Abstract
Purpose
Maintaining high adherence rates (session attendance and compliance) in exercise programs during breast cancer treatment can be challenging. We aimed to identify adherence rates and predictors to an exercise program during adjuvant breast cancer treatment.
Methods
Ninety-two patients with localized breast cancer undergoing chemotherapy were randomly assigned to an 18-week supervised moderate-to-high intensity aerobic and resistance exercise program, including two 1-hour sessions/week. Additionally, participants were asked to be physically active for at least 30 minutes/day on at least three other days. We report median percentages for attendance, compliance with the prescribed duration and intensity of aerobic and muscle strength exercises, and the exercise advice given. Predictors included in univariate and multivariable linear regression models were demographical, tumor- and treatment-related factors, constructs of the theory of planned behavior, psychological and physical factors.
Results
Patients attended 83% (interquartile range: 69–91%) of the supervised sessions. Compliance with the duration of aerobic exercise, high-intensity aerobic exercise (cycling at the ventilatory threshold), muscle strength exercises and the exercise advice were 88%(64–97%), 50%(22–82%), 84%(65–94%) and 61%(33%–79%), respectively. Education, radiotherapy, BMI and physical fatigue were important predictors of adherence to supervised exercise. Beliefs about planned behaviors were important predictors, especially for compliance with the exercise advice.
Conclusions
Attendance to and compliance with an 18-week aerobic and strength exercise program were high. The lowest compliance was found for high-intensity supervised aerobic exercise. The identified predictors should be considered when designing or adapting exercise programs for patients with localized breast cancer to increase adherence.
Trial registration
Current Controlled Trials ISRCTN43801571
Dutch Trial Register NTR2138
Background
In our randomized Physical Activity during Cancer Treatment (PACT) study, we showed that an 18-week exercise program had beneficial effects on fatigue and physical fitness in newly diagnosed breast cancer patients undergoing adjuvant treatment [1]. Several meta-analyses have also reported that supervised exercise interventions during cancer treatment have positive effects on cancer-related fatigue, physical functioning and quality of life [2–6]; however, in general the reported effect sizes were small [1, 7].
An important component facilitating the optimal effectiveness of exercise programs is a high level of exercise adherence, which is defined by the World Health Organization as “the extent to which a person’s behavior corresponds with agreed recommendations” [8]. This definition of adherence incorporates both the number of sessions attended as well as the compliance with the prescribed intensity and duration of the individual training sessions. Adherence to prescribed exercise interventions during cancer treatment is known to be challenging, particularly because of treatment-related barriers [9, 10]. According to a recent review of Neil-Sztramko et al. (2017), studies did not include all components of exercise adherence rates such as attendance and compliance with the prescribed intensity and duration [11]. In previous studies with varying frequencies, durations, and timings of exercise programs among breast cancer patients during treatment, attendance rates for supervised exercise sessions were between 70–79% [12–17]. Little is known about the compliance of breast cancer patients with prescribed programs. Breast cancer patients undergoing treatment who followed a home-based moderate-intensity walking program had a compliance rate of 87% for intensity and 98% for the duration of the program [18]. However, the compliance rates during supervised exercise programs including high-intensity training have not been reported; and so far, it is unknown whether patients comply to the high intensities. Also, compliance with resistance exercises is currently unknown in breast cancer patients participating in exercise trials.
Identifying the factors that predict adherence rates during supervised exercise could lead to improved strategies for enhancing adherence to future interventions, thereby enhancing their effectiveness. It is important to distinguish between trials utilizing exercise interventions during or after cancer treatment because the factors predicting adherence may differ. For example, treatment-related factors may be less important after the cancer treatment has finished. In addition, predictors of adherence may also differ between home-based and supervised exercise. The systematic review of Kampshoff et al. stated that exercise history was a prominent predictor of adherence in cancer patients during or after treatment (19), which is in accordance with a recent study [19, 20]. A growing number of studies have reported predictors of exercise attendance or compliance especially during adjuvant breast cancer treatment [12, 13, 18], three of which applied supervised programs [12, 13].Two of these studies found that a high peak oxygen consumption (VO2peak), a measure of physical fitness, was an important predictor of high attendance rates [12, 13]. Furthermore, quality of life, being employed before treatment and a high personal income predicted high attendance rates in breast cancer patients [21]. However, in these studies predictors of exercise compliance were not addressed. Two studies investigated predictors of compliance, but both applied a home-based program. One study showed that patients who were less fatigued had a higher compliance with both walking duration and intensity [18]. Patients who perceived a higher importance of exercise, who had an early stage of disease, and who were employed had a higher compliance with exercise intensity [18]. The study of Nyrop et al. reported that being Caucasian or reporting higher walking minutes prior to the start of chemotherapy was associated with greater walking steps per week in a home-based exercise intervention [22]. To our knowledge, no studies were performed reporting predictors about compliance in supervised exercise programs during treatment in breast cancer patients. The review of Kampshoff et al. recommended that future studies should specifically focus on both the predictors of attendance and different parts of compliance [19]. Since self-efficacy about participation during the exercise sessions was found as predictor for exercise adherence in cancer survivors [23], this might also be of interest in cancer patient during treatment. Therefore, it is recommended to get insight in behavioral constructs to increase our understanding of exercise behavior in cancer patients. A commonly used framework to understand exercise behavior in cancer patients is Ajzen’s theory of planned behavior which links beliefs and behavior [24]. The patients’ beliefs regarding exercise might influence their adherence to the exercise program and should therefore be assessed when looking for possible factors predicting adherence to physical exercise.
The aim of the present study is to identify adherence rates (both attendance and compliance rates) of patients taking part in an 18-week supervised aerobic and strength exercise intervention and exercise advice during adjuvant breast cancer treatment. Other aims included exploring which demographical, tumor-related, treatment-related, psychological, and physical factors, as well as which constructs of the theory of planned behavior, predicted attendance and compliance.
Methods
Setting and patients
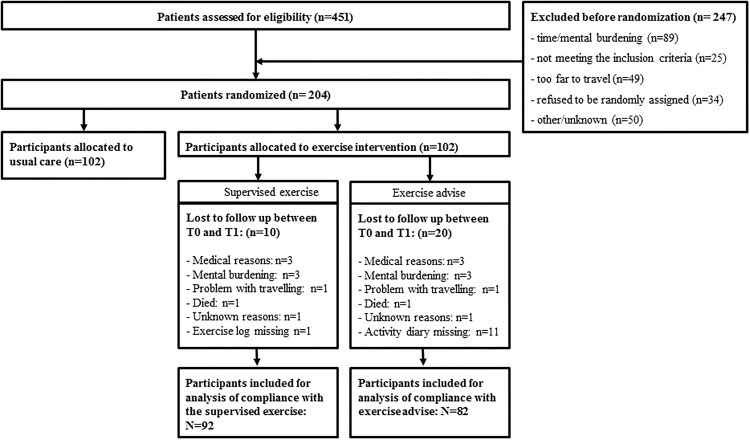
The design of the PACT study (ISRCTN43801571) has been described in detail elsewhere [1, 25, 26]. In short, breast (n = 202) and colon cancer (n = 33) patients were included in the study after written informed consent was signed. A concealed computer-generated randomization, following a 1:1 ratio was used to allocate participants in the exercise intervention group or the control group. Randomization was stratified by age, adjuvant treatment (radiotherapy yes/no before chemotherapy), use of tissue expander and hospital. Sequential balancing was used to allocate participants to the study groups. In the present exploratory analysis, only breast cancer patients who were randomly allocated to the exercise intervention were included (n = 102).
Since 10 exercise logs were not returned, attendance to and compliance with the supervised exercise program was determined for 92 breast cancer patients. Compliance with the exercise advice was determined for 82 patients, due to missing activity diaries (see Fig 1 for the flow chart). Since the original study was not primarily powered for the current analysis, the results should be interpreted as exploratory data. The sample size of the original study was calculated to detect an intervention effect on fatigue of 2 units improvement in the Multiple fatigue Inventory (MFI) questionnaire [25]. Patients were recruited in seven hospitals in the Netherlands between 2010 and 2012. Inclusion criteria were: histological diagnosis of cancer less than six weeks before study recruitment (ten weeks for patients with mastectomy and immediate reconstruction using a tissue expander); stage M0; scheduled for chemotherapy; age 25–75 years; not treated for any other cancer in the five years preceding recruitment (except basal skin cancer); able to read and understand the Dutch language; Karnovsky Performance Status of 60 or higher; able to walk 100 meters or more; no contra-indications for physical activity. The Medical Ethics Committee of the University Medical Centre Utrecht and the local Ethical Boards of the participating hospitals approved the study (07-271/O) and the local Ethical Boards of the participating hospitals (i.e., St. Antonius Hospital, Nieuwegein and Woerden; Diakonessenhuis Hospital, Utrecht; Meander Medical Centre, Amersfoort; Rivierenland Hospital, Tiel; Orbis Medical Centre, Sittard).
Fig 1. Flow of the participants through the study.
Exercise intervention
Patients in the intervention group were offered an 18-week supervised exercise program, in addition to the usual care. The program included two 1-hour sessions per week of combined aerobic and muscle strength exercises supervised by a physiotherapist. In addition, patients were asked to be physically active for at least 30 minutes per day on at least three other days [27]. Several principles of Bandura’s social cognitive theory were incorporated in the exercise program in order to motivate the patients to adhere to the exercise program and to maintain high physical activity levels during and after the supervised exercise program [28].
Supervised exercise program
The exercise program was individualized to the patients’ preferences and fitness levels. Aerobic fitness and muscle strength were determined, respectively, by means of a cardiopulmonary exercise test (CPET) and a one-repetition maximum (1-RM) test at the baseline [29]. The aerobic training included interval training of alternating intensities at or below the ventilatory threshold (VT), as monitored by the heart rate. The exercises were performed on cardiovascular fitness equipment of choice. Muscle strength training was performed for all major muscle groups: arms, legs, shoulders, and trunk. In order to minimize the injury risk of strength assessment, 1-RM strength was predicted by using regression equation prescribed by the American Society of Exercise Physiologists (ASEP)[30]. During all muscle strength assessments, the weight will be estimated that can be lifted between 4 and 10 repetitions. See S1 Table for details. Training intensity was re-assessed every four weeks. The weight used for the muscle strength exercises was adjusted every four weeks based on the estimated 1-RM from a submaximal muscle strength test. If necessary, weight was adjusted between the scheduled tests, although the participant was primarily asked to complete the total repetitions of each exercise prior to adding weight resistance.
The physiotherapists were instructed to closely follow the exercise protocol; however, if patients were not feeling well, adjustments were allowed and documented. If adjustments were necessary, we instructed the physiotherapist to reduce the exercise intensity prior to a reduction of the duration.
Exercise advice
Patients were asked to be physically active at moderate intensity for at least 30 minutes for a total of five days a week, i.e. at least three other days of the week in addition to the two supervised PACT exercise days. Patients were encouraged to embed this activity in their daily lifestyle, in order to make it a habit.
The behavioral component
Bandura’s social cognitive theory emphasizes the role of cognitive processes in behavior [28]. An important construct in the theory is self-efficacy, defined as an individual’s own beliefs in his/her capacity to organize and execute the actions required to reach desirable results [28]. The model gives an insight into how self-efficacy beliefs about physical activity during cancer treatment can influence behavior. Beliefs of self-efficacy are based on actual/mastery experience, vicarious/observational experience, verbal persuasion and emotional arousal (physiological states and affective states). In the PACT supervised exercise program, we addressed the first three determinants. For actual/mastery experience, patients were asked to report their training results in an exercise log. Vicarious/observational experiences included modelling (the use of a role model) and a DVD showing the exercise experiences of other cancer patients. Verbal persuasion included a leaflet showing the effectiveness of exercise in other patients and the supervision by the physiotherapist during the sessions. Physiological states and affective states are part of emotional arousal. Emotional arousal was not addressed in the exercise program, because it can be better targeted through other interventions like cognitive behavioral therapy.
Patients randomized into the control group (not included in the present analysis) received the usual care and were asked to maintain their habitual physical activity patterns up to week 18.
Outcome measures
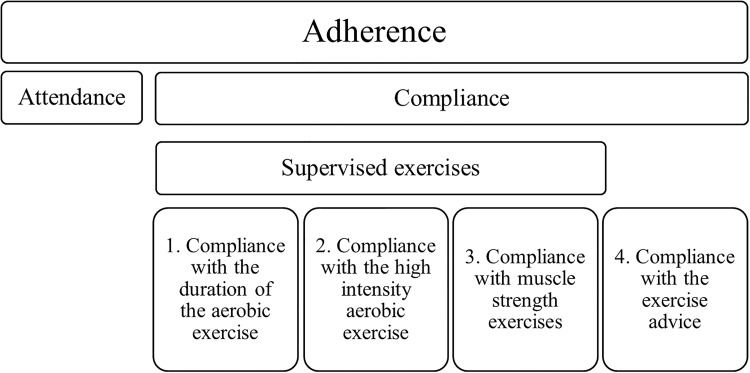
The primary outcome in the present study was adherence, which incorporates both attendance and compliance rates with the PACT intervention. Attendance is simply scoring if someone is present or not independent what the patient during the exercise session. The next step is to assess whether the session was performed according to the exercise protocol, which we defined as compliance. Attendance rates were computed for each patient as the number of supervised sessions attended divided by the number of sessions offered. Compliance rates were computed individually for four measures of the PACT program: (1) the duration of aerobic exercises, (2) the intensity of aerobic exercises, (3) the muscle strength exercises, and (4) compliance with the exercise advice (Fig 2).
Fig 2. Overview of the outcome measures for adherence.
Attendance
Patient attendance of the 36 supervised sessions in the PACT intervention was registered in a Case Record Form by the physiotherapist.
Compliance with the supervised exercise program
During each exercise session, patients registered their performed exercises in an exercise log supervised by the physiotherapist. They reported the total duration of the aerobic exercises in minutes, as well as the duration of the high-intensity intervals (i.e., the number of minutes at or above the VT). The weight used and number of repetitions performed for all muscle strength exercises were also registered. Compliance with the following elements were determined per patient in percentages:
The duration of the aerobic exercises, i.e. performing the total prescribed number of minutes;
The intensity of the aerobic exercises, i.e. performing the prescribed number of minutes at or above the VT;
The muscle strength exercises, i.e. at least three of the five prescribed exercises according to the recommended intensity (weight multiplied by the number of repetitions). We set three as the maximum since an adaption of the protocol was allowed for two exercises because not all locations were suitable for the performance of 1-RM tests for all exercises, due to the limitations of the equipment.
Compliance with the exercise advice
Patients registered the duration of activities they performed at a moderate-to-high intensity in their activity diary on a daily basis. We defined moderate-to high intensity activities according to the Dutch guidelines for physical activity, including activities with a MET-value of at least 4.0 (walking and hiking were also included because at baseline patients were explicitly instructed to register only when walking at a steady pace and when walking at a steady pace and when they had difficulties talking at the same time) [31, 32]. The number of weeks in which patients met the advice for five days of exercise were determined using these diaries [27].
Assessment of predictors
Our second aim was to assess predictors of adherence. The following predictors, which were assessed at baseline, were considered: demographical factors, tumor and treatment factors, constructs of the theory of planned behavior, psychological factors and physical factors.
Demographical factors
Self-reported data were collected for the following factors: age (years), education (high: higher vocational and university education; medium: secondary and secondary vocational education; low: elementary and lower vocational education), and marital status (living alone versus living together).
Tumor and treatment factors
Radiotherapy treatment status (yes or no) and tumor receptor status (triple negative, Her2Neu+ with ER/PR +/-, or Her2Neu- with ER/PR+) were retrieved from medical records. Tumor receptor status was a proxy for treatment type and treatment duration, since actual treatment plan would result in a variable with too many categories.
Constructs of the theory of planned behavior
Ajzen’s theory of planned behavior is the most commonly used framework to understand exercise behavior [24, 33, 34]. The theory links beliefs and behavior. The patients’ beliefs about attending 30 out of 36 exercise sessions and beliefs about being physically active according to the guidelines were assessed as specified by the theory of planned behavior: intention, subjective norm, perceived behavioral control and attitude [24]. The subscales were recorded on a 10-point Likert scale.
Psychological factors
Anxiety and depression were assessed using the validated Dutch language version of the Hospital Anxiety and Depression Scale (HADS) [35]. This questionnaire consists of 14 items; seven items on the depression subscale and seven items on the anxiety subscale resulting in a total depression score and a total anxiety score (both scored 0 (no depressive symptoms/anxiety) to 21 (depressive symptoms/anxiety)).
Health-related quality of life was assessed using the global health status subscale of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) [36]. The global health status subscale consists of two items, regarding overall health and overall quality of life, and ranges from 0 (low score global health status) to 100 (high score global health status).
Self-efficacy was assessed using seven (attending 30 sessions or more) or eight (being physically active for at least 30 minutes for five days per week) items, based upon the social cognitive theory of Bandura [28]. Self-efficacy items were assessed on a 10-point Likert scale with endpoints labelled ‘strongly disagree’ and ‘strongly agree’. Self-efficacy scores range form 1–10, where a higher score means a high belief about self-efficacy for the behavior.
Physical factors
Body weight and height were measured to the nearest 0.5 kg and 0.5 cm, respectively, with the subjects wearing light clothes and no shoes. These data were used to calculate body mass index (BMI; kg/m2).
Physical Activity was measured using the Short Questionnaire to Assess Health-Enhancing Physical Activity (SQUASH). Baseline levels of moderate-to-high intensity leisure and sport activities were calculated in minutes per week for a typical week before diagnosis [37].
Physical fatigue was measured using a subscale of the validated Multidimensional Fatigue Inventory (MFI) [38]. The MFI is a self-report instrument designed to measure multiple fatigue characteristics and the impact on function. The score on the physical fatigue subscale ranges from 4 to 20, with a higher score indicating greater fatigue.
Cardiorespiratory fitness (peak oxygen consumption; liters per minute) was determined using a CPET for patients experiencing an increasing workload on a cycle ergometer with continuous gas analysis of breath. The test was terminated in response to the patients’ symptoms or at the physician’s discretion. The mean of the VO2 values of the last 30 seconds before exhaustion was determined as peak oxygen consumption.
Statistical analyses
Descriptive statistics were used to describe the patients’ characteristics and baseline values of potential predictors. The attendance and compliance rates are reported as medians with interquartile ranges. Descriptive statistics were shown for the total group, and were also stratified for patients attending 0–19 (0–53%), 20–29 (54–80%) and 30–36 (81–100%) sessions in order to explore whether patients with higher attendance rates differed from patients attending fewer exercise sessions.
A univariable linear regression was used to predict the rate of attendance and compliance in order to identify possible predictors for multivariable model building using an intention to treat analysis. The selection of possible predictors was based on p<0.20, and a Pearson’s correlation coefficient of <0.7 among each other. The possible predictors were included in the multivariable model, and the final model was obtained by performing a multivariable linear regression with the stepwise backward selection of factors based on Akaike’s information criterion at p<0.157. Similar p-values are commonly used in prediction research [39]. Predictors are reported as estimated unstandardized regression coefficients (β) with a 95% confidence interval (CI). Model fit of the multivariable model was expressed as R2. All analyses were performed using SPSS version 21.0.
As stated before, the results should be interpreted as exploratory data. Because of this, adjusting for multiple comparisons was not done.
Results
Baseline characteristics
The 92 patients had a mean age of 50.2 ± 7.8 (standard deviation) years and an average BMI of 25.7 ± 4.4 kg/m2. Sixty-four patients (69.6%) received radiotherapy in addition to chemotherapy (Table 1). Patients reported to be physically active for more than 30 minutes a day on 4.9 ± 2.1 days per week prior to diagnosis. Patients who attended fewer than 20 of the prescribed exercise sessions had, on average, a higher BMI, lower quality of life, lower educational level, received more radiotherapy and were more physically fatigued compared with patients who attended more sessions.
Table 1. Baseline characteristics of 92 breast cancer patients, reported for the total group and stratified according to attendance.
| Predictor | Total Group (n = 92) |
0–19 sessions attended (n = 12) |
20–29 sessions attended (n = 33) |
30–36 sessions attended (n = 47) |
Exercise advise group (n = 82) |
|---|---|---|---|---|---|
| Demographical | |||||
| Age (years (mean ± SD)) | 50.2 ± 7.8 | 51.5 ± 5.1 | 47.8 ± 9.2 | 51.6 ± 6.9 | 50.0 ± 7.9 |
| Educational status (n (%)) | |||||
| Low | 4 (4.3%) | 3 (25.0%) | 0 (0.0%) | 1 (2.1%) | 2(2.4%) |
| Medium | 44 (47.8%) | 6 (50.0%) | 17 (51.5%) | 21 (44.7%) | 40 (48.8%) |
| High | 42 (45.7%) | 1 (8.3%) | 16 (48.5%) | 25 (53.2%) | 40 (48.8%) |
| Missing | 2 (2.2%) | 2 (16.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Marital status (n (%)) | |||||
| Together | 73 (79.3%) | 8 (66.7%) | 26 (78.8%) | 39 (83.0%) | 66 (80.5%) |
| Alone | 17 (18.5%) | 2 (16.7%) | 7 (21.2%) | 8 (17.0%) | 16 (19.5%) |
| Missing | 2 (2.2%) | 2 (16.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Tumor and treatment | |||||
| Radiotherapy (n (%)) | 64 (69.6%) | 10 (83.3%) | 23 (69.7%) | 31 (66.0%) | 55 (67.1%) |
| Receptor status (n (%)) | |||||
| Triple negative | 23 (25.0%) | 2 (16.7%) | 10 (30.3%) | 11 (23.4%) | 21 (25.6%) |
| Her2+ and ER+ or PR+ | 8 (8.7%) | 0 (0.0%) | 4 (12.1%) | 4 (8.5%) | 8 (9.8%) |
| Her2+ and ER- and PR- | 10 (10.9%) | 1 (8.3%) | 3 (9.1%) | 6 (12.8%) | 10 (12.2%) |
| Her2- and ER+ or PR+ | 51 (55.4%) | 9 (75.0%) | 16 (48.5%) | 26 (55.3%) | 43 (52.4%) |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Theory of planned behavior | |||||
| Beliefs about attending ≥ 30 exercise sessions | |||||
| Intention | 9.3 ± 1.2 | 8.8 ± 1.3 | 9.3 ± 1.2 | 9.4 ± 1.1 | 9.4 ± 1.1 |
| Subjective norm | 5.9 ± 1.9 | 5.5 ± 2.5 | 5.9 ± 1.7 | 6.0 ± 1.9 | 5.9 ± 1.9 |
| Perceived behavioral control | 8.0 ± 1.5 | 7.8 ± 1.4 | 7.8 ± 1.5 | 8.1 ± 1.5 | 7.9 ± 1.5 |
| Attitude | 8.7 ± 1.5 | 8.4 ± 1.8 | 9.0 ± 1.3 | 8.6 ± 1.5 | 8.7 ± 1.4 |
| Beliefs about being physically active for ≥ five days per week | |||||
| Intention | 8.8 ± 1.3 | 8.4 ± 1.8 | 8.6 ± 1.4 | 9.1 ± 1.0 | 8.8 ± 1.3 |
| Subjective norm | 5.4 ± 1.8 | 5.4 ± 2.1 | 5.5 ± 1.8 | 5.3 ± 1.7 | 5.4 ± 1.7 |
| Perceived behavioral control | 7.9 ± 1.5 | 7.9 ± 1.6 | 7.7 ± 1.8 | 8.0 ± 1.4 | 7.8 ± 1.6 |
| Attitude | 8.5 ± 1.7 | 8.3 ± 1.8 | 8.6 ± 1.7 | 8.4 ± 1.7 | 8.4 ± 1.7 |
| Psychological | |||||
| Anxiety | 4.5 ± 3.4 | 4.6 ± 3.8 | 5.2 ± 4.1 | 4.0 ± 2.8 | 4.4 ± 3.4 |
| Depression | 2.6 ± 3.2 | 2.9 ± 3.6 | 3.0 ± 3.3 | 2.2 ± 2.9 | 2.7 ± 3.1 |
| Health-related quality of life | 74.5 ± 21.1 | 66.7 ± 30.4 | 75.5 ± 19.6 | 75.9 ± 19.3 | 75.5 ± 19.4 |
| Beliefs about self-efficacy | |||||
| Attending ≥ 30 sessions | 7.1 ± 1.9 | 7.3 ± 1.5 | 7.2 ± 2.0 | 7.0 ± 1.9 | 7.0 ± 1.8 |
| Physical activity ≥ five days per week | 7.0 ± 2.0 | 7.3 ± 1.9 | 6.7 ± 2.3 | 7.2 ± 1.9 | 6.9 ± 2.0 |
| Physical | |||||
| BMI (kg/m2) | 25.7 ± 4.4 | 29.0 ± 4.9 | 26.0 ± 4.9 | 24.7 ± 3.5 | 25.4 ± 4.2 |
| Baseline physical activity (minutes/week) | 482,89 ± 449.9 | 163.3 ± 184.7 | 199.2 ± 257.2 | 376.1 ± 573.3 | 284.4 ± 466.6 |
| Physical fatigue | 9.9 ± 4.4 | 12.1 ± 5.1 | 10.2 ± 4.5 | 9.2 ± 4.0 | 10.0 ± 4.4 |
| Peak oxygen consumption (L/min) | 1.7 ± 0.3 | 1.6 ± 0.3 | 1.7 ± 0.4 | 1.7 ± 0.3 | 1.7 ± 0.3 |
Attendance
The median attendance rate for patients was 83% (interquartile range: 69% to 91%); 13% attended fewer than 20 of the supervised exercise sessions, 36% attended 20 to 29 sessions, and 51% attended 30 or more.
Compliance
Compliance with the three measures of the supervised program were 88% (63% to 97%) for the duration of aerobic exercise, 50% (22% to 82%) for the high-intensity aerobic exercises, and 84% (65% to 94%) for the muscle strength exercises (Table 2). Three patients complied with all three components of the supervised program for 30 sessions or more. Patients who attended 19 sessions or fewer had a lower compliance with all three components of the supervised exercise program than those who attended 30 or more sessions.
Table 2. Compliance rates according to the four domains of the PACT intervention.
| Total group (n = 92) |
0–19 exercise sessions attended (n = 12) |
20–29 exercise sessions attended (n = 33) |
30–36 exercise sessions attended (n = 47) |
|||||
|---|---|---|---|---|---|---|---|---|
| Compliance* with the… | Median | IQR | Median | IQR | Median | IQR | Median | IQR |
| Supervised exercises | ||||||||
| Duration of aerobic exercise$ | 87.5% | 63.3–96.8% | 59.7% | 35.4–88.1% | 87.5% | 65.5–96.4% | 90.9% | 77.4–97.0% |
| High-intensity aerobic exercise$ | 49.8% | 22.1–81.6% | 36.6% | 17.8–68.0% | 41.4% | 21.4–77.4% | 63.3% | 29.4–86.1% |
| Muscle strength exercise$ | 83.9% | 65.2–93.7% | 63.3% | 28.4–82.2% | 87.5% | 69.2–96.1% | 83.9% | 73.3–93.8% |
| Exercise advice # | 61.1% | 33.3–79.1% | 33.3% | 8.3–58.3% | 47.2% | 22.2–70.8% | 66.7% | 55.6–88.9% |
* Compliance was defined as following the prescribed exercise protocol
$ Numbers were computed as the (number of sessions complied with)/(number of sessions attended)
# Numbers were computed as the (number of weeks complied with)/(number of weeks of exercise program)
Compliance with the exercise advice was 61% (33% to 79%) and seven patients (9%) fully complied (i.e., completed more than 30 minutes of moderate-to-high intensity physical activity on at least five days a week for 18 consecutive weeks). Compliance with the exercise advice was lower in patients who attended fewer than 20 supervised exercise sessions (33% (8% to 58%)) compared with those attending 30 or more sessions (67% (56% to 89%)) (Table 2).
Predictors of attendance
All univariable results are reported in S2 Table.
The multivariable model retrieved four predictors for attendance: higher educational level predicted a higher attendance (β = 8.24 (95% CI: 0.93, 15.54)); however, receiving radiotherapy in addition to chemotherapy (β = -7.08 (95% CI: -14.91, 0.74), having a high BMI (β = -1.36 (95% CI: -2.21, -0.52)) and high physical fatigue level (β = -0.61 (95% CI: -1.45, 0.24)) predicted low attendance.
Predictors of compliance
In the multivariable analysis, a higher peak oxygen consumption (β = 5.67 (95% CI: -0.27, 11.61)) predicted a higher compliance with the duration of the aerobic exercises, whereas patients with higher self-efficacy beliefs about attending 30 sessions or more (β = -0.89 (95% CI: -1.99, 0.21)), a higher BMI (β = -0.44 (95% CI: -0.89, 0.01)) and higher physical fatigue (β = -0.34 (95% CI: -0.81, 0.12)) showed lower compliance with the duration of aerobic exercises (Table 3).
Table 3. Multivariable linear regression analyses on the association between selected predictors and attendance and compliance.
| Attendance | Compliance with duration of aerobic exercise | Compliance with high-intensity aerobic exercise | Compliance with muscle strength exercise | Compliance with exercise advice | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total group (n = 92) | Total group (n = 92) | Total group (n = 92) | Total group (n = 92) | Total group (n = 82) | ||||||
| Predictor | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P |
| Demographical | ||||||||||
| Age (years) | # | # | - | - | -0.22 (-0.47, 0.02) | 0.08 | # | # | # | # |
| Educational status | ||||||||||
| high vs. low and medium | 8.24 (0.93, 15.54) | 0.03 | - | - | 2.93 (-0.85, 6.70) | 0.13 | - | - | # | # |
| Tumor and treatment | ||||||||||
| Radiotherapy | -7.08 (-14.91, 0.74) | 0.08 | # | # | -5.32 (-9.36, -1.28) | 0.01 | # | # | # | # |
| Tumor receptor status | ||||||||||
| Her2+ and ER or PR+ vs. Triple negative | # | # | # | # | # | # | 6.63 (-0.44, 13.70) | 0.07 | # | # |
| Her2- and ER or PR+ vs. Triple negative | # | # | # | # | # | # | # | # | 1.55 (-0.47; 3.57) | 0.13 |
| Theory of planned behavior | ||||||||||
| Beliefs about physical activity for ≥5 days per week | ||||||||||
| Intention | NA | NA | NA | NA | NA | NA | NA | NA | 0.80 (0.00, 1.60) | 0.05 |
| Subjective norm | NA | NA | NA | NA | NA | NA | NA | NA | 0.59 (0.00, 1.18) | 0.05 |
| Perceived behavioral control | NA | NA | NA | NA | NA | NA | NA | NA | - | - |
| Psychological | ||||||||||
| Anxiety | # | # | - | - | # | # | - | - | # | # |
| Depression | # | # | - | - | # | # | - | - | # | # |
| Health-related quality of life | - | - | - | - | - | - | - | - | # | # |
| Beliefs about self-efficacy | ||||||||||
| Attending 30 sessions | # | # | -0.89 (-1.99, 0.21) | 0.11 | # | # | # | # | - | - |
| Physical | ||||||||||
| BMI (kg/m2) | -1.36 (-2.21, -0.52) | 0.002 | -0.44 (-0.89, 0.01) | 0.06 | - | - | -0.43 (-0.88, 0.03) | 0.07 | # | # |
| Baseline physical activity | - | - | # | # | 0.00 (0.00;0.01) | 0.11 | 0.00 (0.00,0.01) | 0.09 | 0.00 (0.00;0.01) | 0.05 |
| Baseline physical fatigue | -0.61 (-1.45, 0.24) | 0.156 | -0.34 (-0.81, 0.12) | 0.15 | -0.47 (0.90;-0.04) | 0.03 | - | - | # | # |
| Peak O2 consumption (L/min) | # | # | 5.67 (-0.27, 11.61) | 0.06 | - | - | - | - | 3.65 (0.49, 6.80) | 0.02 |
| R square | 0.20 | 0.16 | 0.20 | 0.09 | 0.24 | |||||
Estimated unstandardized regression coefficients with 95% Confidence intervals are mentioned in this table. # Not significant in univariate analysis.—Significant in univariate analysis, however not significant in multivariable analysis. NA: Not applicable
For compliance with the prescribed high intensity part of the aerobic exercises, a higher educational level (β = 2.93 (95% CI: -0.85, 6.70)) and higher baseline physical activity level (β = 0.00(95%CI: 0.00, 0.01)) resulted in higher compliance. Older patients (β = -0.22 (95% CI: 0.47, 0.02), those reporting higher levels of physical fatigue (β = -0.47 (95% CI: -0.90, -0.04)) as well as patients who received radiotherapy in addition to chemotherapy (β = -5.32 (95% CI: -9.36, -1.28)), had a lower compliance.
Higher compliance with the muscle strength exercises was predicted by having a Her2+ and ER or PR+ tumor receptor status versus a triple negative receptor status (β = 6.63 (95% CI: -0.44, 13.70)), a lower BMI (β = -0.43 (95% CI: -0.88, 0.03)) and baseline physical activity level (β = 0.00 (95% CI: 0.00,0.01).
Having a Her2- and ER or PR+ tumor receptor status versus a triple negative receptor status (β = 1.55 (95% CI: -0.47, 3.57), a higher peak oxygen consumption (β = 3.65 (95% CI: 0.49, 6.80)), a higher intention (β = 0.80 (95% CI: 0.00, 1.60)), higher baseline physical activity level (β = 0.00 (95% CI: 0.00, 0.01)) and a higher subjective norm to be physically active for at least five days per week (β = 0.59 (95% CI: 0.00, 1.18)) predicted a higher compliance with the exercise advice.
Discussion
This explorative study shows high adherence rates to an 18-week aerobic and muscle strength exercise program in primary breast cancer patients during treatment, both in terms of attendance rates and compliance rates, with the exception of compliance to the high intensity exercises. The attendance rate (83%) was higher than in previous trials investigating the use of exercise programs in breast cancer patients undergoing chemotherapy, whose attendance rates of twice weekly supervised sessions ranged from 71–79% [15–17]. This might be explained by the incorporation of cognitive behavioral aspects into our exercise program or differences in patient characteristics, such as baseline activity levels, which were high in the PACT population. In addition, the intensity of supervision might have differed. In the PACT study, patients either trained in small groups or alone, and the physiotherapists explicitly encouraged the patients to attend the sessions even though they were not feeling well. Note, that physiotherapists are exercise specialists and internationally other allied health trained exercise professionals can adopt and use this intervention.
If adjustments were necessary in this study, they were made in the high-intensity period of the aerobic supervised exercises, resulting in lower compliance. In this study, the intensity interval training consisted for 2 intervals of 2 to 7 minutes. This high intensity training is longer than other high intensity interval training protocols used in similar trials [40]. It might be that patients would experience the shorter work-rest ratio’s more tolerable and enjoyable which might explain the lower compliance with the high intensity period of the aerobic supervised exercises in our study.
The goal of exercise program was to follow the protocol as precisely as possible. If adjustments were necessary because of wellbeing of the patients, we considered this as a lower compliance. We believe that this is sometimes medically or ethically appropriate, since the patients underwent intensive treatment with chemotherapy. However, we wanted to know if this original protocol was doable for the patients. We would have found higher compliance rates if we had counted adjustments because of medical or ethical reasons as ‘compliant’. In future studies, it might be interesting to investigate whether autoregulation, i.e. allow patients to do less when they feel unwell and do more if they feel well, improves compliance rates.
Factors contributing to higher adherence
We found in this additional explanatory analysis several predictors for both attendance and compliance in breast cancer patients. In this study, a higher educational level, not receiving radiotherapy, a lower BMI and less physical fatigue predicted higher attendance. Other studies in breast cancer patients undergoing treatment found various other predictors of adherence to different exercise prescriptions; a higher peak oxygen uptake, fewer endocrine symptoms, lower durations of exercise, fewer exercise limitations, shorter chemotherapy protocols and exercise facility location all contributed to higher levels of attendance [12, 13]. The authors concluded that predictors of attendance in breast cancer patients are diverse and may differ as a result of the particular exercise prescriptions [12, 13], which also explains the differences between these findings and those of the present study. In addition, the set of possible predictors were not identical across all studies.
To the best of our knowledge, there have been no reports on the predictors of compliance with a supervised exercise program during breast cancer treatment. In our study, physical fatigue predicted a lower compliance with the duration of aerobic exercise and with the high-intensity periods of aerobic exercise, but not with the muscle strength exercises. It might be possible that the physiotherapists first adjusted the aerobic exercises, implying the patients were probably still able to perform the muscle strength exercises despite being fatigued. We did not ask specific reasons for the choice of adjusting the intensity, but our data from the exercise logs suggest that, in general, adjustments were made mainly due to the side effects of treatment such as nausea, fatigue and dizziness.
Patients had a higher compliance with the duration of the aerobic exercises when the peak oxygen consumption was higher. These patients had probably a more physically active lifestyle and were therefore more used to exercise and able to continue the aerobic exercises.
In this study, a remarkable finding was that compliance with the duration of aerobic exercises was lower in patients who reported high self-efficacy beliefs about attending 30 sessions or more at the baseline. It might be that these patients overestimated their own capabilities about physical exercise during cancer treatments; however, this did not affect their compliance with any of the other aspects of the exercise program. On the other hand, our question did not address the patients’ belief of whether they will be able to follow the specific exercises but about their self-efficacy beliefs about attending the sessions.
Patients receiving radiotherapy were less compliant with the high-intensity aerobic exercise. One speculative explanation might be the burden of daily traveling to the hospital for the radiotherapy in combination with attending the PACT exercise sessions, which were not generally held at the same location.
Huang et al. investigated predictors for compliance with both the intensity and duration of a home-based walking program in breast cancer patients [18]. Patients who were less fatigued, perceived a higher importance of exercise, had an early stage of disease and were employed had a higher compliance with exercise intensity. Our study showed that patients who were more physically fit and had a higher intention and higher subjective norm towards being physically active for 30 minutes at least five days per week were more compliant with the exercise advice (i.e., the home-based aspects of the program). In this study, it seems that motivational factors play a more important role in the home-based exercise program than for the supervised exercise program, however this is unclear in previous research [19]. The patient’s beliefs about being physically active in a home-based setting during cancer treatment might be more influenced by the social support within the patient’s environment, such as from a partner, compared with the supervised exercise setting. On the other hand, marital status was not found as a predictor for compliance with the exercise advice. Since we only included marital status in our analysis and no other possible social support from family, friends or carers, this should be included in future trials. Future studies should include more extended information on social support. During supervised exercise, the physiotherapist or other allied health trained exercise professionals and other patients may influence the patient’s motivation; therefore, the patient’s own motivational factors and social context may have been less important [41].
Strengths and limitations
The strengths of the present study are the detailed registration of the different components of compliance and the availability of information on several predictors, including demographical, tumor-, and treatment-related factors, constructs of the theory of planned behavior, psychological and physical factors. The PACT study was a pragmatic trial that resembles daily practice, which increases the generalizability of results. On the other hand, although we cannot exclude overreporting in the questionnaires, patients who participated in this study reported rather high average physical activity levels at the baseline. This probably influenced the generalizability of the predictors to the total population. Furthermore, information about compliance with the exercise advice was extracted from self-reported activity diaries rather than using objective measures and only 80% of the original 102 patients returned their diaries. An overestimation of the performed activities might be reported in the activity diaries, although we explicitly instructed the patients at baseline to fill in activities such as walking or cycling only when they had difficulties talking at the same time. For the supervised sessions, it was not registered whether the high-intensity intervals were adjusted at request of the patient or the physiotherapist. Our analyses should be interpreted as exploratory, since the study was not primarily powered for the current study; consequently, additional predictors might have been missed. In other studies, exercise facility location [20], fewer endocrine symptoms [13], lower durations of exercise [13], fewer exercise limitations [13], shorter chemotherapy protocol [13], exercise history [19], being employed[18, 21], high income [21], early stage disease [18] and being Caucasian [22] were found as predictors for adherence, while these predictors were not assessed in this study. In univariate analyses, several predictors were found to be significant but were not supported in the multivariable analysis, which might have been the result of multicollinearity or restricted statistical power. These unmeasured predictors might also influence attendance and compliance.
Conclusions and clinical implications
The high attendance and compliance rates reported in this study suggest that both supervised aerobic and resistance exercise and exercise advice are feasible for localized breast cancer patients undergoing adjuvant treatment. We observed that high-intensity aerobic exercises were adjusted for a significant number of patients, in preference to making changes to the duration or the strength exercises. Particularly for older patients, patients who were more fatigued at the baseline, patients having a lower educational level or receiving radiotherapy led to difficulties in complying with the high-intensity aerobic exercises. Future studies should consider shorter intervals of high intensity aerobic exercises which might increase compliance rates. Beliefs about exercise behaviors are important for compliance with the exercise advice. The predictors we have identified should be taken into account when designing future exercise programs or adapting present programs for breast cancer patients, to increase compliance. Choosing training locations in the immediate vicinity of the patient’s home and the availability of social support from, for example, a partner or general practitioner, might be additional factors that could increase adherence.
Supporting information
*High intensity: at or above ventilator threshold. **Low intensity: under ventilatory threshold.
(DOCX)
Bold is significant (p<0.2); Estimated unstandardized regression coefficients with 95% Confidence intervals are mentioned in this table. NA: not applicable.
(DOCX)
(PDF)
(DOC)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by The Netherlands Organisation for Health Research and Development (ZonMw, project number: 171002202, https://www.zonmw.nl/nl/onderzoek-resultaten/doelmatigheidsonderzoek/programmas/project-detail/doelmatigheidsonderzoek/physical-activity-during-cancer-treatment-pact-studie-een-gerandomiseerde-studie-naar-de-effecten/, PHMP), the Dutch Cancer Society (KWF Kankerbestrijding. Project number: UU 2009-4473, PHMP), the Dutch Pink Ribbon Foundation (2011.WO02.C100, https://www.pinkribbon.nl/doelbestedingen/wetenschappelijk-onderzoek/2011/pact-studie.html; AMM) and VIOZ (Stichting Vrienden Integrale Oncologische Zorg (2015; MJV). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Travier N, Velthuis MJ, Steins Bisschop CN, van den Buijs B, Monninkhof EM, Backx F, et al. Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med 2015;13:121 10.1186/s12916-015-0362-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ 2012;344:e70 10.1136/bmj.e70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc 2013. November;45(11):2080–90. 10.1249/MSS.0b013e31829a3b63 [DOI] [PubMed] [Google Scholar]
- 4.Ferrer RA, Huedo-Medina TB, Johnson BT, Ryan S, Pescatello LS. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med 2011. February;41(1):32–47. 10.1007/s12160-010-9225-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramp F, James A, Lambert J. The effects of resistance training on quality of life in cancer: a systematic literature review and meta-analysis. Support Care Cancer 2010. November;18(11):1367–76. 10.1007/s00520-010-0904-z [DOI] [PubMed] [Google Scholar]
- 6.Puetz TW, Herring MP. Differential effects of exercise on cancer-related fatigue during and following treatment: a meta-analysis. Am J Prev Med 2012. August;43(2):e1–24. 10.1016/j.amepre.2012.04.027 [DOI] [PubMed] [Google Scholar]
- 7.Mishra SI, Scherer RW, Snyder C, Geigle P, Gotay C. The effectiveness of exercise interventions for improving health-related quality of life from diagnosis through active cancer treatment. Oncol Nurs Forum 2015. January;42(1):E33–E53. 10.1188/15.ONF.E33-E53 [DOI] [PubMed] [Google Scholar]
- 8.De Geest S., Sabate E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs 2003. December;2(4):323 10.1016/S1474-5151(03)00091-4 [DOI] [PubMed] [Google Scholar]
- 9.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Vallance JK, et al. A longitudinal study of exercise barriers in colorectal cancer survivors participating in a randomized controlled trial. Ann Behav Med 2005. April;29(2):147–53. 10.1207/s15324796abm2902_9 [DOI] [PubMed] [Google Scholar]
- 10.Courneya KS, McKenzie DC, Reid RD, Mackey JR, Gelmon K, Friedenreich CM, et al. Barriers to supervised exercise training in a randomized controlled trial of breast cancer patients receiving chemotherapy. Ann Behav Med 2008. February;35(1):116–22. 10.1007/s12160-007-9009-4 [DOI] [PubMed] [Google Scholar]
- 11.Neil-Sztramko SE, Winters-Stone KM, Bland KA, Campbell KL. Updated systematic review of exercise studies in breast cancer survivors: attention to the principles of exercise training. Br J Sports Med 2017. November 21;bjsports-098389. [DOI] [PubMed] [Google Scholar]
- 12.Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, Friedenreich CM, et al. Predictors of supervised exercise adherence during breast cancer chemotherapy. Med Sci Sports Exerc 2008. June;40(6):1180–7. 10.1249/MSS.0b013e318168da45 [DOI] [PubMed] [Google Scholar]
- 13.Courneya KS, Segal RJ, Gelmon K, Mackey JR, Friedenreich CM, Yasui Y, et al. Predictors of adherence to different types and doses of supervised exercise during breast cancer chemotherapy. Int J Behav Nutr Phys Act 2014;11:85 10.1186/s12966-014-0085-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol 2001. February 1;19(3):657–65. 10.1200/JCO.2001.19.3.657 [DOI] [PubMed] [Google Scholar]
- 15.Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. Int J Cancer 2015. July 15;137(2):471–80. 10.1002/ijc.29383 [DOI] [PubMed] [Google Scholar]
- 16.Steindorf K, Schmidt ME, Klassen O, Ulrich CM, Oelmann J, Habermann N, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol 2014. November;25(11):2237–43. 10.1093/annonc/mdu374 [DOI] [PubMed] [Google Scholar]
- 17.van Waart H., Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J Clin Oncol 2015. June 10;33(17):1918–27. 10.1200/JCO.2014.59.1081 [DOI] [PubMed] [Google Scholar]
- 18.Huang HP, Wen FH, Tsai JC, Lin YC, Shun SC, Chang HK, et al. Adherence to prescribed exercise time and intensity declines as the exercise program proceeds: findings from women under treatment for breast cancer. Support Care Cancer 2015. July;23(7):2061–71. 10.1007/s00520-014-2567-7 [DOI] [PubMed] [Google Scholar]
- 19.Kampshoff CS, Jansen F, van MW, May AM, Brug J, Chinapaw MJ, et al. Determinants of exercise adherence and maintenance among cancer survivors: a systematic review. Int J Behav Nutr Phys Act 2014;11:80 10.1186/1479-5868-11-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME. Predictors of adherence to exercise interventions during and after cancer treatment: A systematic review. Psychooncology 2018. March;27(3):713–24. 10.1002/pon.4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bland KA, Neil-Sztramko SE, Kirkham AA, Bonsignore A, Van Patten CL, McKenzie DC, et al. Predictors of attendance to an oncologist-referred exercise program for women with breast cancer. Support Care Cancer 2018. September;26(9):3297–306. 10.1007/s00520-018-4180-7 [DOI] [PubMed] [Google Scholar]
- 22.Nyrop KA, Deal AM, Choi SK, Wagoner CW, Lee JT, Wood A, et al. Measuring and understanding adherence in a home-based exercise intervention during chemotherapy for early breast cancer. Breast Cancer Res Treat 2018. February;168(1):43–55. 10.1007/s10549-017-4565-1 [DOI] [PubMed] [Google Scholar]
- 23.Kampshoff CS, van MW, Schep G, Nijziel MR, Witlox L, Bosman L, et al. Participation in and adherence to physical exercise after completion of primary cancer treatment. Int J Behav Nutr Phys Act 2016. September 9;13(1):100–0425. 10.1186/s12966-016-0425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process 1991;50:179–211. [Google Scholar]
- 25.Velthuis MJ, May AM, Koppejan-Rensenbrink RA, Gijsen BC, van BE, de Wit GA, et al. Physical Activity during Cancer Treatment (PACT) Study: design of a randomised clinical trial. BMC Cancer 2010;10:272 10.1186/1471-2407-10-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Vulpen JK, Velthuis MJ, Steins Bisschop CN, Travier N, van den Buijs B, Backx F, et al. Effects of an exercise program in colon cancer patients undergoing chemotherapy. Med Sci Sports Exerc 2015;accepted for publication. [DOI] [PubMed] [Google Scholar]
- 27.Kemper H, Ooijendijk W, Stiggelbout M. Consensus over de Nederlandse norm voor gezond bewegen. Tijdschrift voor gezondheidswetenschappen 2000;(3):180–3. [Google Scholar]
- 28.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory / Albert Bandura. 1986. [Google Scholar]
- 29.Kraemer W J. RNAFACFDN. Strength training: development and evaluation of methodology. Physiological assessment of human fitness 2006. [Google Scholar]
- 30.Dohoney P., Chrmoniak JA, Lemire D, Abadie B.R., Kovacs C. Prediction of one repetition maximum (1-RM) strength from a 4–6 RM and a 7–10 RM sumaximal strength test in healthy young adult males. Official Journal of the American Society of Exercise Physiologists 2002;5(3):54–9. [Google Scholar]
- 31.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr., Tudor-Locke C, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 2011. August;43(8):1575–81. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 32.Ainsworth BE, Haskell WL, Herrmann SD. Compendium of Physical Activities. Phys Act Track Guid Heal Lifestyles Res Center, Coll Nurs Heal Innov Arizona State Univ 2011;17. [Google Scholar]
- 33.Courneya KS, Friedenreich CM. Utility of the theory of planned behavior for understanding exercise during breast cancer treatment. Psychooncology 1999. March;8(2):112–22. [DOI] [PubMed] [Google Scholar]
- 34.Jones LW, Guill B, Keir ST, Carter K, Friedman HS, Bigner DD, et al. Using the theory of planned behavior to understand the determinants of exercise intention in patients diagnosed with primary brain cancer. Psychooncology 2007. March;16(3):232–40. 10.1002/pon.1077 [DOI] [PubMed] [Google Scholar]
- 35.Osborne RH, Elsworth GR, Sprangers MA, Oort FJ, Hopper JL. The value of the Hospital Anxiety and Depression Scale (HADS) for comparing women with early onset breast cancer with population-based reference women. Qual Life Res 2004. February;13(1):191–206. 10.1023/B:QURE.0000015292.56268.e7 [DOI] [PubMed] [Google Scholar]
- 36.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993. March 3;85(5):365–76. [DOI] [PubMed] [Google Scholar]
- 37.Wendel-Vos GC, Schuit AJ, Saris WH, Kromhout D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol 2003. December;56(12):1163–9. [DOI] [PubMed] [Google Scholar]
- 38.Smets EM, Garssen B, Bonke B, de Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 1995. April;39(3):315–25. [DOI] [PubMed] [Google Scholar]
- 39.Royston P, Moons KG, Altman DG, Vergouwe Y. Prognosis and prognostic research: Developing a prognostic model. BMJ 2009;338:b604 10.1136/bmj.b604 [DOI] [PubMed] [Google Scholar]
- 40.Newton RU, Kenfield SA, Hart NH, Chan JM, Courneya KS, Catto J, et al. Intense Exercise for Survival among Men with Metastatic Castrate-Resistant Prostate Cancer (INTERVAL-GAP4): a multicentre, randomised, controlled phase III study protocol. BMJ Open 2018. May 14;8(5):e022899 10.1136/bmjopen-2018-022899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bassett S F. The assessment of patient adherence to physiotherapy rehabilitation. New Zealand Journal of Physiotherapy 2003;31((2)):60–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
*High intensity: at or above ventilator threshold. **Low intensity: under ventilatory threshold.
(DOCX)
Bold is significant (p<0.2); Estimated unstandardized regression coefficients with 95% Confidence intervals are mentioned in this table. NA: not applicable.
(DOCX)
(PDF)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.