Abstract
Background
Alcohol is classified as a Group 1 carcinogen by the International Agency for Research on Cancer because it induces hepatocellular carcinoma (among other cancers) in humans. An excessive alcohol intake may result in fatty liver, acute/chronic hepatitis, and cirrhosis and eventually lead to hepatocellular carcinoma. It has been reported that alcohol abuse increases the relative risk of hepatocellular carcinoma by 3- to 10-fold.
Aim and Methods
To clarify the known mechanisms of alcohol-related carcinogenesis, we searched Pubmed using the terms alcohol and immune mechanism, alcohol and cancer, and immune mechanism and cancer and summarized the articles as a qualitative review.
Results
From a clinical perspective, it is well known that alcohol interacts with other factors, such as smoking, viral hepatitis, and diabetes, leading to an increased risk of hepatocellular carcinoma. There are several possible mechanisms through which alcohol may induce liver carcinogenicity, including the mutagenic effects of acetaldehyde and the production of ROS due to the excessive hepatic deposition of iron. Furthermore, it has been reported that alcohol accelerates hepatitis C virus-induced liver tumorigenesis through TLR4 signaling. Despite intense investigations to elucidate the mechanisms, they remain poorly understood.
Conclusion
This review summarizes the recent findings of clinical and pathological studies that have investigated the carcinogenic effects of alcohol in the liver.
Keywords: hepatocellular carcinoma, alcohol
Introduction
Worldwide, liver cancer is the second highest cause of cancer-related death in men and the sixth highest cause of cancer-related death in women. Liver cancer is more common in low-income and middle-income countries than in developed countries.1 According to the National Cancer Institute, approximately 40 700 cases of liver cancer are expected to be newly diagnosed, and approximately 29 000 patients will die from liver cancer in the USA in 2017. Besides, the incidence of liver cancer is increasing by approximately 3%–4% per year.2 This means that liver cancer is a major public health problem.
Hepatocellular carcinoma (HCC), which accounts for around 70%–90% of cases, is the most common type of primary liver cancer. Alcohol consumption, the level of which is higher in developed countries, especially in the USA and Europe,3 is one of the frequent causes of HCC in developed countries.4 Conversely, chronic hepatitis B virus (HBV) infection is the major risk factor in low-income and middle-income countries. The ratio of alcohol abuse to all aetiologies of HCC varies according to the country and area; alcohol abuse is reported to be responsible for approximately 15%–30% of HCC.4 5 However, the appropriate methods for surveilling patients with alcohol use disorder (AUD) to facilitate the early-stage diagnosis of HCC remain to be determined, and mechanisms through which alcohol consumption is involved in the initiation of HCC remain unclear.
Understanding the clinical features and the mechanisms of alcohol-based HCC is critically important to the prevention and detection of early-stage HCC and for the development of treatments for HCC. This review summarises the recent clinical and pathological studies investigating the carcinogenic effects of alcohol in the liver.
The risks of liver cirrhosis and HCC
According to a WHO report, approximately 280 million individuals, or 4.1% of the population aged >15 years, meet the definition of AUD (alcohol dependence and the harmful use of alcohol). The prevalence is almost the same as the prevalence of hepatitis B, and is four times higher than the prevalence of hepatitis C.3 6 Because of the large population—HCC screening (eg, ultrasonography or the measurement of serum tumour marker levels) for all of such patients would lead to huge medical costs—it is necessary to select individuals with a high risk of HCC. In this respect, the American Association for the Study of Liver Diseases (AASLD) recommends that patients with Child’s classification A/B cirrhosis undergo surveillance for HCC using ultrasonography with or without alpha-fetoprotein measurement, every 6 months, and does not recommend the modification of the surveillance strategy based on the ‘etiology of liver disease’, the strategy of which is almost the same as that recommended by the European Association for the Study of the Liver.7 8 Incidentally, the previous AASLD guidelines for the management of HCC suggested that HCC surveillance is cost-effective if the annual incidence of HCC is ≥1.5% in patients with cirrhosis. Similar to hepatitis C and hepatitis B, the presence of alcoholic liver cirrhosis is considered to be an important risk factor for the development of HCC. It has been reported that approximately 10%–20% of heavy drinkers develop cirrhosis.9 Furthermore, several previous studies that have assessed the annual incidence of HCC in patients with alcohol-induced liver cirrhosis have revealed the rate to be 1.9%–2.6%.10 11 Thus, it might be appropriate to perform HCC surveillance for patients with alcoholic liver cirrhosis. However, even when guideline-based surveillance was performed, almost 20%–30% of HCC in patients with cirrhosis were diagnosed at a non-early stage.12 It is therefore necessary to determine the traits of patients with alcoholic liver cirrhosis that increase their risk of HCC and the traits of patients with AUD who show the highest risk of HCC.
The amount of ethanol as a risk factor
An excessive alcohol intake has been shown to be a risk factor for liver cirrhosis and HCC. It is considered that there is a linear dose–response relationship between alcohol consumption and the risk of cirrhosis and HCC.13 An alcohol intake of 30–50 g/day increases the OR for liver cirrhosis,14–16 while an alcohol intake of >60–100 g/day increases the OR for HCC.17–20 In terms of the total amount of alcohol intake during a patient’s lifetime, an alcohol consumption of >600 000 mL significantly increased the risk of HCC development (OR=4.52, 95% CI 2.39 to 8.55).21 22 Thus, the amount of ethanol consumption might provide an indication of the risk of liver cirrhosis and HCC.
Gender differences as a risk factor
There might be a gender difference in the volume of alcohol intake that increases the risk of alcohol-induced liver damage and the development of HCC. It has been reported that the risk of developing cirrhosis becomes substantial with the consumption of 60–80 g/day of alcohol for 10 years in men and 20 g/day for 10 years in women.14 23 In addition, women showed a more rapid progression (20 years) to cirrhosis than men (35 years).24 Among individuals who consume more than 80 g/day of alcohol, the risk of HCC development in women has been shown to be almost fivefold higher than that in men.18 However, the overall prevalence of HCC in women is small compared with that in men.
Various mechanisms have been suggested to underlie the higher sensitivity of women to alcohol.25 After the oral administration of alcohol, women show less first-pass metabolism of alcohol, which is defined as the difference in the amount of orally administered ethanol and the quantities in the systemic blood, due to their lower gastric alcohol dehydrogenase (ADH) activity, which results in a higher serum concentration of alcohol.26 Thus, even when the same amount of ethanol is consumed, the female liver may be exposed to more ethanol. Furthermore, oestrogen (a female sex hormone) may play an important role in alcohol-induced liver injury. It has been shown that oestrogen increases the sensitivity of Kupffer cells to lipopolysaccharide (LPS), which results in more severe liver injury.27 28 In fact, many previous studies have reported that more severe inflammatory responses in the liver and fat tissue, which were associated with toll-like receptor (TLR4) signalling, were seen in female patients.29–35
Conversely, several lines of evidence indicate that oestrogen and its downstream signalling protect against HCC development.36–38 In N-nitrosodiethylamine (DEN)-induced HCC mouse model, it has been shown that ablation of interleukin-6 (IL-6) abolished the gender differences in HCC development, and oestrogen attenuates serum IL-6 levels and IL-6 mRNA levels of Kupffer cell. These results suggest that oestrogen reduces the risk of HCC by the inhibition of IL-6 production from Kupffer cells.39 Taken together, the gender disparity in sensitivity to alcohol and HCC development cannot be sufficiently explained by simple mechanism. Although the prevalence of alcoholic liver disease (ALD) in female patients is low,3 they may require more intense surveillance.
Given that the importance of LPS–Kupffer cell interaction has also been reported in a non-alcoholic steatohepatitis (NASH) model,40 41 young females could be severely affected with NASH via oestrogen-related LPS–Kupffer cell interaction worsening. However, several studies have demonstrated that oestrogen protects against liver inflammation and fibrosis in a NASH model, and oestrogen deficiency has been reported to worsen steatohepatitis and liver fibrosis in human.42 43 As oestrogen has multiple functions and the expression profile of the mechanisms might differ in patients’ status, it is difficult to explain the effect simply.
The coexistence of hepatitis virus as a risk factor
In patients with ALD, the coexistence of hepatitis virus has been shown to accelerate the disease course.44 It has been reported that the prevalence of hepatitis C virus (HCV) infection in alcoholic patients is 16.32%,45 which is much higher than that in the general population (1.5%–2.0%).46 47 In patients with a high alcohol intake (>60 g/day to 125 g/day), the coexistence of HCV has been shown to increase the risk of alcohol-associated liver cirrhosis.17 18 24 48–50 Furthermore, heavy alcohol consumption has also been shown to increase the risk of developing HCC.18 44 Patients with coexisting HBV (defined by (hepatitis B s antigen) HBsAg-positivity) are at increased risk of developing fibrosis48 and HCC.51 52 In addition, self-resolved HBV infection (defined as HBsAg-negative, HCVAb-negative and HBcAb-positive) can be a risk factor for developing HCC in patients with alcoholic cirrhosis.53 Although the mechanisms by which the synergistic effect between alcohol and hepatitis virus increases the risk of liver fibrosis and HCC have been the subject of extensive research, they have not been completely elucidated.54–59 Collectively, it might be better to perform surveillance of patients with ALD who show coexisting HBV or HCV.
Diabetes and obesity as risk factors
It has been well recognised that diabetes is a risk factor for HCC. Several meta-analyses have shown that diabetes is significantly associated with HCC (relative risk: 1.87–2.32).60–64 As for the association between obesity and HCC, Larsson and Wolk61 reported that the relative risk of liver cancer among obese (defined as a body mass index of >30) individuals was 1.89 (95% CI 1.51 to 2.36).61 Case–control studies have reported a synergistic interaction between heavy alcohol consumption and diabetes that affects the risk of HCC (OR: 4.2–9.9).44 65 Correspondingly, alcohol use and obesity showed a synergistic interaction with the risk of developing HCC (HR: 3.82, 95% CI 1.94 to 7.52).66 Although the mechanisms of the synergistic effect between ALD and diabetes are unknown, several mechanisms have been suggested to underlie the development of diabetes and/or obesity-induced HCC in patients with non-alcoholic steatohepatitis.67 In terms of cost-effective surveillance, a recent cohort study investigated the potential predictors of HCC in 3544 patients with diabetes without viral hepatitis and suggested that a DM-HCC risk score included age >65 years, low triglyceride levels and high gamma-glutamyl transferase levels.68 In this study, although heavy drinking was found to be a significant predictor in a univariate analysis, it did not remain significant in a multivariate analysis.
In addition to the above-mentioned risk factors, others have been suggested, including genetic polymorphisms,69 race and ethnicity.70 Thus far, unfortunately, there are no definitive hallmarks to narrow down the target patients. Further studies are required to establish clinically beneficial prediction models.
Potential mechanisms of alcohol-induced HCC
The mechanisms underlying the induction of carcinogenesis by alcohol have not been fully elucidated. Because there are no animal models of alcohol-induced HCC alone, a chemical-induced (DEN) HCC model is mainly used to study the effects of alcohol intake on hepatocarcinogenesis.32 71 72 However, the priming effect of hepatocarcinogenesis and the progressive effects have been well investigated.
Alcohol absorption and metabolism
Once ethanol is consumed, almost all of it is absorbed by the small intestine and metabolised by the liver.73 Ethanol is metabolised to acetaldehyde by ADH in the cytoplasm of hepatocytes, acetaldehyde subsequently enters the mitochondria, and is then oxidised to acetate by mitochondrial aldehyde dehydrogenase (ALDH). When a large amount of ethanol is consumed, cytochrome P450 2E1 (CYP2E1), which mainly exists in the endoplasmic reticulum, and catalase of peroxisomes also contribute to the metabolism of ethanol. The CYP2E1-dependent pathway can catalyse ethanol into acetaldehyde while producing reactive oxygen species (ROS), such as hydroxyethyl, superoxide anion and hydroxyl radicals74 (figure 1). In humans, there are at least seven different isozymes of ADH and four isozymes of ALDH. Several previous studies showed ALDH2 polymorphisms to be significantly associated with the development of HCC75; thus, it is possible that the metabolism of alcohol is involved in the mechanisms of HCC development.
Figure 1.
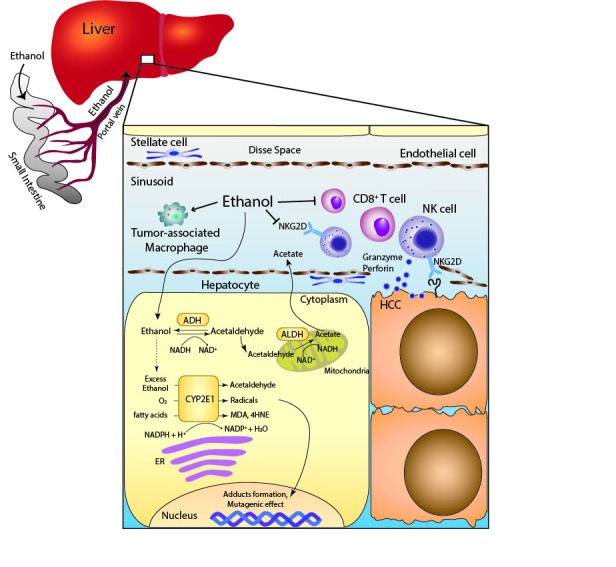
Scheme of the immune system in HCC surveillance and the metabolic effects of alcohol exposure on hepatocyte. The metabolism of ethanol through the CYP2E1-dependent pathway produces acetaldehyde, radicals and lipid peroxidation products, such as MDA and 4HNE. Alcohol consumption reduces the number of CD8+ T cells and NK cells, and reduces the NKG2D expression on NK cells. 4HNE, 4-hydroxy-2-nonenal; ADH, alcohol dehydrogenase; ALDH, aldehyde dehydrogenase; CYP2E1, cytochrome P450 2E1; ER, endoplasmic reticulum; HCC, hepatocellular carcinoma; MDA, malondialdehyde; NK, natural killer; NKG2D, NK group 2D.
The carcinogenic properties of acetaldehyde, an alcohol metabolite
Acetaldehyde has been shown to be a carcinogen in animal studies. With regard to direct DNA mutagenic mechanisms, it has been reported that acetaldehyde increases the point mutation frequency in the hypoxanthine phosphoribosyltransferase (HPRT) gene in lymphocytes,76 and induces sister chromatid exchanges.77 Meanwhile the formation of adducts with DNA, for example, N2-ethyl-deoxyguanosine (N2-Et-dG),78 has been found in patients with ALD. Another DNA adduct, N2-propano-2’-deoxyguanosine (N2-Et-dGTP), has been reported to cause the alternation of DNA integrity.79 Furthermore, the formation of protein adducts plays an important role in carcinogenesis. Acetaldehyde interacts with certain amino acids in proteins, for example, the formation of adducts with O6-methylguanine methyltransferase causes DNA repair system dysfunction.80 With regard to liver fibrosis, acetaldehyde produced in the hepatocytes can enter the hepatic stellate cell (HSC) and induces the expression of type I collagen genes in vitro.81 Acetaldehyde adducts with protein have been shown in HSC.82 Taken together, the direct DNA mutagenic effect of acetaldehyde and the indirect carcinogenic effect through the formation of adducts may modulate hepatocarcinogenesis and liver fibrosis.
Alcohol and oxidative stress
As described above, the metabolism of ethanol through the CYP2E1-dependent pathway produces ROS. Subsequently, the increase of ROS results in the generation of lipid peroxidation products, such as malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4HNE).83 A significant increase in CYP2E1 could be induced for 1 week in patients who consumed more than 40 g of ethanol per day and was further elevated after 4 weeks.84 In an animal model, the induction of CYP2E1 by ethanol causes the production of hydroxyethyl radicals and lipid peroxidation.85 Furthermore, in humans, it has been shown that ethanol-mediated CYP2E1 induction leads to the increased levels of ROS, lipid peroxidation, MDA and 4HNE.86 4HNE, one of the lipid peroxidation products, can cause a mutation at codon 249 of the p53 gene87; this mutation is commonly found in HCC (20%–30%). In addition to the mutagenic effects on DNA, ROS play an important role as mediators of tumour angiogenesis and metastasis. It has been shown that alcohol-induced ROS production results in the activation of NF-kB signalling; conversely the expression levels of VEGF and MCP-1, the tumour growth, angiogenesis and metastasis were suppressed by the inhibition of alcohol-mediated ROS production and NF-kB signalling.88 Apart from above-mentioned mechanisms of CYP2E1-mediated ROS generation, iron overload and tumour necrosis factor-alpha from inflammatory cells also influence ROS production. Chronic ethanol consumption increases intestinal iron absorption and hepatic iron storage. Iron overload has been shown to cause DNA strand breaks and p53 mutation, which could cause hepatocarcinogenesis.89 90
Alcohol and the methylation of DNA and protein
Aberrant DNA methylation and protein methylation may play important roles in the development of various cancers, including HCC. In most cases, DNA methylation is associated with a decreased gene expression because of interference with the interaction of transcription factor and CpG islands of promoter lesions (usually unmethylated).91 It has been shown that alcohol inhibits the synthesis of S-adenosyl-L-methionine (SAMe), which is a universal methyl group donor. The generation of SAMe is induced by the enzyme methionine adenosyltransferase (MAT). MAT is encoded by two different genes: MAT1A and MAT2A. It has been shown that MAT1A knockout mice develop SAMe deficiency, fatty liver and HCC.92 Furthermore, in patients with AUD, the hepatic MAT activity and the expression of the MAT1A gene are reported to be decreased.93 SAMe also works as a methyl group donor for protein methylation. A recent study by Jie Zhao et al94 reported that the activity of PRMT1 in the mouse liver, which is a protein arginine methyltransferase, was inhibited by ethanol feeding. They revealed that the Hnf4α-dependent hepatocyte proliferation was regulated by the balance of methylation and demethylation of Hnf4α promoter, which depends on PRMT1 and JMJD6, respectively.94
Taken together, elucidating the mechanisms of the aberrant DNA and protein methylation in HCC may lead to the discovery of novel therapeutic targets, and SAMe might be a potential candidate for preventive medicine targeting HCC.
The tumour microenvironment
Recent studies have highlighted the cross-talk between tumour cells and the surrounding microenvironment; in the liver, this mainly consists of immune cells, HSC, fibroblasts and sinusoidal endothelial cells. Although there is increasing evidence to show that the tumour microenvironment influences the development of HCC,95 the mechanisms by which alcohol consumption contributes to the progression of HCC remain unclear. In this respect, recent studies have reported that ethanol feeding promoted DEN-induced tumourigenesis in the liver, and reduced the number of antitumour CD8+ T cells and increased the number of tumour-associated macrophages and/or M2 macrophages in mouse models.96 97 These studies strongly indicate that the immune systems of alcohol-induced HCC may also be a potential therapeutic target.
The role of the immune system in tumour surveillance
Natural killer (NK) cells, which are characterised by CD56+ and CD3− lymphocytes in humans, seem to play a role in tumour surveillance. NK cells are abundant in the liver, where they can constitute up to 30% of the intrahepatic lymphocyte population.98 The release of granules (ie, perforin and granzymes) and the secretion of cytokines (ie, Fas and TRAIL) by NK cells have a rapid cytotoxic effect. NK cells express several activating receptors, such as the NK group 2D (NKG2D), natural cytotoxicity receptors, CD226 (DNAX accessory molecule-1) and CD16 (Fcγ receptor III), to recognise ligands. There is increasing evidence to suggest that NK cells contribute to the pathogenesis of HCC.99 In clinical studies, patients with HCC with low levels of tumour-infiltrating lymphocytes showed poor prognosis.100 101 Furthermore, the antitumour ability of NK cells has been shown to be reduced in patients with HCC.102 103 A study using an animal model demonstrated that the depletion of NK cells resulted in a decrease in the antitumour activity of IL-18/IL-12 therapy.104 With regard to the relationship between NK cells and alcohol use, many studies have reported an association between alcohol consumption and NK cell dysfunction. The NK cells in the peripheral blood of patients with alcoholic liver cirrhosis showed reduced cytotoxic activity against cancer cells.105 In an animal model, alcohol ingestion reduced the cytotoxicity of NK cells, which resulted from the reduction of NKG2D.9898 Furthermore, a recent study showed that alcohol consumption reduced the number of cytotoxic NK cells defined as Eomes+CD3−NK1.1+ in the liver.106 However, further studies are needed as it is difficult to completely clarify the association between NK cells and alcohol-induced HCC.
TLR4 and hepatocarcinogenesis in alcohol users
TLR4 recognises the lipid A motif of LPS, which is a component of Gram-negative bacteria. The plasma LPS levels were elevated in ethanol-fed animals and the plasma endotoxin levels were found to be elevated in patients with ALD.107 108 Machida et al109 reported that an HCV-derived protein, NS5A, induced the expression of the TLR4 gene in hepatocytes and B cells.109 They also showed that alcohol induced the progression of HCC through LPS-TLR4signaling activation. The LPS-TLR4 pathway was regulated by the Nanog-mediatedmodulation of the mitochondrial metabolism in NS5A Tg mice.110 These studies suggest the crucial involvement of TLR4 and NANOG in the induction of HCC that is mediated by alcohol and HCV infection.
Gene polymorphisms and HCC development in ALD
Several studies have demonstrated that genetic susceptibility is associated with the alcohol-induced cancer risk. There are several genetic polymorphisms that affect the clinical course of ALD as they can induce psychological behavioural changes, alcohol metabolism, lipid metabolism, oxidative stress-related pathway activation and inflammatory responses.69 As described above in the Alcohol absorption and metabolism section, the gene polymorphisms of ADH2 and ALDH2 have been reported to correlate with HCC development in a Japanese cohort study,21 111 but several studies conversely found no association with HCC69; thus, further validation is needed. Concerning alcohol-induced HCC development, the gene polymorphism of patatin-like phospholipase 3 domain containing 3 (PNPLA3) was found to be an important risk factor. Several cohort studies revealed the risk of PNPLA3 (rs738409) on alcohol-induced HCC in patients with ALD.112–114 A recent genome-wide association study in Europe revealed that the gene polymorphisms of TM6SF2 and PNPLA3 could be regarded as potential genetic risk factors for the development of alcohol-related HCC.115
Conclusion
Several clinical factors increase the risk of alcohol-induced HCC. A large alcohol intake, coexistence of diabetes and hepatitis virus infection, and female gender are established factors. The precise mechanisms of hepatocarcinogenesis are not clearly understood; however, there is increasing evidence to suggest that many factors are involved. Alcohol metabolites and adducts have been shown to induce oxidative stress, direct mutagenesis, and the aberrant methylation of DNA or protein on hepatocytes. Furthermore, the immune system is also implicated in the development and progression of HCC. Obviously, the best approach for resolving this complex pathogenesis is the cessation of alcohol consumption. However, as this is often difficult, further studies are needed to reduce the risk of HCC.
Acknowledgments
The authors wish to acknowledge Dr Hiroyuki Okada, Professor at the Department of Gastroenterology and Hepatology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan.
Footnotes
Contributors: HM and AT contributed to the writing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Torre LA, Bray F, Siegel RL, et al. . Global Cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. 67 CA: A Cancer Journal for Clinicians, 2017: 7–30. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization, a. W. H. O. M. o. S. A. U Global status report on alcohol and health, 2014. World Health Organization, 2014. [Google Scholar]
- 4.Testino G, Leone S, Borro P. Alcohol and hepatocellular carcinoma: a review and a point of view. World J Gastroenterol 2014;20:15943–54. 10.3748/wjg.v20.i43.15943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hlady RA, Tiedemann RL, Puszyk W, et al. . Epigenetic signatures of alcohol abuse and hepatitis infection during human hepatocarcinogenesis. Oncotarget 2014;5:9425–43. 10.18632/oncotarget.2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Global hepatitis report, 2017. [Google Scholar]
- 7.Heimbach J, Kulik LM, Finn R, et al. . Aasld guidelines for the treatment of hepatocellular carcinoma. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 8.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer . EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–43. 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 9.Grant BF, Dufour MC, Harford TC. Epidemiology of alcoholic liver disease. Semin Liver Dis 1988;8:12–25. 10.1055/s-2008-1040525 [DOI] [PubMed] [Google Scholar]
- 10.Mancebo A, González-Diéguez ML, Cadahía V, et al. . Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clin Gastroenterol Hepatol 2013;11:95–101. 10.1016/j.cgh.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 11.Lin CW, Lin CC, Mo LR, et al. . Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J Hepatol 2013;58:730–5. 10.1016/j.jhep.2012.11.045 [DOI] [PubMed] [Google Scholar]
- 12.Trinchet J-C, Chaffaut C, Bourcier V, et al. . Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology 2011;54:1987–97. 10.1002/hep.24545 [DOI] [PubMed] [Google Scholar]
- 13.Scoccianti C, Cecchini M, Anderson AS, et al. . European code against cancer 4th Edition: alcohol drinking and cancer. Cancer Epidemiol 2016;45:181–8. 10.1016/j.canep.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 14.Bellentani S, Saccoccio G, Costa G, et al. . Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 1997;41:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corrao G, Aricò S, Zambon A, et al. . Is alcohol a risk factor for liver cirrhosis in HBsAg and anti-HCV negative subjects? collaborative groups for the study of liver diseases in Italy. J Hepatol 1997;27:470–6. [DOI] [PubMed] [Google Scholar]
- 16.Savolainen VT, Liesto K, Männikkö A, et al. . Alcohol consumption and alcoholic liver disease: evidence of a threshold level of effects of ethanol. Alcoholism Clin Exp Res 1993;17:1112–7. 10.1111/j.1530-0277.1993.tb05673.x [DOI] [PubMed] [Google Scholar]
- 17.Corrao G, Aricò S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology 1998;27:914–9. 10.1002/hep.510270404 [DOI] [PubMed] [Google Scholar]
- 18.Donato F, Tagger A, Gelatti U, et al. . Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol 2002;155:323–31. 10.1093/aje/155.4.323 [DOI] [PubMed] [Google Scholar]
- 19.Corrao G, Bagnardi V, Zambon A, et al. . A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med 2004;38:613–9. 10.1016/j.ypmed.2003.11.027 [DOI] [PubMed] [Google Scholar]
- 20.Persson EC, Schwartz LM, Park Y, et al. . Alcohol consumption, folate intake, hepatocellular carcinoma, and liver disease mortality. Cancer Epidemiol Biomarkers Prev 2013;22:415–21. 10.1158/1055-9965.EPI-12-1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munaka M, Kohshi K, Kawamoto T, et al. . Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and the risk of hepatocellular carcinoma. J Cancer Res Clin Oncol 2003;129:355–60. 10.1007/s00432-003-0439-5 [DOI] [PubMed] [Google Scholar]
- 22.Tanaka K, Tsuji I, Wakai K, et al. . Alcohol drinking and liver cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol 2008;38:816–38. 10.1093/jjco/hyn108 [DOI] [PubMed] [Google Scholar]
- 23.Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis 2004;24:217–32. 10.1055/s-2004-832936 [DOI] [PubMed] [Google Scholar]
- 24.Poynard T, Mathurin P, Lai C-L, et al. . A comparison of fibrosis progression in chronic liver diseases. J Hepatol 2003;38:257–65. 10.1016/S0168-8278(02)00413-0 [DOI] [PubMed] [Google Scholar]
- 25.Thurman RG. II. alcoholic liver injury involves activation of Kupffer cells by endotoxin. American Journal of Physiology-Gastrointestinal and Liver Physiology 1998;275:G605–G611. 10.1152/ajpgi.1998.275.4.G605 [DOI] [PubMed] [Google Scholar]
- 26.Baraona E, Abittan CS, Dohmen K, et al. . Gender differences in pharmacokinetics of alcohol. Alcoholism Clin Exp Res 2001;25:502–7. 10.1111/j.1530-0277.2001.tb02242.x [DOI] [PubMed] [Google Scholar]
- 27.Ikejima K, Enomoto N, Iimuro Y, et al. . Estrogen increases sensitivity of hepatic Kupffer cells to endotoxin. American Journal of Physiology-Gastrointestinal and Liver Physiology 1998;274:G669–G676. 10.1152/ajpgi.1998.274.4.G669 [DOI] [PubMed] [Google Scholar]
- 28.Enomoto N, Yamashina S, Schemmer P, et al. . Estriol sensitizes rat Kupffer cells via gut-derived endotoxin. American Journal of Physiology-Gastrointestinal and Liver Physiology 1999;277:G671–G677. 10.1152/ajpgi.1999.277.3.G671 [DOI] [PubMed] [Google Scholar]
- 29.Yamada S, Matsuoka H, Harada Y, et al. . Effect of long-term ethanol consumption on ability to produce cytokine-induced neutrophil chemoattractant-1 in the rat liver and its gender difference. Alcohol Clin Exp Res 1999;23(4 Suppl):61S–6. 10.1111/j.1530-0277.1999.tb04536.x [DOI] [PubMed] [Google Scholar]
- 30.Nanji AA, Jokelainen K, Fotouhinia M, et al. . Increased severity of alcoholic liver injury in female rats: role of oxidative stress, endotoxin, and chemokines. Am J Physiol Gastrointest Liver Physiol 2001;281:G1348–G1356. 10.1152/ajpgi.2001.281.6.G1348 [DOI] [PubMed] [Google Scholar]
- 31.Gallucci RM, Sloan DK, O'Dell SJ, et al. . Differential expression of liver interleukin-6 receptor-?? in female versus male Ethanol-Consuming rats. Alcoholism: Clinical & Experimental Research 2004;28:365–73. 10.1097/01.ALC.0000118316.20560.0D [DOI] [PubMed] [Google Scholar]
- 32.Brandon-Warner E, Walling TL, Schrum LW, et al. . Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis. Alcohol Clin Exp Res 2012;36:641–53. 10.1111/j.1530-0277.2011.01660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagnerberger S, Fiederlein L, Kanuri G, et al. . Sex-specific differences in the development of acute alcohol-induced liver steatosis in mice. Alcohol Alcohol 2013;48:648–56. 10.1093/alcalc/agt138 [DOI] [PubMed] [Google Scholar]
- 34.Kasztelan-Szczerbińska B, Surdacka A, Celiński K, et al. . Prognostic significance of the systemic inflammatory and immune balance in alcoholic liver disease with a focus on gender-related differences. PLoS One 2015;10:e0128347 10.1371/journal.pone.0128347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulham MA, Mandrekar P. Sexual dimorphism in alcohol induced adipose inflammation relates to liver injury. PLoS One 2016;11:e0164225 10.1371/journal.pone.0164225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sukocheva OA, Estrogen SOA. Estrogen, estrogen receptors, and hepatocellular carcinoma: are we there yet? World J Gastroenterol 2018;24:1–4. 10.3748/wjg.v24.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H, Yao S, Zhang S, et al. . Elevated expression of ERBIN destabilizes ERα protein and promotes tumorigenesis in hepatocellular carcinoma. Journal of Hepatology 2017;66:1193–204. 10.1016/j.jhep.2017.01.030 [DOI] [PubMed] [Google Scholar]
- 38.Ren J, Chen GG, Liu Y, et al. . Cytochrome P450 1A2 metabolizes 17β-estradiol to suppress hepatocellular carcinoma. PLoS One 2016;11:e0153863 10.1371/journal.pone.0153863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naugler WE, Sakurai T, Kim S, et al. . Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007;317:121–4. 10.1126/science.1140485 [DOI] [PubMed] [Google Scholar]
- 40.Csak T, Velayudham A, Hritz I, et al. . Deficiency in myeloid differentiation factor-2 and Toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol 2011;300:G433–G441. 10.1152/ajpgi.00163.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivera CA, Adegboyega P, van Rooijen N, et al. . Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. Journal of Hepatology 2007;47:571–9. 10.1016/j.jhep.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klair JS, Yang JD, Abdelmalek MF, et al. . A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology 2016;64:85–91. 10.1002/hep.28514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamada Y, Kiso S, Yoshida Y, et al. . Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am J Physiol Gastrointest Liver Physiol 2011;301:G1031–G1043. 10.1152/ajpgi.00211.2011 [DOI] [PubMed] [Google Scholar]
- 44.Yuan JM, Govindarajan S, Arakawa K, et al. . Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer 2004;101:1009–17. 10.1002/cncr.20427 [DOI] [PubMed] [Google Scholar]
- 45.Novo-Veleiro I, Calle CL, Domínguez-Quibén S, et al. . Prevalence of hepatitis C virus infection in alcoholic patients: cohort study and systematic review. Alcohol Alcohol 2013;48:564–9. 10.1093/alcalc/agt044 [DOI] [PubMed] [Google Scholar]
- 46.Bruguera M, Forns X. Hepatitis C in Spain. Med Clin 2006;127:113–7. [DOI] [PubMed] [Google Scholar]
- 47.Armstrong GL, Wasley A, Simard EP, et al. . The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006;144:705–14. 10.7326/0003-4819-144-10-200605160-00004 [DOI] [PubMed] [Google Scholar]
- 48.Stroffolini T, Cotticelli G, Medda E, et al. . Interaction of alcohol intake and cofactors on the risk of cirrhosis. Liver Int 2010;30:867–70. 10.1111/j.1478-3231.2010.02261.x [DOI] [PubMed] [Google Scholar]
- 49.Lim JK, Tate JP, Fultz SL, et al. . Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C Virus–Infected, and uninfected patients. Clin Infect Dis 2014;58:1449–58. 10.1093/cid/ciu097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiley TE, McCarthy M, Breidi L, et al. . Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology 1998;28:805–9. 10.1002/hep.510280330 [DOI] [PubMed] [Google Scholar]
- 51.Walter SR, Thein HH, Gidding HF, et al. . Risk factors for hepatocellular carcinoma in a cohort infected with hepatitis B or C. J Gastroenterol Hepatol 2011;26:1757–64. 10.1111/j.1440-1746.2011.06785.x [DOI] [PubMed] [Google Scholar]
- 52.Ikeda K, Saitoh S, Suzuki Y, et al. . Interferon decreases hepatocellular carcinogenesis in patients with cirrhosis caused by the hepatitis B virus: a pilot study. Cancer 1998;82:827–35. [DOI] [PubMed] [Google Scholar]
- 53.Uetake S, Yamauchi M, Itoh S, et al. . Analysis of risk factors for hepatocellular carcinoma in patients with HBs antigen- and anti-HCV antibody-negative alcoholic cirrhosis: clinical significance of prior hepatitis B virus infection. Alcoholism: Clinical & Experimental Research 2003;27(Supplement):47S–51. 10.1097/01.ALC.0000079449.47468.B0 [DOI] [PubMed] [Google Scholar]
- 54.Kohgo Y, Ohtake T, Ikuta K, et al. . Iron accumulation in alcoholic liver diseases. Alcohol Clin Exp Res 2005;29(11 Suppl):189S–93. 10.1097/01.alc.0000189274.00479.62 [DOI] [PubMed] [Google Scholar]
- 55.Otani K, Korenaga M, Beard MR, et al. . Hepatitis C virus core protein, cytochrome P450 2E1, and alcohol produce combined mitochondrial injury and cytotoxicity in hepatoma cells. Gastroenterology 2005;128:96–107. 10.1053/j.gastro.2004.10.045 [DOI] [PubMed] [Google Scholar]
- 56.Zekry A, McHutchison JG, Diehl AM. Insulin resistance and steatosis in hepatitis C virus infection. Gut 2005;54:903–6. 10.1136/gut.2004.059873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aloman C, Gehring S, Wintermeyer P, et al. . Chronic ethanol consumption impairs cellular immune responses against HCV NS5 protein due to dendritic cell dysfunction. Gastroenterology 2007;132:698–708. 10.1053/j.gastro.2006.11.016 [DOI] [PubMed] [Google Scholar]
- 58.Bala S, Marcos M, Kodys K, et al. . Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem 2011;286:1436–44. 10.1074/jbc.M110.145870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dolganiuc A. Alcohol and viral hepatitis: role of lipid rafts. Alcohol Res 2015;37:299–309. [PMC free article] [PubMed] [Google Scholar]
- 60.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol 2006;4:369–80. 10.1016/j.cgh.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 61.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer 2007;97:1005–8. 10.1038/sj.bjc.6603932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang C, Wang X, Gong G, et al. . Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int. J. Cancer 2012;130:1639–48. 10.1002/ijc.26165 [DOI] [PubMed] [Google Scholar]
- 63.Wang P, Kang D, Cao W, et al. . Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev 2012;28:109–22. 10.1002/dmrr.1291 [DOI] [PubMed] [Google Scholar]
- 64.Yang W-S, Va P, Bray F, et al. . The role of pre-existing diabetes mellitus on hepatocellular carcinoma occurrence and prognosis: a meta-analysis of prospective cohort studies. PLoS ONE 2011;6:e27326 10.1371/journal.pone.0027326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hassan M, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 2002;36:1206–13. 10.1053/jhep.2002.36780 [DOI] [PubMed] [Google Scholar]
- 66.Loomba R, Yang H-I, Su J, et al. . Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol 2013;177:333–42. 10.1093/aje/kws252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reeves HL, Zaki MY, Day CP. Hepatocellular carcinoma in obesity, type 2 diabetes, and NAFLD. Dig Dis Sci 2016;61:1234–45. 10.1007/s10620-016-4085-6 [DOI] [PubMed] [Google Scholar]
- 68.Si WK, Chung JW, Cho J, et al. . Predictors of increased risk of hepatocellular carcinoma in patients with type 2 diabetes. PLoS One 2016;11:e0158066 10.1371/journal.pone.0158066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anstee QM, Daly AK, Day CP. Genetics of alcoholic liver disease. Semin Liver Dis 2015;35:361–74. 10.1055/s-0035-1567832 [DOI] [PubMed] [Google Scholar]
- 70.Douds AC, Cox MA, Iqbal TH, et al. . Ethnic differences in cirrhosis of the liver in a British city: Alcoholic cirrhosis in South Asian men. Alcohol Alcohol 2003;38:148–50. 10.1093/alcalc/agg040 [DOI] [PubMed] [Google Scholar]
- 71.Yan G, Wang X, Sun C, et al. . Chronic alcohol consumption promotes diethylnitrosamine-induced hepatocarcinogenesis via immune disturbances. Sci Rep 2017;7 10.1038/s41598-017-02887-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ambade A, Satishchandran A, Szabo G. Alcoholic hepatitis accelerates early hepatobiliary cancer by increasing stemness and miR-122-mediated HIF-1α activation. Sci Rep 2016;6 10.1038/srep21340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holt S. Observations on the relation between alcohol absorption and the rate of gastric emptying. Can Med Assoc J 1981;124:267-77–97. [PMC free article] [PubMed] [Google Scholar]
- 74.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health 2006;29:245–54. [PMC free article] [PubMed] [Google Scholar]
- 75.Chang JS, Hsiao J-R, Chen C-H. ALDH2 polymorphism and alcohol-related cancers in Asians: a public health perspective. J Biomed Sci 2017;24 10.1186/s12929-017-0327-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He SM, Lambert B. Acetaldehyde-induced mutation at the HPRT locus in human lymphocytes in vitro. Environ Mol Mutagen 1990;16:57–63. [DOI] [PubMed] [Google Scholar]
- 77.Obe G, Jonas R, Schmidt S. Metabolism of ethanol in vitro produces a compound which induces sister-chromatid exchanges in human peripheral lymphocytes in vitro: Acetaldehyde not ethanol is mutagenic. Mutation Research Letters 1986;174:47–51. 10.1016/0165-7992(86)90075-8 [DOI] [PubMed] [Google Scholar]
- 78.Brooks PJ, damage DNA. DNA damage, DNA repair, and alcohol toxicity--a review. Alcohol Clin Exp Res 1997;21:1073–82. [PubMed] [Google Scholar]
- 79.Matsuda T, Terashima I, Matsumoto Y, et al. . Effective utilization of N2-ethyl-2'-deoxyguanosine triphosphate during DNA synthesis catalyzed by mammalian replicative DNA polymerases. Biochemistry 1999;38:929–35. 10.1021/bi982134j [DOI] [PubMed] [Google Scholar]
- 80.Collier JD, Bassendine MF, Burt AD, et al. . Characterisation of the DNA repair enzyme for O6-methylguanine in cirrhosis. Journal of Hepatology 1996;25:158–65. 10.1016/S0168-8278(96)80068-7 [DOI] [PubMed] [Google Scholar]
- 81.Siegmund SV, Dooley S, Brenner DA. Molecular mechanisms of alcohol-induced hepatic fibrosis. Dig Dis 2005;23:264–74. 10.1159/000090174 [DOI] [PubMed] [Google Scholar]
- 82.Paradis V, Scoazec JY, Köllinger M, et al. . Cellular and subcellular localization of acetaldehyde-protein adducts in liver biopsies from alcoholic patients. J Histochem Cytochem. 1996;44:1051–7. 10.1177/44.9.8773571 [DOI] [PubMed] [Google Scholar]
- 83.Haorah J, Ramirez SH, Floreani N, et al. . Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med 2008;45:1542–50. 10.1016/j.freeradbiomed.2008.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oneta CM, Lieber CS, Li J, et al. . Dynamics of cytochrome P4502E1 activity in man: induction by ethanol and disappearance during withdrawal phase. J Hepatol 2002;36:47–52. 10.1016/S0168-8278(01)00223-9 [DOI] [PubMed] [Google Scholar]
- 85.Albano E, Clot P, Morimoto M, et al. . Role of cytochrome P4502E1-dependent formation of hydroxyethyl free radical in the development of liver damage in rats intragastrically fed with ethanol. Hepatology 1996;23:155–63. 10.1002/hep.510230121 [DOI] [PubMed] [Google Scholar]
- 86.Aleynik SI, Leo MA, Aleynik MK, et al. . Increased circulating products of lipid peroxidation in patients with alcoholic liver disease. Alcoholism Clin Exp Res 1998;22:192–6. 10.1111/j.1530-0277.1998.tb03637.x [DOI] [PubMed] [Google Scholar]
- 87.Hu W, Feng Z, Eveleigh J, et al. . The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis 2002;23:1781–9. 10.1093/carcin/23.11.1781 [DOI] [PubMed] [Google Scholar]
- 88.Wang F, Yang J-L, Yu K-ke, et al. . Activation of the NF-κB pathway as a mechanism of alcohol enhanced progression and metastasis of human hepatocellular carcinoma. Mol Cancer 2015;14 10.1186/s12943-014-0274-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Asare GA, Mossanda KS, Kew MC, et al. . Hepatocellular carcinoma caused by iron overload: a possible mechanism of direct hepatocarcinogenicity. Toxicology 2006;219:41–52. 10.1016/j.tox.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 90.Marrogi AJ, Khan MA, van Gijssel HE, et al. . Oxidative stress and p53 mutations in the carcinogenesis of iron overload-associated hepatocellular carcinoma. J Natl Cancer Inst 2001;93:1652–5. 10.1093/jnci/93.21.1652 [DOI] [PubMed] [Google Scholar]
- 91.Lu SC, Mato JM. Role of methionine adenosyltransferase and S-adenosylmethionine in alcohol-associated liver cancer. Alcohol 2005;35:227–34. 10.1016/j.alcohol.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 92.Santamaría E, Muñoz J, Fernández-Irigoyen J, et al. . Molecular profiling of hepatocellular carcinoma in mice with a chronic deficiency of hepatic S-adenosylmethionine: relevance in human liver diseases. J Proteome Res 2006;5:944–53. 10.1021/pr050429v [DOI] [PubMed] [Google Scholar]
- 93.Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. Faseb J 2001;15:1335–49. 10.1096/fj.00-0650rev [DOI] [PubMed] [Google Scholar]
- 94.Zhao J, Adams A, Roberts B, et al. . PRMT1 and JMJD6 dependent arginine methylation regulate HNF4alpha expression and hepatocyte proliferation in mice. Hepatology 2018;67:1109–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tahmasebi Birgani M, Carloni V, Microenvironment T. Tumor microenvironment, a paradigm in hepatocellular carcinoma progression and therapy. Int J Mol Sci 2017;18 10.3390/ijms18020405. [Epub ahead of print: 14 Feb 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang F, Little A, Zhang H. Chronic alcohol consumption inhibits peripheral NK cell development and maturation by decreasing the availability of IL-15. J Leukoc Biol 2017;101:1015–27. 10.1189/jlb.1A0716-298RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ambade A, Satishchandran A, Gyongyosi B, et al. . Adult mouse model of early hepatocellular carcinoma promoted by alcoholic liver disease. WJG 2016;22:4091–108. 10.3748/wjg.v22.i16.4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 2006;43:S54–S62. 10.1002/hep.21060 [DOI] [PubMed] [Google Scholar]
- 99.Yu M, Li Z. Natural killer cells in hepatocellular carcinoma: current status and perspectives for future immunotherapeutic approaches. Front Med 2017;11:509–21. 10.1007/s11684-017-0546-3 [DOI] [PubMed] [Google Scholar]
- 100.Chew V, Chen J, Lee D, et al. . Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012;61:427–38. 10.1136/gutjnl-2011-300509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chew V, Tow C, Teo M, et al. . Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol 2010;52:370–9. 10.1016/j.jhep.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 102.Cai L, Zhang Z, Zhou L, et al. . Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 2008;129:428–37. 10.1016/j.clim.2008.08.012 [DOI] [PubMed] [Google Scholar]
- 103.Hoechst B, Voigtlaender T, Ormandy L, et al. . Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009;50:799–807. 10.1002/hep.23054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Subleski JJ, Hall VL, Back TC, et al. . Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Research 2006;66:11005–12. 10.1158/0008-5472.CAN-06-0811 [DOI] [PubMed] [Google Scholar]
- 105.Laso FJ, Almeida J, Torres E, et al. . Chronic alcohol consumption is associated with an increased cytotoxic profile of circulating lymphocytes that may be related with the development of liver injury. Alcohol Clin Exp Res 2010;34:876–85. 10.1111/j.1530-0277.2010.01160.x [DOI] [PubMed] [Google Scholar]
- 106.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology 2008;134:248–58. 10.1053/j.gastro.2007.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mathurin P, Deng QG, Keshavarzian A, et al. . Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology 2000;32:1008–17. 10.1053/jhep.2000.19621 [DOI] [PubMed] [Google Scholar]
- 108.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol 1987;4:8–14. 10.1016/S0168-8278(87)80003-X [DOI] [PubMed] [Google Scholar]
- 109.Machida K, Cheng KT, Sung VM, et al. . Hepatitis C virus induces Toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol 2006;80:866–74. 10.1128/JVI.80.2.866-874.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen CL, Uthaya Kumar DB, Punj V, et al. . Nanog metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab 2016;23:206–19. 10.1016/j.cmet.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sakamoto T, Hara M, Higaki Y, et al. . Influence of alcohol consumption and gene polymorphisms of ADH2 and ALDH2 on hepatocellular carcinoma in a Japanese population. Int J Cancer 2006;118:1501–7. 10.1002/ijc.21505 [DOI] [PubMed] [Google Scholar]
- 112.Guyot E, Sutton A, Rufat P, et al. . PNPLA3 rs738409, hepatocellular carcinoma occurrence and risk model prediction in patients with cirrhosis. J Hepatol 2013;58:312–8. 10.1016/j.jhep.2012.09.036 [DOI] [PubMed] [Google Scholar]
- 113.Nischalke HD, Berger C, Luda C, et al. . The PNPLA3 rs738409 148M/M genotype is a risk factor for liver cancer in alcoholic cirrhosis but shows no or weak association in hepatitis C cirrhosis. PLoS One 2011;6:e27087 10.1371/journal.pone.0027087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trepo E, Guyot E, Ganne-Carrie N, et al. . PNPLA3 (rs738409 C>G) is a common risk variant associated with hepatocellular carcinoma in alcoholic cirrhosis. Hepatology 2012;55:1307–8. 10.1002/hep.25518 [DOI] [PubMed] [Google Scholar]
- 115.Falleti E, Cussigh A, Cmet S, et al. . PNPLA3 rs738409 and TM6SF2 rs58542926 variants increase the risk of hepatocellular carcinoma in alcoholic cirrhosis. Dig Liver Dis 2016;48:69–75. 10.1016/j.dld.2015.09.009 [DOI] [PubMed] [Google Scholar]


