Abstract
Pyoderma gangrenosum (PG)-like ulcerations are a rare clinical manifestation of methylenetetrahydrofolate reductase (MTHFR) mutation. We describe a patient considered to have PG who was treated with long-term high doses of systemic corticosteroids and multiple immunosuppressive agents for several years. In spite of this continuous aggressive therapy, the lesions did not improve but continued to get worse. She developed many significant and catastrophic side effects to them. When referred to our dermatology centre, on investigation, it was discovered that she has an MTHFR mutation. It seemed reasonable to presume that PG-like lesions were related to it. Treatment with a biologically active form of folate—[6S]-5-MTHF—with vitamins B6 and B12 was initiated. It was considered to be beneficial and capable of reducing hyperhomocysteinaemia and endothelial damage consequent from it. Since the institution of this treatment, the patient has begun to show very gradual but slow and incremental improvement.
Keywords: genetics, dermatology
Background
Pyoderma gangrenosum (PG) is a debilitating chronic skin disease with necrotising ulcerations that gradually enlarge and heal very slowly, causing considerable morbidity. An incidence of 3–10 per million per year has been reported.1 Some patients report preceding trauma and others have associated systemic disorders, such as autoimmune diseases, inflammatory bowel diseases, polyarthritis and haematological disorders, among others.1 Currently, there are no laboratory tests specific for the diagnosis of PG. Therefore, it needs to be emphasised and clearly understood that PG is a diagnosis of exclusion in most patients.2 Conventional treatment most frequently consists of systemic corticosteroids and immunosuppressive agents.1 In addition, many patients need to have topical therapy in specialised ‘wound care centres’, wherein special dressings and frequent systemic antibiotics are administered. This frequent and regular combined treatment imposes a financial burden on the family and the healthcare system.
In the literature, there are a few cases of PG-like cutaneous ulcerations that are observed in patients with methylenetetrahydrofolate reductase (MTHFR) mutation.3 4 These patients frequently have high levels of plasma homocysteine (figure 1) and demonstrate endothelial damage.3 In such patients, instead of systemic immunosuppressive therapy, treatment with biologically active form of folate [6S]-5-MTHF,5 not synthetic folic acid, along with vitamins B6 and B12, has produced significant and satisfactory clinical improvement and resolution of lesions.3
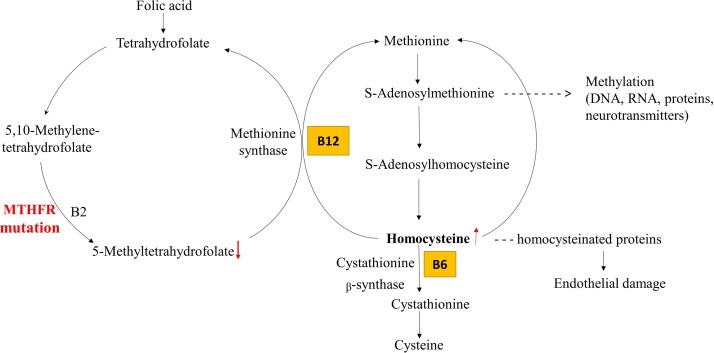
Figure 1.
Simplified scheme of folate-dependent homocysteine metabolism. Due to deficiency of methylenetetrahydrofolate reductase (MTHFR), 5,10-MTHF is not able to convert into 5-MTHF, which leads to the development of hyperhomocysteinaemia. Supplementation with [6S]-5-MTHF is an adequate alternative to folate for decreasing homocysteine concentration.
There are other features of MTHFR mutation that are noteworthy. Some of these are developmental delays, vascular complications and psychiatric abnormalities with increased risk for cardiovascular disease, depression and Alzheimer’s disease.6 Recent meta-analysis studies found a significant correlation between the C667T mutation and an increased risk for oesophageal and gastric cancers.6 The most severely affected patients with MTHFR deficiency do not survive.6
This case is important because the patient has PG-like lesions, a documented MTHFR mutation but does not have any other clinical abnormalities associated with the mutation. She did not respond to conventional immunosuppressive therapy, but demonstrated slow and progressive improvement with the treatment recommended for patients with MTHFR mutation.
Case presentation
We present a case of a 59-year-old Caucasian woman who developed PG-like lesions on the right leg for over 5 years. She was treated with prednisone at 60 mg/day and showed some improvement. As the doses were reduced, the lesion worsened. Hence, the dose of prednisone was increased. Mycophenolate mofetil was added and its dose increased to 3 g daily. The patient demonstrated clinical improvement. Within 3 months, the lesions returned to their original extent and severity in spite of the treatment. She has been treated at the wound centre with soaking, topical agents and special dressings. Whenever pathogenic bacteria were cultured, systemic antibiotics were administered. Hydroxychloroquine (400 mg/day) was added, but significant benefit was not demonstrated. No systemic symptoms were observed. There were no known associated diseases. There was no relevant family history.
Thus, in spite of 5 years of systemic corticosteroids, immunosuppressive and aggressive topical therapy, a complete sustained remission was not achieved. The patient was referred to our dermatology centre for further management.
A physical examination demonstrated a 15×20 cm asymmetric ulceration with irregular violaceous border, serosanguinous discharge, pink and yellow granulation tissue was present on the right leg covering entire shin, while skipping a small area posteriorly (figure 2). There was no oedema around the ulcer. Posterior tibial and dorsalis pedis pulses were palpable bilaterally.
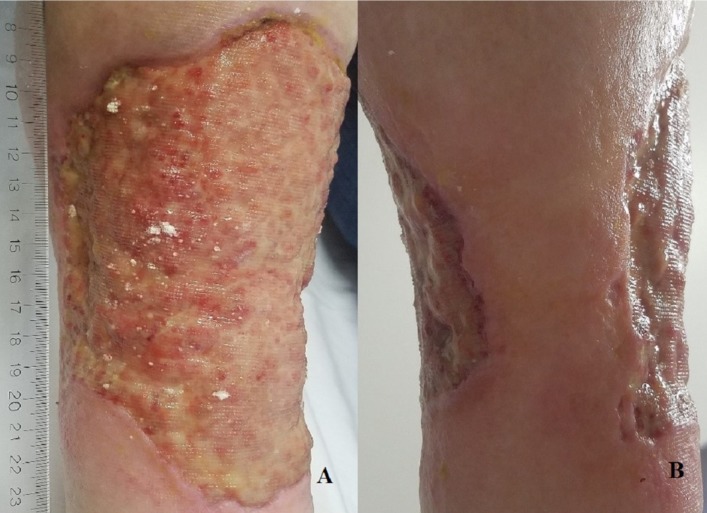
Figure 2.
(A) Anterior view of the ulceration 15×20 cm with violaceous border and yellow–pink granulation base on the right lower extremity. (B) Posterior view of the right lower extremity demonstrating the circumferential extend of the ulceration.
Investigations
Prior to evaluation at our dermatology centre, the patient had three skin biopsies from the peripheral edge of the lesions in April 2013, June 2015 and August 2016. The three biopsies demonstrated similar observations, which were necrotising changes in vessel walls with focal thrombosis, predominantly lymphocytic infiltrate extending from the dermis into subcutaneous tissue. The vasculopathy observed on histology strongly suggested the vascular nature of the lesions. Since three biopsies done over a 3-year period had similar histopathological findings, a skin biopsy was not performed by us at the time of evaluation. The patient’s complete blood count, urinalysis, liver and kidney function tests were within normal limits. The quantitative immunoglobulins, subsets of T and B cells, immune complexes, complement levels, p-ANCA, ANA, cryoglobulin, factor V Leiden, antithrombin III antigen, hepatitis profile and HIV test were normal. Laboratory investigations revealed homocysteinaemia 22.7 μmol/L (range 5–15). Genetic analysis demonstrated compound heterozygous mutation at C677T and A1298C, in the gene for MTHFR.
Differential diagnosis
Differential diagnosis of PG includes infections (bacterial, viral and fungal), malignancies (cutaneous lymphoma and squamous cell carcinoma), vascular ulcerations (venous or arterial disease and antiphospholipid syndrome) and systemic diseases (systemic lupus erythematosus, Behcet’s disease and Wegener’s granulomatosis).7
Treatment
The treatment with methylfolate ([6S]-5-MTHF) at a dose of 400 μg/day, B6 100 mg/day and B12 1000 μg/day was initiated 4 months ago.
Outcome and follow-up
The patient has observed that there is no further deterioration of the lesions. She has noticed the beginning in the reduction of the size of the lesion, which was filling in from the sides and a definitive decrease in the discomfort and pain. However, these improvements could not be photographically documented. The authors anticipate that the full recovery of such a large lesion may take a long time.
Discussion
Genetic analysis of our patient revealed a compound heterozygous mutation at C677T and A1298C, in the gene for MTHFR, which resulted in hyperhomocysteinaemia. Homocysteine can be methylated to produce methionine or be converted to cystathionine during time of excess dietary methionine.6 Homocysteine is converted to cysteine with vitamin B6 as coenzyme. Recent studies demonstrated that hyperhomocysteinaemia results in 3–5-fold increased risk for thrombosis and correlates with 5-fold incidence of chronic venous ulcerations,8 possibly caused by accumulation of homocysteinylated proteins in vessel wall.9 The homocysteinylated proteins are recognised by macrophages, leading to phagocytosis of these cells, resulting in endothelial damage.9 Few patients with MTHFR mutation and PG-like lesions have been previously reported.3 These patients demonstrated healing of cutaneous ulcerations with folic acid, vitamin B6 and vitamin B12,3 with no recurrence at 6 months follow-up.4
On the basis of the biochemical pathways involved, hyperhomocysteinaemia may manifest with neurological dysfunction, vascular lesions, retinal detachment, secondary glaucoma, ectopia lentis, optic atrophy, osteoporosis, and thrombotic and cardiovascular disease, among others.9 [6S]-5-MTHF is a natural and biologically active form of folate.5 Patients with MTHFR mutation need treatment with [6S]-5-MTHF, not synthetic folic acid,5 because excess folic acid intake can cause toxicity in patients with MTHFR mutation due to presence of unmetabolised folic acid.10 Supplementation with [6S]-5-MTHF may be more efficient in reducing the plasma homocysteine levels in patients with MTHFR mutation.5
Since corticosteroids and immunosuppressants are frequently the first-line therapy for patients with PG, it may be advisable to screen patients suspected to have PG for MTHFR mutation. [6S]-5-MTHF with vitamins B6 and B12 may be more beneficial and save patients from the hazardous and consequence of prolonged immunosuppression.5 11 In all likelihood, patients with MTHFR mutations may need these supplements lifelong to prevent hyperhomocysteinaemia and damage resulting from it.
Learning points.
High level of homocysteine characteristic of methylenetetrahydrofolate reductase (MTHFR) mutation can result in thrombosis, which correlates with high incidence of chronic venous ulcerations.
It is advisable to consider MTHFR mutation and hyperhomocysteinaemia in the differential diagnosis of chronic leg ulcerations.
[6S]-5-MTHF is a natural and biologically active form of folate.
Patients with MTHFR mutation need treatment with [6S]-5-MTHF, not synthetic folic acid, since excess folic acid intake can cause toxicity due to presence of unmetabolised folic acid.
Patients with PG-like lesions and MTHFR mutation may need vitamin B6, vitamin B12 and oral [6S]-5-MTHF lifelong to prevent hyperhomocysteinaemia and damage occurring from it.
Footnotes
Contributors: ARA designed the case study, collected the clinical data and laboratory tests, and participated in their analysis and writing of this manuscript. SR participated in the treatment of the patient, acquisition of the clinical data and biopsies, and analysis and interpretation of results. He was also involved in the oversight and review of the writing. YT was involved in ordering the various laboratory tests including genetic testing. She played a critical role in the interpretation of the genetic data, collection of other ancillary tests and information from other physicians involved in the care of the patient. She also played an important role in preparing the figures and writing the manuscript. All the three authors had access to all the material at all times and opportunity to provide their input at every stage.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Wollina U, Tchernev G. Pyoderma gangrenosum: pathogenetic oriented treatment approaches. Wien Med Wochenschr 2014;164:263–73. 10.1007/s10354-014-0285-x [DOI] [PubMed] [Google Scholar]
- 2. Kim YS, Kim HK, Han YS. Importance of accurate diagnosis in pyoderma gangrenosum. Arch Craniofac Surg 2014;15:138–41. 10.7181/acfs.2014.15.3.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. New D, Eaton P, Knable A, et al. The use of B vitamins for cutaneous ulcerations mimicking pyoderma gangrenosum in patients with MTHFR polymorphism. Arch Dermatol 2011;147:450–3. 10.1001/archdermatol.2011.77 [DOI] [PubMed] [Google Scholar]
- 4. Rubio-González B, Castellanos-González M, Alegría-Landa V, et al. Skin ulcers mimicking pyoderma gangrenosum in a patient with MTHFR polymorphism. J Am Acad Dermatol 2012;67:e282–4. 10.1016/j.jaad.2012.06.024 [DOI] [PubMed] [Google Scholar]
- 5. Prinz-Langenohl R, Brämswig S, Tobolski O, et al. [6S]-5-methyltetrahydrofolate increases plasma folate more effectively than folic acid in women with the homozygous or wild-type 677C-->T polymorphism of methylenetetrahydrofolate reductase. Br J Pharmacol 2009;158:2014–21. 10.1111/j.1476-5381.2009.00492.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trimmer EE. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Curr Pharm Des 2013;19:2574–93. 10.2174/1381612811319140008 [DOI] [PubMed] [Google Scholar]
- 7. Brooklyn T, Dunnill G, Probert C. Diagnosis and treatment of pyoderma gangrenosum. BMJ 2006;333:181–4. 10.1136/bmj.333.7560.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grzela T, Bialoszewska A. Genetic risk factors of chronic venous leg ulceration: can molecular screening aid in the prevention of chronic venous insufficiency complications? Mol Med Rep 2010;3:205–11. 10.3892/mmr_000000241 [DOI] [PubMed] [Google Scholar]
- 9. Ramakrishnan S, Sulochana KN, Lakshmi S, et al. Biochemistry of homocysteine in health and diseases. Indian J Biochem Biophys 2006;43:275–83. [PubMed] [Google Scholar]
- 10. Servy E, Menezo Y. The Methylene Tetrahydrofolate Reductase (MTHFR) isoform challenge. High doses of folic acid are not a suitable option compared to 5 Methyltetrahydrofolate treatment. Clin Obstet Gynecol Reprod Med 2017;3 10.15761/COGRM.1000204 [DOI] [Google Scholar]
- 11. Lamers Y, Prinz-Langenohl R, Moser R, et al. Supplementation with [6S]-5-methyltetrahydrofolate or folic acid equally reduces plasma total homocysteine concentrations in healthy women. Am J Clin Nutr 2004;79:473–8. 10.1093/ajcn/79.3.473 [DOI] [PubMed] [Google Scholar]