.
Figure 3. Numerous densely cellular foci surrounding spore-like adiaspores are present throughout the lung. H&E.
Figure 4. Adiaspores exhibit a bi- to trilaminar wall (arrow) comprising a thin, brightly eosinophilic outer layer, and a thick, pale eosinophilic inner layer surrounding a core of basophilic granular-to-foamy material (asterisk). Adiaspores are surrounded by heterophils, macrophages, multinucleate giant cells (arrowhead), lymphocytes, and plasma cells, in variable proportions, with interspersed fibroblasts. H&E.
Figure 5. The adiaspore wall is stained intensely positive with periodic acid–Schiff stain. Multifocally, adiaspores are surrounded by multinucleate giant cells with >40 nuclei (arrowhead).
Figure 6. The adiaspore wall exhibits intense staining with Grocott methenamine silver stain (arrow).
Figure 7. Similar adiaspores (arrow) are present within the tracheobronchial lymph node. Large multinucleate giant cells are also evident multifocally in this case within the subcapsular sinus (arrowheads).
The lungs, tracheobronchial lymph nodes, liver, kidneys, heart, esophagus, and trachea were fixed in 10% neutral-buffered formalin, and were subsequently processed and sectioned. Staining with hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), and Grocott methenamine silver (GMS) stains followed standard protocols. An H&E histologic section of the left caudal lung lobe was scanned at 40× (NanoZoomer 2.0RS, C10730, Hamamatsu Photonics, Hamamatsu City, Japan). For determination of an adiaspore count, and mean adiaspore diameter, the scanned histologic section was analyzed with viewing software (NDP.view2, Hamamatsu Photonics). A 50-mm2 area of the left caudal lung was randomly selected, and adiaspores within this area were counted. Partially degenerate, but histologically identifiable, adiaspores, and those falling only partially within the designated area, were included in the analysis. To determine mean and median adiaspore diameter, adiaspores within the same 50-mm2 area were analyzed, but only adiaspores considered to be sufficiently intact to be representative were included. Diameter was measured in micrometers in the widest plane judged to bisect the center of the spore. Mean diameter (± standard deviation) and median diameter were calculated from the acquired measurements. Formalin-fixed tissue was submitted to the Mycology Reference Laboratory, Public Health England (Bristol, UK), for microdissection of adiaspores, DNA purification, and PCR amplification using both panfungal and Emmonsia-specific primers as described previously.4
Histologic examination revealed that the lung was expanded by myriad densely cellular granulomatous foci surrounding spore-like adiaspores (Fig. 3). Adiaspores were considered to be present at a similar frequency in all lung lobes. In the left caudal lung lobe, 80 adiaspores were counted in a 50-mm2 area. Thirty-one (38%) of the adiaspores in the selected area were considered sufficiently intact to be used for measurement of the adiaspore diameter. The mean adiaspore diameter was 240 ± 52 μm, and the median diameter was 255 μm.
Adiaspores had a bi- or trilaminar wall comprising a thin, brightly eosinophilic outer layer, ~3 μm thick; an ~30–50 μm thick, or multifocally thicker, pale eosinophilic layer; and a variably distinct 2–3 μm inner basophilic layer, surrounding a core of basophilic granular-to-foamy material. Adiaspores were surrounded by large numbers of heterophils, macrophages, multinucleate giant cells, lymphocytes, and plasma cells, in variable proportions, with interspersed fibroblasts (Fig. 4). Many of the multinucleate giant cells exhibited >40 nuclei (Fig. 5). The adiaspore wall exhibited intense positive staining with PAS (Fig. 5) and GMS (Fig. 6). In other foci, inflammatory cells, similar to those already described, surrounded degenerate adiaspore remnants, and there was scattered necrotic debris. In some foci, large aggregates of degenerate heterophils predominated. Moderate numbers of adiaspores similar to those already described were present in the tracheobronchial lymph node in both the cortex and the medulla, and were accompanied by very large numbers of epithelioid macrophages and multinucleate giant cells (Fig. 7). The final diagnoses were granulomatous pneumonia and tracheobronchial lymphadenitis both with intralesional adiaspores, and cortical lymphoid hyperplasia. Histologic examination also confirmed mild hepatic eimeriosis, which was considered to be an incidental finding. No other lesions were detected on gross or histologic examination.
On the basis of the histopathologic findings, the cause of the granulomatous pneumonia was considered most likely E. crescens. Microdissection of adiaspores, DNA purification, and PCR amplification using both panfungal and Emmonsia-specific primers failed to identify Emmonsia-specific DNA, although sequencing revealed a spectrum of other saprobic fungi, including both molds and yeasts.
Adiaspiromycosis was proposed as a term used to describe the pulmonary lesions caused by Emmonsia spp. A cardinal feature of this disease is that “there is no multiplication or dissemination of the fungus beyond its original site of implantation.”9 This is in contrast to a number of other pulmonary mycoses of veterinary significance, in which lung lesions result from systemic spread and embolic dissemination from a distant portal of entry, such as a site of gastrointestinal ulceration14 or, as reported previously, more unusual primary foci of infection such as a surgical site.7
E. parva has been suggested to be geographically restricted to arid areas including the southwestern United States,28 Africa, and Israel.13 By contrast, E. crescens has been implicated as the main etiologic agent of adiaspiromycosis in Europe.4 Histologic analysis and measurement of spore diameter have traditionally been important in the diagnosis of infections with Emmonsia spp., with E. parva suggested to have spores of 10–40 μm diameter and E. crescens stated to have larger spores with diameter 250–500 μm.5,10 In our case, the mean adiaspore diameter of spores in the left caudal lobe of 240 ± 52 μm was considered consistent with a diagnosis of E. crescens.
A number of phylogenetic studies, based on multilocus sequence typing and whole genome sequencing, have revealed that Emmonsia is, in fact, polyphyletic, leading to major taxonomic revisions of Emmonsia spp. and several other members of family Ajellomycetaeae.8,23 The type species of Emmonsia, E. parva, was shown to cluster in genus Blastomyces (as B. parvus), making the genus Emmonsia a synonym of Blastomyces. Several other former members of Emmonsia, which have a yeast rather than adiaspore tissue form, now comprise a novel genus Emergomyces,8 and genus Adiaspiromyces has been proposed to accommodate the separate, relatively invariable clade formerly known as E. crescens.
In our case, the macroscopic pathology associated with adiaspiromycosis was particularly striking, with profound granulomatous changes recognizable grossly in all lung lobes. This contrasts with some previous cases in wild animals in which gross anatomic lesions were not observed,12,20 although in other cases, white-to-cream, 0.5–1 mm diameter lung lesions have been recorded.4 It is notable that the gross lesions were particularly marked in our case, although as the animal was wild, no clinical history is available to indicate whether respiratory compromise was evident. Another unusual aspect of this case is the presence of adiaspores within the tracheobronchial lymph nodes, which may, again, reflect the severity of the infection in this rabbit. Adiaspores have been previously described in the mediastinal lymph node of a Eurasian beaver (Castor fiber)22 and in the tracheobronchial and mediastinal lymph nodes of striped skunks (Mephitis mephitis),2 but lymph node involvement in adiaspiromycosis is generally uncommon.5
From a diagnostic pathology viewpoint, it is perhaps pertinent to note that some authors have suggested that the spores of Emmonsia spp. may be potentially confused with Besnoitia spp. cysts.11 However, as the latter is an apicomplexan protozoan, cyst development occurs within a parasitophorous vacuole within a host cell, and so identification of the host cell nucleus should be possible in some cysts. In addition, Besnoitia spp. cysts contain tachy- or bradyzoites,18 whereas clearly fungal adiaspores do not. In our case, PAS and GMS stains were utilized to further highlight the adiaspore wall as in previous cases.4,20 Although GMS has been reported to stain the outer and inner capsules of Besnoitia spp. cysts as well as the host cell cytoplasm, staining was reported to be weak, although the bradyzoites stained moderately.3 By contrast, intense staining of the cyst wall is observed with Emmonsia spp. spores, as in our case.
A limitation of our case is that microbial culture of fresh tissue was not attempted. However, culture of E. crescens is frequently unsuccessful, even following prolonged culture of fresh tissue,24 and particularly when cadavers have been previously frozen,4 but successful microdissection of adiaspores, DNA extraction, PCR amplification, and subsequent sequencing has been described previously as a useful diagnostic confirmatory technique.4 Our attempt at this confirmation was unsuccessful, in spite of the presence of numerous adiaspores in the submitted material. This was ascribed to difficulties associated with PCR amplification of DNA from formalin-fixed tissue, particularly when adiaspores are mature and may be multifocally mineralized (unpublished observation). The presence of a spectrum of other unrelated molds and yeasts was attributed to inhalation of soil-associated fungi by small mammals, and is not uncommon with PCR of respiratory tissue from small, soil-dwelling mammals (unpublished observation).
There are multiple reports of adiaspiromycosis in the family Mustelidae, leading authors to suggest that this family may be particularly susceptible to infection.20 Infection considered to be most likely caused by E. crescens has been reported in a male Eurasian otter (Lutra lutra) in Italy.20 Granulomatous lesions surrounding adiaspores have also been reported in otters, stoats (syn. ermines; Mustela erminea), weasels (Mustela nivalis), European pine martens (Martes martes), and European polecats (Mustela putorius) in the United Kingdom.4,27 Interestingly, in one study, the mean adiaspore diameter between species was similar (39, 30, and 36 μm for stoats, weasels, and polecats, respectively) raising questions regarding the potential species of Emmonsia spp., given that smaller adiaspores are typically associated with E. parva, which is not found in Britain.27 Further reports of pulmonary adiaspiromycosis affecting members of family Mustelidae include documentation of cases in the former Czechoslovakia,17 and 3 cases in European mink (Mustela lutreola) from Spain.25
In the United Kingdom, natural infections of E. crescens have also been reported in other mammals including European moles (Talpa europaea), red foxes (Vulpes vulpes), Eurasian red squirrels (Sciurus vulgaris), and a European water vole (Arvicola terrestris; syn. Eurasian water mole, Arvicola amphibius).4,6,26 Worldwide, a range of wild animal species have been diagnosed with adiaspiromycosis including the crested porcupine (Hystrix cristata),21 Franklin’s ground squirrels (Poliocitellus franklinii; syn. Spermophilus franklinii),29 raccoons (Procyon lotor),12 and American pikas (Ochotona princeps).29 It is self-evident that many of the affected species of wild animals are small animals, dwelling close to the ground. Occurrence in domestic species has been reported less frequently, although canine,1,16 equine,24 and caprine15 cases have been recorded.
We document the gross and microscopic pathology of a severe case of adiaspiromycosis, with lymph node involvement, in a wild European rabbit, and raise the possibility that confirmation of the etiologic agent by PCR using formalin-fixed tissue may not be possible in all cases of this disease, in spite of success in many cases. Data from whole genome sequencing offers diagnostic pathologists new insights into the phylogeny of the etiologic agents. Digital image analysis may be employed to aid accurate assessment of adiaspore density and morphology. The latter may therefore represent a useful tool for future surveys of the prevalence of adiaspiromycosis in wildlife populations, and characterization of the associated lesions.
Acknowledgments
We thank V Owenson and L Grimson of the Department of Veterinary Medicine, University of Cambridge, for technical assistance in the preparation of tissue sections, and Howard Davies, Department of Physiology, Development and Neuroscience, University of Cambridge, for his assistance in slide scanning. The rabbit in our case was part of a postmortem study of the pathology of wild rabbits, and the authors gratefully acknowledge the Ethics and Welfare Committee of the Department of Veterinary Medicine, University of Cambridge for their review of the study plan (reference: CR240).
Footnotes
Funding: Publication costs were supported by Girton College, University of Cambridge.
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Katherine Hughes  https://orcid.org/0000-0002-3331-1249
https://orcid.org/0000-0002-3331-1249
References
- 1. al-Doory Y, et al. Adiaspiromycosis in a dog. J Am Vet Med Assoc 1971;159:87–90. [PubMed] [Google Scholar]
- 2. Albassam MA, et al. Adiaspiromycosis in striped skunks in Alberta, Canada. J Wildl Dis 1986;22:13–18. [DOI] [PubMed] [Google Scholar]
- 3. Ayroud M, et al. The morphology and pathology of Besnoitia sp. in reindeer (Rangifer tarandus tarandus). J Wildl Dis 1995;31:319–326. [DOI] [PubMed] [Google Scholar]
- 4. Borman AM, et al. Adiaspiromycosis due to Emmonsia crescens is widespread in native British mammals. Mycopathologia 2009;168:153–163. [DOI] [PubMed] [Google Scholar]
- 5. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 2 St. Louis, MO: Elsevier, 2016:584–585. [Google Scholar]
- 6. Chantrey JC, et al. Emmonsia crescens infection in a British water vole (Arvicola terrestris). Med Mycol 2006;44:375–378. [DOI] [PubMed] [Google Scholar]
- 7. Curtis B, et al. Embolic mycotic encephalitis in a cow following Mortierella wolfii infection of a surgery site. J Vet Diagn Invest 2017;29:725–728. [DOI] [PubMed] [Google Scholar]
- 8. Dukik K, et al. Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). Mycoses 2017;60:296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emmons CW, Jellison WL. Emmonsia crescens sp. n. and adiaspiromycosis (haplomycosis) in mammals. Ann N Y Acad Sci 1960;89:91–101. [DOI] [PubMed] [Google Scholar]
- 10. Flatt RE, et al. Metabolic, traumatic, mycotic, and miscellaneous diseases of rabbits. In: Weisbroth SH, ed. The Biology of the Laboratory Rabbit. New York, NY: Academic Press, 1974:443–444. [Google Scholar]
- 11. Gardiner CH, et al. Fungi. In: An Atlas of Protozoan Parasites in Animal Tissues. Washington, DC: Armed Forces Institute of Pathology, 1998:77–80. [Google Scholar]
- 12. Hamir AN. Pulmonary adiaspiromycosis in raccoons (Procyon lotor) from Oregon. J Vet Diagn Invest 1999;11:565–567. [DOI] [PubMed] [Google Scholar]
- 13. Hubalek Z, et al. Emmonsiosis of subterranean rodents (Bathyergidae, Spalacidae) in Africa and Israel. Med Mycol 2005;43:691–697. [DOI] [PubMed] [Google Scholar]
- 14. Hughes K, Mueller K. Pathologic lesions of mycotic pneumonia in an alpaca following third compartment ulceration. J Vet Diagn Invest 2008;20:672–675. [DOI] [PubMed] [Google Scholar]
- 15. Koller LD, Helfer DH. Adiaspiromycosis in the lungs of a goat. J Am Vet Med Assoc 1978;173:80–81. [PubMed] [Google Scholar]
- 16. Koller LD, et al. Adiaspiromycosis in the lungs of a dog. J Am Vet Med Assoc 1976;169:1316–1317. [PubMed] [Google Scholar]
- 17. Krivanec K, Otcenasek M. Importance of free living mustelid carnivores in circulation of adiaspiromycosis. Mycopathologia 1977;60:139–144. [DOI] [PubMed] [Google Scholar]
- 18. Langenmayer MC, et al. Naturally acquired bovine besnoitiosis: histological and immunohistochemical findings in acute, subacute, and chronic disease. Vet Pathol 2015;52:476–488. [DOI] [PubMed] [Google Scholar]
- 19. Lello J, et al. The effect of single and concomitant pathogen infections on condition and fecundity of the wild rabbit (Oryctolagus cuniculus). Int J Parasitol 2005;35:1509–1515. [DOI] [PubMed] [Google Scholar]
- 20. Malatesta D, et al. First description of adiaspiromycosis in an Eurasian otter (Lutra lutra) in Italy. Vet Ital 2014;50:199–202. [DOI] [PubMed] [Google Scholar]
- 21. Morandi F, et al. Disseminated pulmonary adiaspiromycosis in a crested porcupine (Hystrix cristata Linnaeus, 1758). J Wildl Dis 2012;48:523–525. [DOI] [PubMed] [Google Scholar]
- 22. Morner T, et al. Adiaspiromycosis in a European beaver from Sweden. J Wildl Dis 1999;35:367–370. [DOI] [PubMed] [Google Scholar]
- 23. Peterson SW, Sigler L. Molecular genetic variation in Emmonsia crescens and Emmonsia parva, etiologic agents of adiaspiromycosis, and their phylogenetic relationship to Blastomyces dermatitidis (Ajellomyces dermatitidis) and other systemic fungal pathogens. J Clin Microbiol 1998;36:2918–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pusterla N, et al. Disseminated pulmonary adiaspiromycosis caused by Emmonsia crescens in a horse. Equine Vet J 2002;34:749–752. [DOI] [PubMed] [Google Scholar]
- 25. Sanchez-Migallon Guzman D, et al. Aleutian disease serology, protein electrophoresis, and pathology of the European mink (Mustela lutreola) from Navarra, Spain. J Zoo Wildl Med 2008;39:305–313. [DOI] [PubMed] [Google Scholar]
- 26. Simpson VR, et al. Causes of mortality and pathological lesions observed post-mortem in red squirrels (Sciurus vulgaris) in Great Britain. BMC Vet Res 2013;9:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simpson VR, et al. A post-mortem study of respiratory disease in small mustelids in south-west England. BMC Vet Res 2016;12:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taylor RL, et al. Adiaspiromycosis in small mammals of New Mexico. Mycologia 1967;59:513–518. [PubMed] [Google Scholar]
- 29. Tobon JL, et al. Adiaspiromycosis in the Franklin’s ground squirrel, Spermophilus franklini, and pika, Ochotona princeps, from Alberta, Canada. J Wildl Dis 1976;12:97–100. [DOI] [PubMed] [Google Scholar]