Abstract
The precipitation of mucin using high concentrations of ethanol has been used by many researchers while others have questioned the validity of the technique. In this study, analysis of an ethanol precipitate, from the soluble fraction of ileal digesta from pigs was undertaken using molecular weight profiling and polyacrylamide gel electrophoresis. The precipitate contained 201 mg·g-1 protein, 87% of which had a molecular weight >20 KDa. Polyacrylamide gel electrophoresis stained with Coomassie blue and periodic acid/Schiff, revealed that most glycoprotein had a molecular weight between 37–100 KDa. The molecular weight of glycoprotein in the precipitate was therefore lower than that of intact mucin. These observations indicated that the glycoprotein in the ethanol precipitate was significantly degraded. The large amount of protein and carbohydrate in the supernatant from ethanol precipitation indicated that the precipitation of glycoprotein was incomplete. As a method for determining the concentration of mucin in digesta, ethanol precipitation is unreliable.
The epithelial tissues of the gastrointestinal tract are subject to degradative challenges from extremes in pH, enzymatic hydrolysis, attack from pathogenic bacteria and physical erosion1,2. The gastrointestinal mucosa is protected by layers of secreted or membrane-bound mucus formed from polymeric glycoproteins, mucins, secreted by specialist cells of the underlying mucosa. Secreted mucins are high molecular mass, (2 × 106 Da3,) polydisperse molecules that contain a central polypeptide core of 1500 to 4500 amino acids and 100–200 covalently bound oligosaccharide side chains containing 1 to 20 or more monosaccharides. Likened to the structure of a bottlebrush the oligosaccharides, arranged around the protein core make up 50 to 80% of the mucin's mass. The carbohydrate side chains are bound to the polypeptide by O-glycosidic linkages between N-acetylgalactosamine and the hydroxylated amino acids serine and threonine4. The glycosylated regions of the polypeptide are rich in serine, threonine and proline whilst the non-glycosylated regions are rich in cysteine which allows the formation of disulphide bridges between glycoprotein polymeric sub-units to form a cohesive viscoelastic gel across the surface of the gastrointestinal tract5.
All along the gastrointestinal tract a dynamic equilibrium exists between the synthesis of mucins and their degradation by proteolysis and physical erosion. As mucins are somewhat resistant to mammalian digestive processes they represent a significant fraction of endogenous losses at the terminal ileum. In our own work6 the mucin content of digesta, collected from the terminal ileum of pigs fed a casein-based diet, equalled nearly 16% of the overall dry matter and mucins were the single most abundant, truly endogenous component secreted into the gastrointestinal tract. As mucin is so important in the protection of the gastrointestinal mucosa any dietary or physiological factor that affects the amount of mucin secreted into the digestive tract will have important implications on both metabolism and the integrity of the defensive mechanism.
There are few studies that detail the quantification of gastrointestinal mucin because it is particularly difficult to assay7. In a previous study8 a number of methods to determine the concentration of mucin in ileal digesta were evaluated, including the gravimetric method of precipitating mucins using high concentrations of ethanol and the determination of mucin based on the markers N-acetylgalactosamine (GalNAc) and N-acetylglucosamine (GluNAc)7. In our study, values obtained by ethanol precipitation were on average 43% lower than for the hexosamine assay, whilst Piel et al.7 found the difference was as much as 33%.
The precipitation of glycoproteins using high concentrations of ethanol is considered by many researchers to be non-specific, as Leterme et al.9 concede, because the precipitate does not only contain raw mucus but may be contaminated by non-covalently bound proteins. This is corroborated by considering the various compounds ethanol has been used to precipitate (e.g., α-galactosidase from a fungus10, human-immunodeficiency-virus-inhibitory glycoprotein from aqueous extracts of a Caribbean sponge11, gonadotropins, follicle-stimulating hormone and luteinising hormone from halibut pituitary gland12). Although the validity of this assay for the determination of mucin in human gastric juices has been accepted by some workers (e.g., Azuumi et al.13), its effectiveness has been questioned by others7,11.
The results from our previous study8 and that of others7 call into question the reliability of the ethanol precipitation method for the quantification of mucin in ileal digesta. The aim of this study was to investigate ethanol precipitates from ileal digesta as the source of mucins to evaluate the ethanol precipitation of digesta as a method for the quantification of mucin.
Results
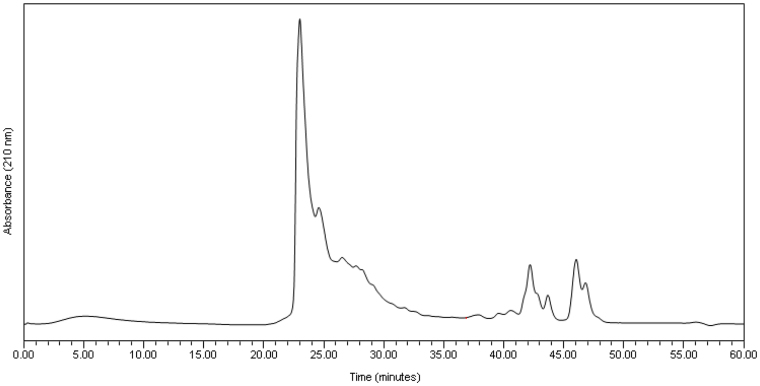
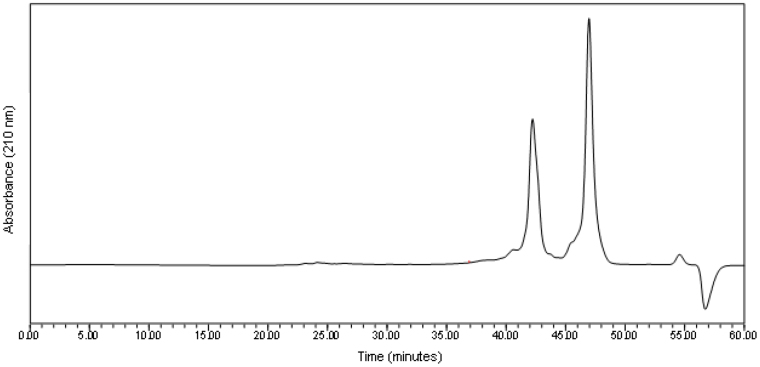
The average mass of the ethanol precipitate was 69.7 mg·g−1 digesta dry matter ([DDM] coefficient of variation = 19.1%; n = 6) The molecular weight profile (Figure 1, Table 2) of the residue from the ethanol precipitation showed that 87% of the material consisted of protein molecules with a molecular weight greater than 20 KDa, with a further 10% of the material consisting of molecules with a molecular weight less than 1 KDa. In comparison, the supernatant contained predominantly protein molecules with a molecular weight less than 1 KDa, (Figure 2, Table 2). Only a small amount of material in the supernatant (4%) had a molecular weight greater than 20 KDa.
Figure 1. Molecular weight profile (absorbance versus time) of the residue after ethanol precipitation of the 14,500 RCF digesta supernatant.
Table 1. Composition of the experimental diet (g/kg air dry weight).
| Ingredient | Experimental Diet 1. |
|---|---|
| Maltodextrin | 453 |
| Sucrose | 161 |
| Soyabean oil | 154 |
| Sodium Hydrogen Carbonate | 18 |
| Titanium Dioxide | 3 |
| Lactic casein | 211 |
1No vitamins, minerals or fibre were added to this diet as the same diet was used in an acute feeding study with human subjects.
Table 2. Molecular weight distribution within the residue and supernatant following ethanol precipitation of the 14,500 RCF digesta supernatant.
| Ethanol-precipitation fraction | |||
|---|---|---|---|
| Molecular weight grouping | Molecular weight (KDa) | Residue 1. | Supernatant 1. |
| 1 | > 20 | 87.2 | 4.0 |
| 2 | 5–20 | 2.9 | 0.1 |
| 3 | 1–5 | 0.01 | 0.1 |
| 4 | < 1 | 9.9 | 95.8 |
1Recorded as a percentage of total mass within each fraction.
Figure 2. Molecular weight profile (absorbance versus time) of the supernatant after ethanol precipitation of the 14,500 RCF digesta supernatant.
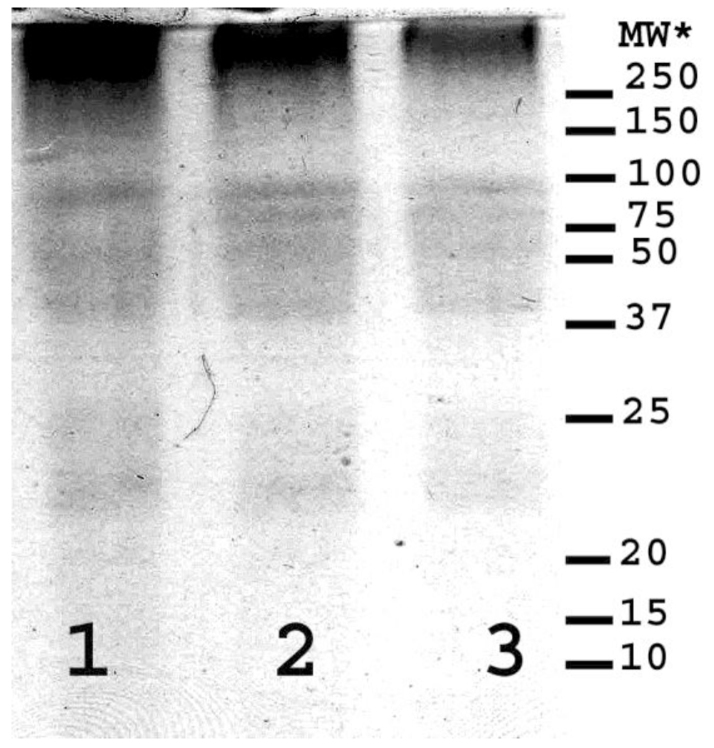
SDS-PAGE analysis with Coomassie staining (Figure 3) revealed banding that indicated that the residue contained proteins with molecular weights ranging from less than 10 to just over one hundred KDa and confirmed what was observed with the molecular weight profiling. Bands more heavily stained than others were seen at <10,12, 20, 31, 37, 69, and 120 KDa (Table 3). When the SDS-PAGE analysis was repeated, using the same conditions but using a periodic acid/Schiff stain (Figure 4) for sugars that are oxidizable by periodate, densely stained bands were observed equivalent to 23, 40, 63, 80 and 100 KDa (Table 3). However, there was diffuse staining in two separate bands between 21 and 28 and between 37 and100 KDa which also matched areas of diffuse staining on the Coomassie blue plate.
Figure 3. Polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the residue from ethanol precipitation of the 14,500 RCF digesta supernatant using Coomassie Blue staining for protein.
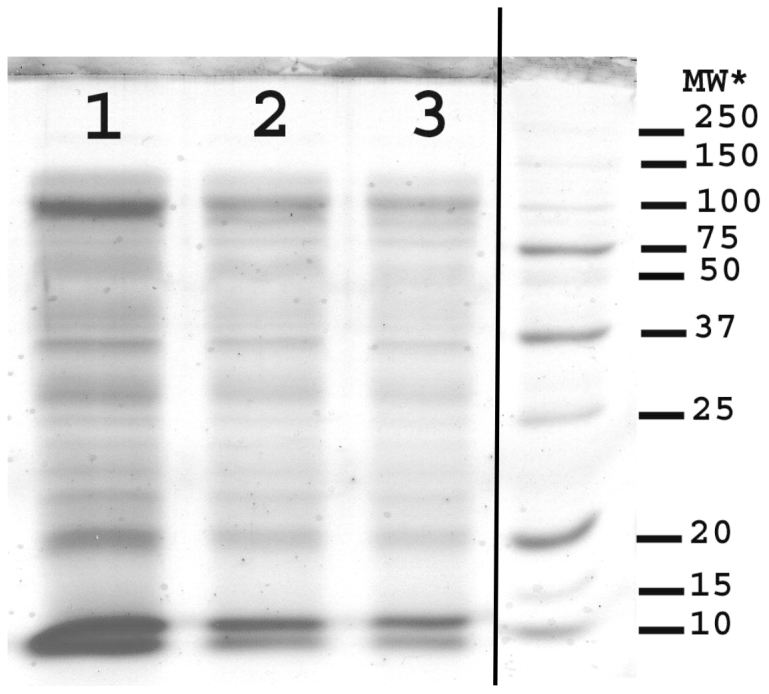
* MW = Molecular weight (kDa) 1, 2 and 3 represent the volume of solubilised ethanol precipitate applied to the electrophoresis gel. 1 = 5 μL, 2 = 2.5 μL and 3 = 1 μL run against the marker in the right hand column.
Table 3. The molecular weights of observed bands within the polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the residue from ethanol precipitation of the 14,500 RCF digesta supernatant using Coomassie Blue staining for protein and periodic acid Schiff staining for sugar.
| Band | Molecular weight (KDa) | |
|---|---|---|
| Coomassie Blue | Periodic Acid Schiff | |
| 1 | <10* | 2123*a |
| 2 | 12* | |
| 3 | 20* | 28 |
| 4 | 22 | 3740*63*80*b |
| 5 | 23 | |
| 6 | 24 | |
| 7 | 27 | |
| 8 | 31* | 100* |
| 9 | 37* | |
| 10 | 47 | |
| 11 | 69* | |
| 12 | 80 | |
| 13 | 88 | |
| 14 | 120* | |
*Significantly dense stained bands.
a & b areas with diffuse bands stained by periodic acid Schiff.</tfootnote3a>
Figure 4. Polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the residue from ethanol precipitation of the 14,500 RCF digesta supernatant using periodic acid Schiff staining for carbohydrate.
* MW = Molecular weight (kDa) 1, 2 and 3 represent the volume of solubilised ethanol precipitate applied to the electrophoresis gel. 1 = 5 μL, 2 = 2.5 μL and 3 = 1 μL.
In an earlier study8 it was determined that terminal ileal digesta from pigs contains a mixture of gastric and small intestinal mucins in a ratio of nearly 2:1. Using this ratio, and data published by Allen et al.14 on the composition of both porcine gastric and small intestinal glycoproteins, the expected composition of the ethanol precipitate was calculated (Table 4). The ester sulphate content of the ethanol precipitate was not determined during the present study. This expected composition was then compared with the determined composition of the ethanol precipitate.
Table 4. Composition of pure pig gastric and small intestinal glycoprotein together with the expected composition of glycoprotein in the ethanol precipitate (of the 14,500 RCF digesta supernatant fraction) found in the present study.
| Dry weight composition (%)1 | Expected composition of ethanol precipitate (%)2 | |||
|---|---|---|---|---|
| Constituent | Gastric | Small intestinal | Dry | Wet |
| Carbohydrate | 82.9 | 77.5 | 81.6 | 71.8 |
| Protein | 13.2 | 19.6 | 15.3 | 13.5 |
| Ester sulphate | 3.2 | 2.8 | 3.1 | 2.7 |
| Water | – | – | – | 123 |
| Total | 99.3 | 99.9 | 100 | 100 |
1Taken from Allen et al., (20).
2Calculated using the estimated ratio of gastric and small intestinal mucin (2.05:1) present in the digesta from data from a previous study (Miner-Williams et al., 2008).
3Calculated using data from (38;39).
The determined chemical composition of the ethanol precipitate is given in Table 5. If the ester sulphate content of porcine gastric and small intestinal mucin is assumed to be 2.7%14 (Table 4), then only 1.4% of the dry matter remained unaccounted for. During a similar analysis of the supernatant the carbohydrate and protein concentrations were estimated to be 25.6 and 14.1 mg·g−1 DDM respectively. When the mass of the precipitate (69.7 mg·g−1 DDM) was compared with the cumulative mass of the components determined individually (69.2 mg·g−1 DDM) only a small (0.7%) deficit was observed. However when the concentration of carbohydrate in the ethanol supernatant is taken as the difference between that in the ethanol precipitate (65% of its mass, Table 5) and the total concentration of carbohydrate in the original 14,500 RCF supernatant, a concentration of 21.1 mg·g−1 DDM should be present, 6.7% less than what was actually found.
Table 5. Composition of the ethanol precipitate (from the 14,500 RCF digesta supernatant fraction).
| Constituent | mg·g−1 DDM | SEM | % of total |
|---|---|---|---|
| Carbohydrate | 45.6 | 1.1 | 65.0 |
| Protein | 13.9 | 0.83 | 20.1 |
| Ester sulphate1 | 1.91 | – | 2.7 |
| Water | 7.8 | 0.70 | 10.8 |
| Total | 69.2 | 98.6 |
1Estimate from Table 2.
The dry weight of the precipitate was determined to be 32.5% of the dry matter present in the 14,500 RCF digesta supernatant. The concentration of protein in the ethanol precipitate and supernatant were estimated to be 13.9 and 14.1 mg·g−1 DDM respectively. Less than 50% of the total protein and 64% of the total carbohydrate present in the 14,500 RCF digesta supernatant were precipitated by the ethanol.
Over 70% of the total sialic acid in the 14,500 RCF supernatant was free.
Discussion
Pig gastric mucin is thought to contain an average of four glycoprotein subunits, of 5 × 105 Da, joined together by covalent disulphide bridges, whereas pig small intestine has an average of eight subunits each with a molecular weight of 2 × 105 Da. During proteolysis the glycoprotein sub-units separate and some degradation of the non-glycosylated regions of the glycoprotein core occurs. Although proteases may degrade polymeric mucins into smaller glycosylated units, any further breakdown of the protein core cannot occur until the carbohydrate side chains are removed. This occurs primarily in the colon, mediated by bacterial exoenzymes15.
Sialic acid residues usually occur as the terminal non-reducing residues16 of the oligosaccharide chains of the glycoconjugate and their electronegativity is known to significantly influence mucin rheology and degradation17,18. As the glycosidic linkages between sialic acids and the oligosaccharide chains are easily hydrolysed, the concentration of free sialic acid present in the digesta may be used as an estimate of mucin degradation17. With more than 70% of the sialic acid in the 14,500 RCF supernatant found to be unbound, this degree of desialylation represents significant mucin degradation and supports the SDS PAGE data that the glycoproteins detected in the ethanol precipitate were degraded mucin subunits of lower molecular weight.
Less than 50% of the total protein and 64% of the total carbohydrate present in the 14,500 RCF digesta supernatant were precipitated by the ethanol. Such ineffectual precipitation might explain why, in an earlier study comparing different methods of quantifying mucin in ileal digesta8, the ethanol precipitation method gave an estimated mucin concentration less than half of that determined using the mucin markers GalNAc and GluNAc.
The determined carbohydrate concentration of the ethanol precipitate (65%, Table 5), is 7% less than expected (72% estimated from the composition data of Allen et al.14, Table 4), and the protein concentration is nearly 7 percentage units higher than expected (20% determined in this study compared to 13.5% derived from the data of Allen et al.14). This together with the free sialic acid data indicate that the glycoprotein present in the ethanol precipitate, and the original digesta, had been degraded and that some sugar moieties had been stripped off the bottlebrush structure by both α- and β-glycosidases. Such degradation of the glycoprotein subunits would also explain the range of smaller glycoproteins observed in the SDS PAGE analysis where the bulk of the proteins, with associated sugar residues, had molecular weights ranging from between 37 and100 KDa.
Mucin in ileal effluent would be expected to contain predominantly a mixture of gastric and small intestinal mucins. It has been assumed that there is minimal digestion of mucin in the small intestine and that this largely takes place in the colon3,19. However the data from this study show that considerable degradation has occurred proximal to the terminal ileum. Mucin degradation by proteolysis and physical erosion is a multistep process initiated by the enzymatic proteolysis of the non-glycosylated regions of the mucin oligomers by host and microbial proteases: this process, together with the disruption of the intermolecular disulphide bridges, occurs continuously throughout the gastrointestinal tract20. As a result the chyme will contain fragments of the non-glycosylated apomucin and an accumulation of the heavily glycosylated STP domains (>500 KDa) now termed mucin glycopeptides or T-domains (T = trypsin) where the oligosaccharide side chains protect the polypeptide chain from further proteolytic attack4,20.
It is therefore anticipated that although the T-domains are conserved some sugar moieties from the termini of the oligosaccharide chains will have been degraded and only small remnants of the non-glycosylated apomucin remain in the ileal effluent. The data from this study largely corroborates these conclusions.
The precipitation of crude mucin using high concentrations of ethanol is not an easily reproducible assay as a coefficient of variation of 19% found in this study suggests. As reported previously8 the final ethanol concentration is critical and any deviation from the standard conditions can lead to spurious results. Ethanol precipitation is not specific to mucin10,11,12, and its effectiveness has been called into question by a number of researchers7,11. Moughan et al.21, using perchloric and trichloroacetic acids as protein precipitants and indeed Björling22 suggested that some highly glycosylated glycoproteins remain soluble even in high concentrations of ethanol. In the light of this evidence, the high concentration of carbohydrate and protein found in the ethanol supernatant in the present study may represent either non-precipitated mucin or other non-associated sugars and proteins found in the soluble fraction of digesta.
The ineffective precipitation of degraded glycoprotein subunits present in the digesta may explain why the ethanol precipitation procedure appears to underestimate digesta mucin concentration7,23. This procedure for the quantification of mucin is likely to lead to considerable error.
If accurate estimations are to be made, any estimation using mucin markers must involve those markers that are best conserved, even though mucin degradation has been demonstrated proximal to the ileocaecal junction. In our earlier study23 it was demonstrated that using the ileal concentrations of neutral sugars and sialic acid underestimated the concentration of mucin in ileal digesta. Data from this study confirms that such mucin markers are unreliable as mucin degradation lowers the concentration of many carbohydrate moieties and leads to the underestimation of mucin in the ileal effluent. However, as every oligosaccharide side chain begins with one GalNAc residue and that these residues are conserved more than any other, we suggest that using GalNAc as a mucin marker is justifiably more reliable.
Methods
Terminal ileal digesta samples were collected during the conduct of another study6, from 6 Large White × Duroc pigs of mean body weight 79 (± 4.8) kg, (± standard error of mean [SEM]) fitted with a post-valve T caecum cannula24. The pigs were fed a lactic casein-based diet mixed with water, (Table 1). Ethics approval for the trial was received from Massey University Animal Ethics Committee (protocol 05/29).
The fresh digesta were fractionated by differential centrifugation following the method of Metges et al.25 Samples were centrifuged first at 250 RCF for 15 minutes at 4°C to separate food particles and porcine cells, then at 14,500 RCF for 30 minutes at 4°C to separate microbial cells and porcine cellular detritus. It was assumed that the mucins, which are soluble, were quantitatively recovered in the supernatant, together with other soluble materials including proteins, peptides, free amino acids, neutral sugars, urea, creatinine and ammonia.
The precipitation of crude mucin by ethanol followed a method adapted from that of Piel et al.7 Digesta (3 mL) were added to 25 mL of 0.15 M aqueous sodium chloride and centrifuged at 14,500 RCF for 30 minutes at 4°C. After centrifugation, 15 mL of the supernatant were added to 22 mL of absolute ethanol at 0°C and kept at −20°C overnight. The tubes were then centrifuged again at 14,500 RCF (at 4°C) before the supernatant was removed and the precipitate dissolved in a further 15 mL of 0.15 M aqueous sodium chloride. The ethanol precipitation was then repeated before the final precipitate was dissolved in 10 mL of deionised water, freeze-dried and weighed. All the ethanol supernatant was recovered, dried and redissolved in 10 mL of deionised water.
Polyacrylamide gel electrophoresis (SDS-PAGE) followed the protocol used by Piel et al.7 for analysing the purity of protein, using 7.5% and 4% polyacrylamide gels for migrating and stacking gels respectively under conditions described by Laemmli26. Mucins on SDS-PAGE gels were detected using periodic acid/Schiff (PAS) staining, using a glycoprotein detection kit (GLYCO-PRO, Sigma, St Louis, MO, USA), and Coomassie Blue staining, using 0.1% Coomassie Brilliant Blue R-250.
Molecular weight profiling was adapted from the method described by Swergold and Rubin27 using high performance liquid chromatography with a size exclusion column. Briefly, peptides and amino acids in a hydrolysate were separated according to their size using two 2 × 30 cm TSK G2000 SWXL columns in series, with a TSK SWXL guard column, with a mobile phase that contained acetonitrile to denature the peptides and to promote better separation in the 0–10,000 molecular weight range. The column flow rate was 0.5 mL/minute (isocratic) at ambient temperature with a run length of 65 minutes. Peptides were detected by monitoring the absorbance at 205 or 210 nm. A calibration standard was obtained by using nine molecular weight markers to establish a plot of log molecular weight versus retention time (r2 = 0.995). The curve allowed the estimation of molecular weights corresponding to specific retention times.
Soluble protein was determined using the Bradford method28. The detection of neutral sugars, using phenol-sulphuric acid, and of sialic acid, using periodate-thiobarbituric acid, was undertaken using an adaptation of the methods described by Beeley16. Dry matter was determined by drying material to a constant mass in a forced-air oven at 95°C.
Footnotes
The authors declare no competing financial interests.
Author Contributions W. M.-W. designed and conducted the research. W. M.-W. conducted the chemical analysis, analyzed the data, wrote the paper and had primary responsibility for final content. The research was supervised by P.J. M. The paper was reviewed by P.J.M. and M.F.F. All authors read and approved the final manuscript.
References
- Bevins C. L., Martin-Porter E. & Ganz T. Defensins and innate host defence of the gastrointestinal tract. Gut 271, 14038–14045 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson I. R. & Walker W. A. in Handbook of Mucosal Immunology eds Ogra R., J., et al.) 5–17 (Academic Press, 1999). [Google Scholar]
- Montagne L., Piel C. & Lalles J. P. Effect of diet on mucin kinetics and composition: Nutrition and health implications. Nutrition Reviews 62, 105–114 (2004). [DOI] [PubMed] [Google Scholar]
- Roussel P. & Delmotte P. The diversity of epithelial secreted mucins. Current Organic Chemistry 8, 413–437 (2004). [Google Scholar]
- Krause W. J. Brunner's glands: A structural, histochemical and pathological profile. Progress in Histochemistry and Cytochemistry 35, 259–367 (2000). [PubMed] [Google Scholar]
- Miner-Williams W., Moughan P. J. & Fuller M. F. Endogenous components of digesta protein from the terminal ileum of pigs fed a casein based diet. Journal of Agricultural and Food Chemistry (2008). [DOI] [PubMed] [Google Scholar]
- Piel C., Montagne L., Salgado P. & Lalles J. P. Estimation of ileal output of gastro-intestinal glycoprotein in weaned piglets using three different methods. Reproduction Nutrition Development 44, 419–435 (2004). [DOI] [PubMed] [Google Scholar]
- Miner-Williams W., Moughan P. J. & Fuller M. F. Methods for mucin analysis - a comparative study. Journal of Agricultural and Food Chemistry 57, 6029–6035 (2009). [DOI] [PubMed] [Google Scholar]
- Leterme P., Seve B. & Thewis A. The current N-15-Leucine infusion technique is not suitable for quantitative measurements of ileal endogenous amino acid flows in pigs. Journal of Nutrition 128, 1961–1968 (1998). [DOI] [PubMed] [Google Scholar]
- Kotwal S. M., Gote M. M., Khan M. I. & Khire J. M. Production, purification and characterization of a constitutive intracellular alpha-galactosidase from the thermophilic fungus Humicola sp. Journal of Industrial Microbiology & Biotechnology 23, 661–667 (1999). [DOI] [PubMed] [Google Scholar]
- O'Keefe B. R. et al. Isolation and characterization of niphatevirin, a human-immunodeficiency-virus-inhibitory glycoprotein from the marine sponge Niphates erecta. European Journal of Biochemistry 245, 47–53 (1997). [DOI] [PubMed] [Google Scholar]
- Weltzien F. A., Norberg B. & Swanson P. Isolation and characterization of FSH and LH from pituitary glands of Atlantic halibut (Hippoglossus hippoglossus L.). General and Comparative Endocrinology 131, 97–105 (2003). [DOI] [PubMed] [Google Scholar]
- Azuumi Y., Ichikawa T., Ishihara K. & Hotta K. The Validity of the Ethanol Precipitation Method for the Measurement of Mucin Content in Human Gastric Juices and Its Possible Relationship to Gastroduodenal Diseases. Clinica Chimica Acta 221, 219–225 (1993). [DOI] [PubMed] [Google Scholar]
- Allen A., Bell A., Mantle M. & Pearson J. P. The Structure and Physiology of Gastrointestinal Mucus. Advances in Experimental Medicine and Biology 144, 115–133 (1982). [DOI] [PubMed] [Google Scholar]
- Mantle M. & Allen A. in Gastrointestinal Secretions (ed Davison J. S., ed.) 202-229 (Butterworth and Co, 1989). [Google Scholar]
- Beeley J. G. Glycoprotein and Proteoglycan Techniques. Vol. 16 (Elsevier Science Publishers B. V., 1985). [Google Scholar]
- Yusuf S., Nok A. J., Ameh D. A., Adelaiye A. B. & Balogun E. O. Correlation of gastric mucosal damage with sialic acid profile in rats: effect of hydrochloric acid, pepsin and hypertonic saline. Cell Biochemistry and Function 23, 339–345 (2005). [DOI] [PubMed] [Google Scholar]
- Pasquier M. C., Vatier J., Poitevin C., Vallot T. & Mignon M. Assessment of Mucus Glycoprotein Erosion by Measurement of Sialic-Acid in Gastric Secretions - Pathophysiologic and Therapeutic Aspects. Journal of Clinical Gastroenterology 13, S22–S31 (1991). [DOI] [PubMed] [Google Scholar]
- Morel P. C. H., Padilla R. M. & Ravindran G. Effect of non-starch polysaccharides on mucin secretion and endogenous amino acid losses in pigs. Asian-Australasian Journal of Animal Sciences 16, 1332–1338 (2003). [Google Scholar]
- Deplancke B. & Gaskins H. R. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. American Journal of Clinical Nutrition 73, 1131S–1141S (2001). [DOI] [PubMed] [Google Scholar]
- Moughan P. J., Smith W. C. & Stevens E. V. J. Allometric Growth of Chemical Body Components and Several Organs in the Pig (20–90 Kg Liveweight). New Zealand Journal of Agricultural Research 33, 77–84 (1990). [Google Scholar]
- Björling H. in Plasma Proteins eds Blombäck B., & Hanson L. A., eds.) 29–37 (Wiley, 1976). [Google Scholar]
- Miner-Williams W., Moughan P. J. & Fuller M. F. Methods for Mucin Analysis - a Comparative Study. Journal of Agricultural and Food Chemistry (2008). [DOI] [PubMed] [Google Scholar]
- van Leeuwen P., van Kleef D. J., van Kempen G. J. M., Huisman J. & Verstegen M. W. A. The Post Valve T-Cecum Cannulation Technique in Pigs Applicated (sic) to Determine the Digestibility of Amino-Acid in Maize, Groundnut and Sunflower Meal. Journal of Animal Physiology and Animal Nutrition-Zeitschrift Fur Tierphysiologie Tierernahrung Und Futtermittelkunde 65, 183–193 (1991). [Google Scholar]
- Metges C. C. et al. Incorporation of urea and ammonia nitrogen into ileal and fecal microbial proteins and plasma free amino acids in normal men and ileostomates. American Journal of Clinical Nutrition 70, 1046–1058 (1999). [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of Structural Proteins During Assembly of Head of Bacteriophage-T4. Nature 227, 680–& (1970). [DOI] [PubMed] [Google Scholar]
- Swergold G. D. & Rubin C. S. High-Performance Gel-Permeation Chromatography of Polypeptides in a Volatile Solvent - Rapid Resolution and Molecular-Weight Estimations of Proteins and Peptides on a Column of Tsk-G3000-Pw. Analytical Biochemistry 131, 295–300 (1983). [DOI] [PubMed] [Google Scholar]
- Bradford M. M. Rapid and Sensitive Method for Quantitation of Microgram Quantities of Protein Utilizing Principle of Protein-Dye Binding. Analytical Biochemistry 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]