Abstract
Domestic and wild sheep are the natural reservoirs for ovine gammaherpesvirus 2 (OvHV-2), the causative agent of sheep-associated malignant catarrhal fever (SA-MCF). Virtually all adult sheep are infected with OvHV-2 under natural flock conditions, and infection is normally subclinical. MCF-like clinical signs and typical histologic lesions in sheep have been linked during case investigations at veterinary diagnostic laboratories; however, the confirmation of naturally occurring MCF in sheep is problematic. To date, the assays for detection of OvHV-2–specific antibodies or DNA are usually positive in sheep, regardless of health status, so mere detection of antibodies or the agent is of minimal diagnostic significance in this species. We document herein a naturally occurring MCF case in a 4-mo-old domestic lamb and demonstrate that the affected animal had 100–1,000 times more OvHV-2 copy numbers in tissues than in healthy adult and age-matched sheep. These results indicate that high copy numbers of viral DNA in tissues associated with characteristic lesions can be used to confirm the diagnosis of MCF in sheep.
Keywords: Malignant catarrhal fever, ovine gammaherpesvirus 2, sheep
Malignant catarrhal fever (MCF) is a fatal viral disease resulting in multisystem lesions in ruminants and infrequently in pigs.7,14 The condition is caused by a collection of gammaherpesviruses in the genus Macavirus, including ovine gammaherpesvirus 2 (OvHV-2).8 Although susceptibility to the disease varies among individual species, when these viruses are transmitted to clinically susceptible hosts, MCF may occur.7 Consistent clinical findings in diseased animals include lymphadenomegaly, oral and gastrointestinal ulcerations with mild-to-severe hemorrhage, pneumonia, conjunctivitis, encephalitis, and dermatitis.12 The hallmark of MCF pathology includes lymphoproliferative inflammatory infiltrates, systemic vasculitis, and epithelial necrosis.12,14
Domestic and wild sheep are the reservoir hosts for OvHV-2, the causative agent of sheep-associated (SA)-MCF.7 Under natural conditions, virtually all adult sheep are infected with OvHV-2, and infection is lifelong and usually subclinical.7 Aerosol is the major mode of OvHV-2 transmission among sheep.10 Lambs are usually not infected until after 2 mo of age, and the infection rates in lambs are dependent on exposure to the concentration of virus shed from adult sheep.7 OvHV-2 initially replicates in sheep lung epithelial cells, maintains its latency in lymphocytes, and final replication in turbinate epithelial cells propagates shedding.7 Although there is a long-held belief that OvHV-2 does not cause disease in sheep, histologic lesions consistent with MCF have been observed occasionally in both domestic and wild sheep.5,15 The fact that MCF-like disease can be induced in sheep by experimental inoculation of OvHV-2 suggests that MCF can also occur in sheep following natural infection.9
Because almost all sheep have life-long infection with OvHV-2, detection of antibodies to OvHV-2 or viral DNA in a clinically affected sheep has little value to confirm the diagnosis of MCF.6 However, studies in bison and rabbits with experimentally-induced MCF showed that OvHV-2 DNA levels in tissues are positively associated with lesion severity,3,4 suggesting that quantification of OvHV-2 DNA in tissues associated with MCF-like lesions in sheep might be a valuable indicator to support the diagnosis. Herein we report a naturally occurring OvHV-2–induced MCF case in a lamb with clinical signs and lesions that remarkably resembled those seen in SA-MCF in clinically susceptible species. The confirmation of MCF in this lamb was based on histopathologic findings and supported by the detection of higher levels of OvHV-2 DNA in affected tissues compared to clinically normal sheep. Additionally, levels of OvHV-2 DNA were positively correlated with lesion severity in the diseased lamb.
The affected animal was a 4-mo-old crossbred lamb from a small farm in northwestern Oregon, which housed 18 sheep (8 adult sheep and 10 lambs) and a few other non-ruminant species, including pigs, alpacas, and chickens. In June of 2016, the lamb developed clinical signs including coughing, fever, and nasal discharge. Antibiotic treatment rendered no clinical response, and the lamb was euthanized by barbiturate injection 1 wk after onset of clinical signs. The carcass was sent to the Oregon Veterinary Diagnostic Laboratory for postmortem examination.
The carcass was in poor body condition. Abundant subcutaneous and intermuscular edema extended from the ventral neck into the thoracic inlet. The lungs were diffusely firm, gray-brown, and edematous; pleural rib impressions were observed; and lung tissue sank in 10% neutral-buffered formalin. Yellow viscous material filled ectatic bronchioles. The submandibular and tracheobronchial lymph nodes were enlarged, pale, and bulged on cut surface. Abomasal and esophageal mucosae were disrupted by numerous round-to-linear ulcers and associated minor hemorrhages.
Most organs examined, including those from the respiratory tract, adrenal and thyroid glands, liver, gastrointestinal tract, heart, pancreas, kidney, and thymus, exhibited various severities of transmural lymphocytic arteritis with fibrinoid necrosis and thrombosis. Affected vessels ranged from arterioles to large arteries. In most tissues, mural infiltrates were small lymphocytes with condensed nuclei; in some of the largest arteries, the infiltrate included small numbers of larger, atypical lymphocytes with a more blastic morphology. In addition to pulmonary vascular damage, alveoli were filled with various combinations of foamy macrophages, neutrophils, erythrocytes, fibrin, mucus, and necrotic cellular debris. Tracheal, bronchiolar, and bronchial epithelia were frequently sloughed, exposing marked submucosal lymphoplasmacytic infiltration. Pulmonary interlobular septa were expanded by edema and a few lymphocytic infiltrates. Several vessels in the tongue demonstrated the typical diffuse, transmural, lymphocytic vasculitis with a few lymphoblasts and segmental fibrinoid necrosis of the vascular wall seen in many of the organs examined (Fig. 1). The adrenal glands had multifocal lymphocytic infiltrates targeting vessels in the cortex, capsule, and periadrenal connective tissue (Fig. 2). The liver had similar inflammatory infiltrates within the periportal regions and several foci of acute coagulative necrosis (Fig. 3). In addition to vasculitis, the esophagus had multifocal ulcers accompanied by neutrophils and fibrin, perivascular infiltrates of mixed leukocytes in the submucosa, and a few lymphocytes in the epithelial layer (Fig. 4). Additional esophageal findings included rare apoptotic cells in the basal layer of the intact inflamed mucosa and transmural edema. Omasal, ruminal, abomasal, intestinal, and colonic lamina propria were expanded by increased numbers of small- and medium-sized lymphocytes with fewer plasma cells, in addition to the aforementioned vasculitis of larger arteries in other tunics. The thymus had focal transmural lymphocytic arteritis and was diffusely edematous and hypocellular, with poor corticomedullary distinction. Although brain was not extensively sampled, foci of perivascular cuffing by small lymphocytes were identified in the meninges and in the white matter of the cerebral cortex. Morphologic diagnoses included systemic (multi-organ) lymphocytic vasculitis, lymphohistiocytic-to-necrotizing bronchointerstitial pneumonia, lymphocytic and ulcerative esophagitis, lymphoplasmacytic gastroenteritis, and lymphocytic perivascular meningoencephalitis.
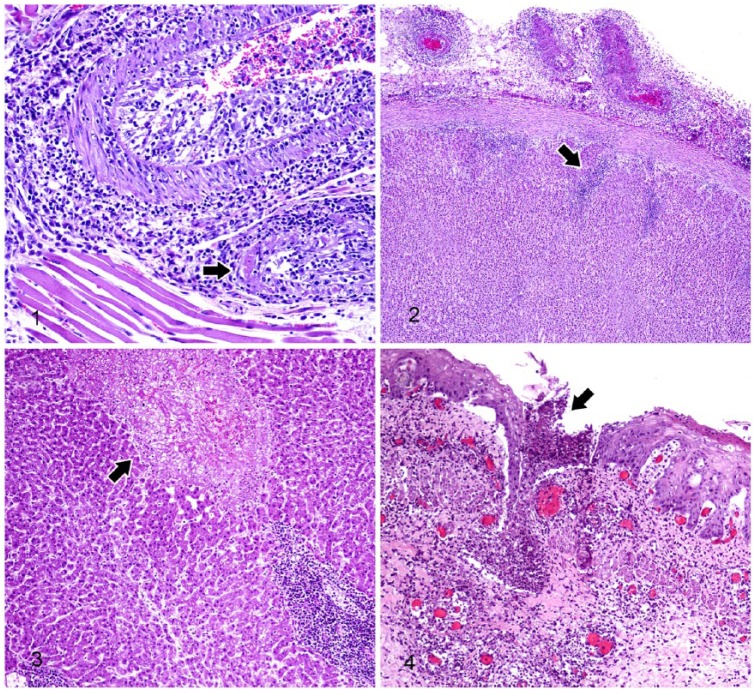
Figures 1–4.
Tissue from lamb affected with malignant catarrhal fever. H&E.
Figure 1. Lymphocytes of various sizes are visible in the subintimal, medial, and adventitial tunics of a large and a small lingual artery in the tongue. Fibrinoid necrosis is seen in the small artery (arrow).
Figure 2. Extracapsular lymphocytic vasculitis with lymphocytic infiltrates extending into the surrounding adipose tissue and adrenal cortex (arrow).
Figure 3. A focus of hepatic coagulative necrosis (arrow) and adjacent periportal lymphocytic aggregates.
Figure 4. Focal ulceration (arrow) and subtending lymphocytic vasculitis in the esophagus.
The character of the alveolar exudate in some areas (neutrophilic with necrotic debris) suggested a bacterial component to the pneumonia, but aerobic culture of lung tissue yielded only a mixed population of Escherichia coli, interpreted as postmortem contamination. Fecal flotation for gastrointestinal parasites was negative.
Because the lesions were characteristic of those seen in cattle and bison with SA-MCF, OvHV-2 was investigated in this lamb. Fresh frozen tissue samples and formalin-fixed, paraffin-embedded tissues blocks were sent to the Animal Disease Research Unit (ADRU), USDA-ARS and to the Washington Animal Disease Diagnostic Laboratory (WADDL), both located at Washington State University, Pullman, WA. Lung tissue submitted to the WADDL and tested by PCR analyses were negative for border disease virus (BDV), bluetongue virus (BTV), and epizootic hemorrhagic disease virus (EHDV). Immunohistochemistry on lung sections was also negative for bovine alphaherpesvirus 1 (BoHV-1) and inconclusive for bovine respiratory syncytial virus (BRSV; species Bovine orthopneumovirus). Six tissues (brain, lung, liver, kidney, small intestine, and large intestine) were sent to ADRU for investigation of OvHV-2. Total DNA was extracted from the tissues and subjected to OvHV-2 nested (n)PCR, herpesvirus consensus PCR, and OvHV-2 quantitative (q)PCR as described previously.1,16,17 OvHV-2 qPCR data are reported as viral DNA copy numbers per 5 ng of total tissue DNA.
All tissues were positive by OvHV-2 nPCR. A DNA band of relevant size was amplified by the herpesvirus consensus PCR, and sequencing of 177 base pairs of the amplicon showed that it shared 100% homology to a portion of the OvHV-2 DNA polymerase gene (GenBank DQ198083.1), confirming the virus identity. The OvHV-2 qPCR results revealed that the 6 tissues examined from this lamb had high copy numbers of viral DNA (811–457,000 OvHV-2 genome copies [mean = 209,000; SD = 190,000]), which were 100–1,000 times more OvHV-2 copy numbers in tissues than in clinically healthy sheep.
A 2017 published report of a MCF-like skin disease in a free-ranging bighorn sheep, another natural OvHV-2 reservoir host, identified higher levels of OvHV-2 DNA in affected skin relative to unaffected skin.15 In addition, studies in bison and rabbits with experimentally induced SA-MCF revealed that OvHV-2 DNA levels are positively correlated with lesion severity scores.3,4 In order to determine if there was a similar association in the clinically affected lamb described in our study, tissue lesions were scored using a score criterion previously developed for bison with MCF.3 The 6 tissues examined were ranked by lesion severity, from most to least severe, in the following order: lung, small intestine, large intestine, kidney, liver, and brain. The tissues with higher lesion scores were also among the ones with higher OvHV-2 genome copy numbers. The lungs had 433,000 OvHV-2 genome copies, the small intestine had 457,000, and the large intestine had 28,900. The liver and kidneys had similar lesion severity scores and 51,500 and 283,000 viral genome copies, respectively. The brain tissues had the lowest level of OvHV-2 DNA at 811 copies. Although no statistical analysis was performed because the data were from only a single animal, an apparently positive association between lesion severities and levels of viral DNA in the tissues was observed. The detection of high copy numbers of OvHV-2 DNA in tissues and its association with lesion severity supports the diagnosis of OvHV-2–induced MCF in this lamb.
To further evaluate levels of OvHV-2 in tissues as a reliable indicator of OvHV-2–induced MCF in sheep, we quantified OvHV-2 DNA in tissues of healthy sheep that carry the virus and compared them with the levels present in tissues of the clinically affected lamb described in our study. Tissue samples from small intestine, lung, kidney, and liver were obtained at slaughterhouse from 3 groups of sheep: 1) 3 age-matched lambs from the same farm where the lamb with MCF was housed (cohort group); 2) 7 age-matched lambs from a population of 10 adult and 12 lambs located on a farm in southeastern Washington; and 3) 16 adults, 1–3-y-old, also located in southeastern Washington. All animals were clinically normal.
All tissues (n = 104) of the 26 sheep from these 3 groups were positive for OvHV-2 by nPCR, confirming that all sheep were infected with OvHV-2. OvHV-2 DNA levels in each sample were quantified by qPCR (Table 1). Considering the 4 tissues (small intestine, lung, kidney, and liver; Table 1) together, the clinically affected lamb had an average of 306,000 OvHV-2 genome copies (SD = 161,000, n = 4), whereas the cohort lamb group had 2,240 (SD = 3,400, n = 12), the age-matched lambs from a different location had 209 (SD = 429, n = 28), and the adult sheep 129 (SD = 264, n = 64). There was a significant difference (p < 0.0001, ordinary one-way ANOVA) in the levels of OvHV-2 DNA among the groups. Post-test analysis, using the Tukey multiple comparison test, indicated that the levels of OvHV-2 DNA in the sick lamb were significantly higher than in all other groups. No significant difference was found among the 3 clinically normal groups. Statistical analysis was performed using Graph Pad Prism 6.07 (GraphPad Software, La Jolla, CA). These data indicate that OvHV-2 DNA levels in the 4 tissues examined from all 3 groups of healthy sheep (both lambs and adults) were ~100–1,000 times lower than the levels in the tissues of the lamb with MCF. Together, these results indicate that high copy numbers of OvHV-2 DNA in tissues can be used as to corroborate the diagnosis of OvHV-2–induced MCF in sheep.
Table 1.
Ovine gammaherpesvirus 2 (OvHV-2) DNA levels in tissues of the malignant catarrhal fever (MCF)-affected lamb and of clinically normal sheep.
| Group | OvHV-2 DNA levels* |
||||
|---|---|---|---|---|---|
| Intestine | Lung | Kidney | Liver | Total† | |
| MCF | |||||
| Lamb (n = 1) | 457,000 | 433,000 | 283,000 | 51,500 | 306,000 (161,000) |
| Clinically normal | |||||
| Cohort lambs (n = 3) | 3,870 (3,280) | 4,050 (6,280) | 334 (491) | 727 (983) | 2,240 (3,400) |
| Lambs (n = 7) | 574 (714) | 210 (333) | 14 (24) | 37 (58) | 209 (429) |
| Adults (n = 16) | 428 (403) | 36 (44) | 16 (24) | 35 (64) | 129 (264) |
OvHV-2 DNA levels are expressed as viral genome copies/5 ng of total tissue DNA, as measured by quantitative PCR. Mean viral DNA copy number for clinically normal groups are listed with standard deviations in parentheses.
Mean (SD) of all samples from each group: MCF lamb (n = 4), clinically normal cohort lambs (n = 12), clinically normal lambs (n = 28), and clinically normal adults (n = 64).
Sheep usually carry OvHV-2 without any clinical manifestation, clearly indicating resistance to MCF. Although MCF can be induced in sheep experimentally,9 it requires a very high dose of OvHV-2, at ~105 times more virus than that needed to induce MCF in bison, a highly disease-susceptible host.7 It is unlikely that the dose of virus required to cause MCF in sheep is normally reached in natural conditions.10 Therefore, naturally occurring MCF in sheep is likely associated with factors other than just viral dose, such as host immunologic status and/or OvHV-2 virulence.
In our case, the overall condition of the flock was poor when the lamb developed MCF. Lamb weight gains were slower than usual, at an approximate delay of 2 mo to reach market weight. Edema along the ventral jaw line (“bottle jaw”) was found in an adult sheep, and Haemonchus contortus eggs were identified in pooled fecal samples, indicating that internal parasites persisted in the flock. Ventral edema was also noted in the clinically affected lamb, possibly as a result of parasite-induced hypoproteinemia. The animals were in a deworming program, so the negative fecal flotation result in this lamb may reflect recent treatment. Failure to control internal parasites in this flock may have partly been the result of re-exposure on heavily infected pastures; the pastures are located in a valley with relatively mild temperatures and moisture that favor parasite survival. Internal parasitism, especially H. contortus, may have been an important underlying factor contributing to the poor health condition. In order to determine other factors that might cause immune suppression in the sheep flock, exposure to, or infection with, other viral and bacterial pathogens were investigated using sera from 8 adult sheep from the farm. Serum samples were tested for BoHV-1, bovine parainfluenza virus 3 (BPIV-3; species Bovine respirovirus 3), and BRSV by virus neutralization; for ovine progressive pneumonia virus (OPPV; species Visna/maedi virus) and Mycoplasma ovipneumoniae by competitive ELISA; and for Mycoplasma ovis by PCR. All testing was performed at the WADDL, except for the cELISA for OPPV and PCR for M. ovis, which were conducted at the ADRU. All 8 sheep were seronegative for BoHV-1 and OPPV, but positive for BPIV-3. Two of the 8 sheep were seropositive for BRSV, 2 were seropositive for M. ovipneumoniae, and 2 were PCR-positive for M. ovis. Trace mineral analysis, performed by the WADDL on a liver sample from the clinically affected lamb described in our study, showed that all minerals were within reference intervals. Based on the ancillary testing performed on sheep from this flock, a common cause for the overall poor health of the flock was not definitively identified.
It is interesting to note that the levels of OvHV-2 DNA in the cohort lamb group, especially in 1 lamb, were higher than in the other 2 clinically normal sheep groups. The reason for that was not clear; it could be because this particular lamb was in a preclinical stage of MCF and/or this viral strain was more virulent. The question about strain virulence has been raised before when a high number of cattle died from MCF when exposed to sheep at a state livestock exhibition.11 Unfortunately, the investigation of OvHV-2 inter-strain virulence is difficult because the virus cannot be propagated in vitro. There is limited information regarding the segments of OvHV-2 genome that may be associated with virulence. Studies on corneal infection by herpes simplex 1 showed that different viral genes cooperate to influence disease severity and confirmed that a constellation of genes within a particular strain determines the disease phenotype.2 It is possible that certain OvHV-2 genes function in a similar way to determine virulence, but further investigation is needed.
Diagnosis of clinical MCF in sheep has always been a challenge for veterinarians and diagnostic laboratories. Although characteristic histologic lesions of vasculitis are highly suggestive of OvHV-2–induced MCF, there is a similarity at the histologic level between MCF lesions in clinically susceptible species and systemic necrotizing polyarteritis (SNP) in sheep. SNP in sheep is an infrequently reported syndrome of unknown etiology, although several viruses, including BDV, BTV, EHDV, and OvHV-2, have been speculated.13 A 2017 published report on a case of SNP in sheep clearly demonstrates how difficult it is to confirm a diagnosis associated with such lesions,18 reinforcing the need to rely on other parameters such as agent identification to establish a definitive diagnosis. Confirmation of OvHV-2–induced MCF in species other than sheep can be routinely carried out in veterinary diagnostic laboratories with the detection of OvHV-2 DNA in blood or tissue samples by PCR, in combination with compatible histologic lesions.6,16 However, the detection of OvHV-2 DNA in blood or tissues of sheep provides little information about any associated clinical disease, given that sheep are the natural host for the virus and infected sheep have detectable viral DNA in blood and tissues.6 The overall high copy numbers of OvHV-2 DNA in tissues of the clinically affected lamb as compared to clinically normal sheep, plus the apparent association between the amount of viral DNA and severity of lesions, support the diagnosis of OvHV-2–induced MCF in our case. Although more cases need to be examined, these findings suggest that OvHV-2 qPCR may be a useful tool in the diagnosis of clinical MCF in sheep. Development of an in situ assay, such as immunohistochemistry or in situ hybridization, would provide means to directly correlate the presence of OvHV-2–infected cells within lesions, providing a more definitive assay for the diagnosis of MCF in sheep.
Acknowledgments
We thank Erika Czerniejewski for collection of blood and tissue samples from the cohort sheep, George Barrington for the tissue samples from age-matched lambs, and Jan R. Busboom for the tissue samples from adult sheep. We also thank Shirley Elias, Nicholas Durfee, Paige Grossman, and Xiaoya Chen for excellent technical assistance.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: CW Cunha and H Li disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: USDA-ARS CWU 2090-32000-037-00D.
ORCID iD: Cristina W. Cunha  https://orcid.org/0000-0002-8168-5448
https://orcid.org/0000-0002-8168-5448
References
- 1. Baxter SIF, et al. PCR detection of the sheep-associated agent of malignant catarrhal fever. Arch Virol 1993;132:145–159. [DOI] [PubMed] [Google Scholar]
- 2. Brandt CR. The role of viral and host genes in corneal infection with herpes simplex virus type 1. Exp Eye Res 2005;80:607–621. [DOI] [PubMed] [Google Scholar]
- 3. Cunha CW, et al. Ovine herpesvirus 2 infection in American bison: virus and host dynamics in the development of sheep-associated malignant catarrhal fever. Vet Microbiol 2012;159:307–319. [DOI] [PubMed] [Google Scholar]
- 4. Cunha CW, et al. Are rabbits a suitable model to study sheep-associated malignant catarrhal fever in susceptible hosts? Vet Microbiol 2013;163:358–363. [DOI] [PubMed] [Google Scholar]
- 5. Gaudy J, et al. Possible natural MCF-like disease in a domestic lamb in Scotland. Vet Rec 2012;171:563–564. [DOI] [PubMed] [Google Scholar]
- 6. Li H, et al. Malignant catarrhal fever: understanding molecular diagnostics in context of epidemiology. Int J Mol Sci 2011;12:6881–6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li H, et al. Malignant catarrhal fever: inching toward understanding. Annu Rev Anim Biosci 2014;2:209–233. [DOI] [PubMed] [Google Scholar]
- 8. Li H, et al. A novel subgroup of rhadinoviruses in ruminants. J Gen Virol 2005;86:3021–3026. [DOI] [PubMed] [Google Scholar]
- 9. Li H, et al. Malignant catarrhal fever-like disease in sheep after intranasal inoculation with ovine herpesvirus-2. J Vet Diagn Invest 2005;17:171–175. [DOI] [PubMed] [Google Scholar]
- 10. Li H, et al. Shedding of ovine herpesvirus 2 in sheep nasal secretions: the predominant mode for transmission. J Clin Microbiol 2004;42:5558–5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore DA, et al. Outbreak of malignant catarrhal fever among cattle associated with a state livestock exhibition. J Am Vet Med Assoc 2010;237:87–92. [DOI] [PubMed] [Google Scholar]
- 12. O’Toole D, et al. The pathology of malignant catarrhal fever, with an emphasis on ovine herpesvirus 2. Vet Pathol 2014;51:437–452. [DOI] [PubMed] [Google Scholar]
- 13. Rae CA. Lymphocytic enteritis and systemic vasculitis in sheep. Can Vet J 1994;35:622–625. [PMC free article] [PubMed] [Google Scholar]
- 14. Russell GC, et al. Malignant catarrhal fever: a review. Vet J 2009;179:324–335. [DOI] [PubMed] [Google Scholar]
- 15. Slater OM, et al. Sheep-associated malignant catarrhal fever-like skin disease in a free-ranging bighorn sheep (Ovis canadensis), Alberta, Canada. J Wildl Dis 2017;53:153–158. [DOI] [PubMed] [Google Scholar]
- 16. Traul DL, et al. Validation of nonnested and real-time PCR for diagnosis of sheep-associated malignant catarrhal fever in clinical samples. J Vet Diagn Invest 2007;19:405–408. [DOI] [PubMed] [Google Scholar]
- 17. VanDevanter DR, et al. Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol 1996;34:1666–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wessels M, et al. Systemic necrotizing polyarteritis in three weaned lambs from one flock. J Vet Diagn Invest 2017;29:733–737. [DOI] [PubMed] [Google Scholar]