On May 16, 2018, two chicken carcasses and oropharyngeal swabs originating from a small flock of backyard exhibition chickens in Los Angeles County, CA were submitted to the California Animal Health and Food Safety Laboratory, San Bernardino and Davis branches, respectively. The clinical history mentioned high mortality (9 of 10) in a period of one week. Autopsy of the 2 birds revealed conjunctivitis, edema of the head and neck, petechial cutaneous hemorrhages of the head, linear-to-ecchymotic hemorrhages of pharynx, proventriculus, trachea, intestine and cloaca, and necrosis of the cecal tonsils. Real-time PCR testing of the oropharyngeal swabs utilizing protocols provided by the National Animal Health Laboratory Network was positive for virulent Newcastle disease (vND) virus. With this information, a presumptive diagnosis of vND (formerly known as exotic Newcastle disease) was established. The diagnosis was confirmed by the U.S. Department of Agriculture–Animal and Plant Health Inspection Service on May 17, 2018. As of May 31, 2018, the origin of the index case has not been determined, and several infected premises have now been identified in Los Angeles and San Bernardino counties. This is the first presentation of vND reported in the United States since 2003.
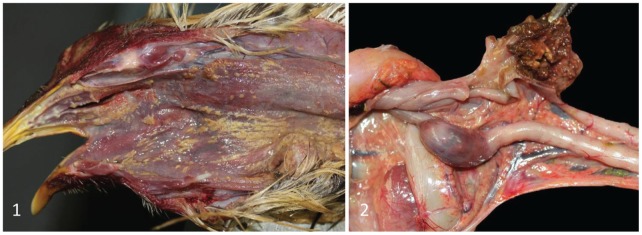
Gross lesions identified to date in birds submitted for autopsy from 6 cases that tested positive by vND PCR (multiple birds per case), included one or more of the following: conjunctival, tracheal, proventricular, intestinal and cloacal hemorrhages; fibrinonecrotizing stomatitis pharyngitis, esophagitis, and laryngotracheitis (Fig. 1); and cecal tonsil necrosis (Fig. 2). In one case, no gross lesions were observed, but a high virus concentration was detected by PCR.
Figures 1 and 2.
Chickens with virulent Newcastle disease.
Figure 1. Multifocal-to-coalescing fibrinonecrotizing stomatitis, pharyngitis, and esophagitis.
Figure 2. Diffuse, bilateral cecal tonsil necrosis and hemorrhage. Notice that while this lesion is very evident in the open cecum (top), it can also be observed from the serosal surface of the cecum (bottom).
Virulent ND is caused by avian paramyxovirus 1, a nonsegmented, single-stranded, negative-sense enveloped RNA virus (order Mononegavirales, family Paramyxoviridae, subfamily Paramyxovirinae, genus Avulavirus). Virulent ND is a highly fatal disease of birds, including 236 pet and wild, in addition to domestic avian species,3 and can cause significant economic impact to the poultry industry given high morbidity and mortality in chickens, which are among the species most susceptible to the disease.2 The development of clinical signs and gross lesions is dependent on many factors, and neither the clinical presentation nor the autopsy findings are fully diagnostic. Necrosis and hemorrhages of the cecal tonsils was frequently observed in chickens during the 2002–2003 outbreak.1 Other gross lesions observed included conjunctivitis; fibrinonecrotizing stomatitis, pharyngitis, esophagitis, and tracheitis; splenomegaly; and hemorrhages of proventriculus, trachea, thymus, and cloaca.2 Histologically, a lesion that is common to most avian species is necrosis of lymphoid tissue throughout the body, including the spleen, cecal tonsils, and lymphoid tissue of the esophageal-proventriculus junction. Other histologic lesions that may be observed are fibrinonecrotizing tracheitis, airsacculitis, encephalitis with gliosis and perivascular cuffing, necrotizing myocarditis, and necrosis of the bone marrow, pancreas, thymus, and liver.1,2
Clinical history, gross examination, and rapid reliable molecular testing are considered key for the accurate diagnosis of vND. During the last outbreak of the disease in California in 2002–2003, prior to the development and validation of a PCR assay, the regulatory agencies dealing with the eradication of vND relied heavily on gross presumptive diagnoses, which were later confirmed by virus isolation (VI). Because results of VI take several days or weeks, field measures initially had to be based on gross findings, later confirmed by VI. When the real-time PCR assay became available, it was used in the diagnosis of vND during the final stages of the outbreak and later for surveillance testing to prove freedom-of-disease to our trading partners.
In the current outbreak, with a reliable and fast PCR technique available to detect vND virus, most field diagnoses are confirmed directly by PCR. The clear advantages of this approach are the speed and accuracy of the diagnosis. In addition, reducing the number of birds that are transported to the laboratory for autopsy reduces the risk of contamination and disease transmission. Gross diagnosis remains, however, a fundamental pillar of early detection, as demonstrated in the current outbreak in which the initial recognition of compatible gross lesions prompted molecular testing and confirmation of the disease in a few hours. In addition, autopsies are still being performed when immediate results, albeit presumptive, are required. Finally, training of pathologists for lesion recognition remains critical, and outbreaks such as the current one provide a unique opportunity for this task.
Francisco R. Carvallo, Janet D. Moore, Akinyi C. Nyaoke, Linda
Huang
California Animal Health and Food Safety Laboratory system, San
Bernardino Branch, University of California, Davis
Beate M. Crossley
California Animal Health and Food Safety Laboratory
system, Davis Branch, University of California, Davis
Francisco A. Uzal
California Animal Health and Food Safety Laboratory, San
Bernardino Branch, University of California, Davis, San Bernardino, CA
fuzal@cahfs.ucdavis.edu
References
- 1. Cattoli G, et al. Newcastle disease: a review of field recognition and current methods of laboratory detection. J Vet Diagn Invest 2011;23:637–656. [DOI] [PubMed] [Google Scholar]
- 2. Miller PJ, et al. Newcastle disease. In: Swayne DE, et al., eds. Diseases of Poultry. 13th ed. Oxford, UK: Wiley-Blackwell, 2013:89–107. [Google Scholar]
- 3. Wakamatsu N, et al. Experimental pathogenesis for chickens, turkeys and pigeons of exotic Newcastle disease virus from an outbreak in California during 2002–2003. Vet Pathol 2006;43:925–933. [DOI] [PubMed] [Google Scholar]