Abstract
Background
Acute intestinal infections are common conditions causing high morbidity and mortality especially in the young and elderly, resulting in a significant burden on health service resources and the economy. Current National Institute for Health and Care Excellence guidance are fluid and nutritional management; however, this does not reduce the duration of diarrhoea and the challenge of treating diarrhoea itself remains. We investigated the efficacy, tolerability and safety of intestinal adsorbent Enterosgel (polymethylsiloxane polyhydrate) compared with standard care in adults with acute diarrhoea.
Methods
This was a randomised controlled trial enrolling 105 subjects to receive the medical device Enterosgel up to six times daily for up to 8 days with standard care (oral rehydration solution), or standard care alone. The primary endpoint was the duration of diarrhoea (hours) from randomisation to first non-loose stool in the Enterosgel versus control group.
Results
A total of 51 subjects were randomised into the Enterosgel group and 54 into the control group, after excluding missing data, the data from 43 subjects in each group were analysed. Duration of diarrhoea was significantly shorter in the Enterosgel group at 27 hours versus 39 hours in the control group (HR was 1.74 [95% CI 1.06 to 2.87]) (p=0.03). This yielded a number needed to treat value of 5. Enterosgel was well tolerated and safe with no serious adverse events. One serious diarrhoea-related event resulting in hospitalisation was reported in the control group.
Conclusions
Enterosgel treatment was associated with a significant reduction in the duration of diarrhoea in adults with patient-reported acute diarrhoea, compared with standard care. These findings support the role of Enterosgel in acute diarrhoea especially in vulnerable groups where rapid resolution of symptoms is required. Reduction in symptom duration could translate to less healthcare costs and socioeconomic burden.
Trial registration number
Keywords: bacterial infection, diarrhoea, dietary–gastrointestinal infections, infectious diarrhoea
Summary box.
What is already known about this subject?
Acute intestinal infection is a common condition in the UK; there are approximately 17 million cases each year, which result in one million GP consultations.
The treatments recommended for acute diarrhoea by the National Institute for Health and Care Excellence guidance are fluid and nutritional management, with antidiarrhoeal (antimotility) drugs considered unnecessary and only advisable for specified groups.
Oral intestinal adsorbents, such as Enterosgel, offer a safe drug-free alternative to treat the symptoms of diarrhoea but require further exploration.
What are the new findings?
Enterosgel treatment significantly reduced the duration of diarrhoea in adults with patient-reported acute diarrhoea, compared with oral rehydration solution alone.
The first randomised trial of Enterosgel use in adults with acute diarrhoea in the UK.
How might it impact on clinical practice in the foreseeable future?
This study provides data suggesting that Enterosgel can be used to reduce the duration of diarrhoea in acute cases and could be a safe over-the-counter self-treatment option, including vulnerable groups, such as children, where antidiarrhoeal drugs are not recommended. Thereby reducing the impact on healthcare resources in both primary and secondary care.
Introduction
WHO and Unicef claim that there are approximately two billion cases of diarrhoeal disease every year worldwide, and in children under 5 years of age, 1.9 million die from diarrhoea each year, most often in developing countries. Annually, in the UK, there are 17 million cases and 1 million physician consultations attributed to acute infectious diarrhoea.1
Acute infectious diarrhoea is characterised by sudden onset diarrhoea, with or without vomiting. Most cases are due to an enteric virus, but some are caused by bacterial or protozoal infections.2 The illness usually resolves without treatment within days and is often managed at home without seeking professional advice. However, it still poses a significant burden on health service resources and the economy with many patients and parents missing time from work and seeking advice from healthcare professionals in primary or secondary care.1 3 4 Current National Institute for Health and Care Excellence (NICE) guidance is fluid and nutritional management.1 3 However, standard rehydration management does not reduce the duration of diarrhoea5 and the challenge of treating diarrhoea itself remains. There is a need for treatments, which can decrease the duration of illness and reduce attendances to primary care or emergency departments.
Oral intestinal adsorbents, also called enterosorbents, are used in many countries for the treatment of diarrhoea.6 A meta-analysis found that diosmectite, a natural clay intestinal adsorbent, significantly decreased the duration of acute diarrhoea in comparison with placebo, although further studies were recommended.7 Several studies have suggested that Enterosgel, an intestinal adsorbent consisting of an organosilicon compound, polymethylsiloxane polyhydrate, could be effective in the treatment of gastrointestinal disorders.8–12
Enterosgel has been available in Europe as an over-the-counter medical device since 2011. The performance and safety have been documented through clinical studies and postmarketing safety surveillance for over 30 years. However, these studies have suffered from inherent shortcomings with regards to methodological design and reporting.
The aim of this randomised controlled study was to evaluate the efficacy, tolerability and safety of Enterosgel in the treatment of acute diarrhoea in adults in a real-life, primary care setting.
Materials and methods
Study design
This was a randomised, controlled, multicentre pragmatic study to assess efficacy, tolerability and safety of Enterosgel in the treatment of acute diarrhoea in adults compared with standard care. In line with many medical device studies, the use of a placebo is rare, most studies compare to approved therapies. The specific gel-like formulation and organoleptic properties of Enterosgel are difficult to simulate in a placebo, which is both safe and has no impact on the study outcomes. Hence, this was a pragmatic study utilising patient-reported outcomes compared with NICE guidance standard of care.
Ten primary care practices participated to recruit 105 subjects, randomised in 1:1 ratio into two treatment arms for an 8-day treatment phase. Randomisation was completed using a computer-based stratified permuted blocks randomisation tool in the electronic case report form (Sealed Envelope Ltd, London, UK). Patients completed a daily diary to record symptoms and bowel movements. Stool samples collected at screening visit were evaluated for the presence of rotavirus, norovirus and common diarrhoea causing bacteria.
Eligibility criteria
Inclusion criteria included aged 18–70 years with a patient-reported episode of acute diarrhoea, defined as at least three watery stools within the last 48 hours. Subjects with other potential causes of diarrhoea, such as any underlying condition that could cause chronic diarrhoea (gastroduodenal ulcer, ulcerative colitis or Crohn’s disease), were excluded from the study. Additional exclusions included use of antibiotics, blood in stools, known cancer of any localisation, pregnancy and history of intestinal atony or clinically significant allergic reactions.
Interventions
Subjects randomised to the experimental group received Enterosgel (n=51) to be taken according to instructions dependent on stool frequency and consistency and standard care, that is, oral rehydration solution (ORS) for 6–8 days. Dosage was two tablespoons immediately mixed in a glass of water, followed by one tablespoon (or one sachet) 1–6 times per day for 7–8 days (see online supplementary appendix 1 for the complete dosage instructions). The control group (n=54) received standard treatment (ORS) only. Use of antidiarrhoeal medications, such as loperamide, was prohibited during the treatment phase.
bmjgast-2019-000287supp001.docx (12.2KB, docx)
Measures of clinical efficacy and safety
The primary efficacy endpoint was the duration of diarrhoea defined as the time (hours) from randomisation to first non-watery stool (soft or firm) based on bowel movement data, recorded in the patient daily diary. Secondary efficacy endpoints included the duration of diarrhoea defined as the time (hours) from randomisation to last watery stool, percentage of patients with diarrhoea resolved on day 3 (ie, first soft/firm stool recorded on day 0–3), stool frequency defined as average number of stools/day from randomisation to first soft or firm stool and duration (days) of nausea, vomiting, fever defined as ≥38°C and abdominal pain. A subgroup analysis was conducted in subjects whose stool samples were positive for an infectious agent. The tolerance and safety of Enterosgel were assessed via adverse events (AEs) and the percentage of patients with diarrhoea-related complications resulting in hospitalisation, accident and emergency visit, nurse/GP home visit or unscheduled visit to the medical practice.
Sample size calculation
The sample size calculation was based on demonstrating superiority of the interventional group on the primary outcome. Power was calculated for a continuous outcome superiority trial, assuming a SD for the duration of diarrhoea of 32 hours in line with previous studies.13 14 To detect an average decrease of 24 hours in the interventional group compared with the control group, 36 subjects in each group were required for 90% power to demonstrate superiority at 5% significance. Accounting for a combined subject withdrawal of 30%, 52 subjects were required per arm.
Statistical analysis
Analyses were performed using SPSS V.25 and STATA/SE V.14.0. The baseline characteristics of the groups were summarised as mean (SD) and median (IQR) by trial arm.
Primary analysis was conducted on an intention-to-treat basis. For the primary outcome, unadjusted and adjusted (baseline characteristics [age and gender], duration of diarrhoea before randomisation, symptoms, use of medication at baseline and study site), Cox regression was used to compare groups and the time (hours) from randomisation to first non-watery stool (soft or firm). The number needed to treat was calculated.
For the secondary outcomes, continuous data were compared using independent t-tests or Mann-Whitney U test (MW). Analysis of covariance (ANCOVA) was used to adjust for baseline characteristics. Categorical data were compared initially using χ2 or Fisher’s exact test and adjusted logistic regression models with ORs and 95% CIs. A p value of <0.05 was considered to indicate statistical significance. Safety analysis included all subjects who were randomised to the study and was based on the treatment received.
Results
Participants
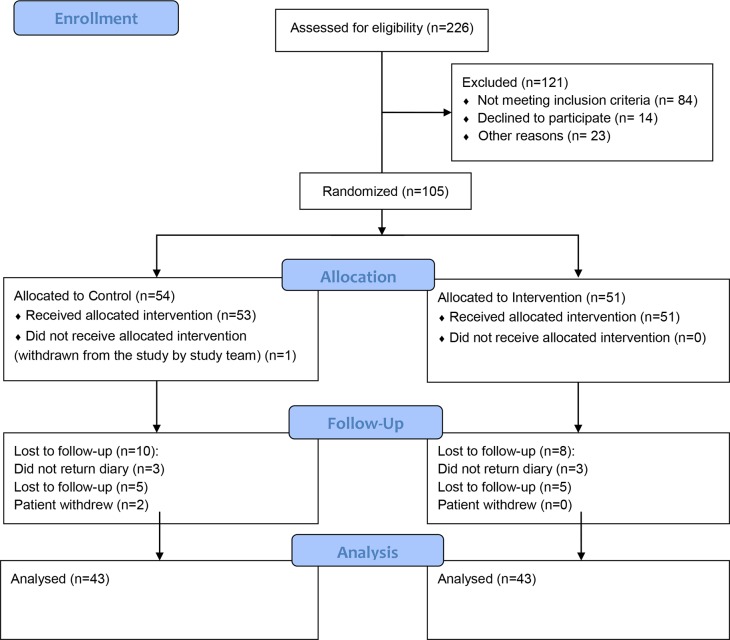
The study CONSORT flow diagram (figure 1) shows the progress through the parallel randomised trial. A total of 105 adults were randomised between January 2017 and June 2018, 51 subjects were treated with Enterosgel and ORS, and 53 subjects in the control group with ORS alone (one subject was withdrawn). Forty-three subjects from each group completed the 8-day study: 8 subjects in the Enterosgel group discontinued and 10 in the control group. The reasons for discontinuation were: subjects did not return diary (n=6), were lost to follow-up (n=10) or withdrew (n=2).
Figure 1.
CONSORT flow diagram of the randomised controlled trial of adults with acute diarrhoea. After 226 were assessed for eligibility, 105 were randomised to either the control group or the intervention group receiving Enterosgel. 86 subjects completed the study and were analysed.
The baseline characteristics were generally comparable between the treatment groups (table 1). The Enterosgel group had longer diarrhoea duration prior to randomisation; however, this was based on a self-reported time so may have recall bias. The perceived difference was determined not to be significantly different between groups (MW, p=0.398) and was not significant in the fully adjusted model (p=0.352) for the primary outcome (table 2). The Enterosgel group had a lower proportion of participants with fever at baseline (16% vs 49%) but a higher proportion of abdominal pain (86% vs 70%) and a higher baseline reported use of medications for the current diarrhoea episode prior to randomisation (21% vs 16%).
Table 1.
Participant demographics and baseline data summarised by trial arm as analysed (ITT)
| Variable | Enterosgel (n=43) | Control (n=43) |
| Age in years, mean (SD) | 44.0 (14.2) | 43.3 (14.5) |
| Age in years, median (IQR) | 44 (32–55) | 46 (31–56) |
| Sex: male, n (%) | 22 (51) | 22 (51) |
| Ethnicity, n (%) | ||
| White | 41 (95) | 39 (91) |
| Asian British | 0 (0) | 3 (7) |
| Black/African/Caribbean/Black British | 0 (0) | 1 (2) |
| Mixed | 1 (2) | 0 (0) |
| Other | 1 (2) | 0 (0) |
| Diarrhoea duration in hours*, mean (SD) | 138.6 (138.3) | 115.9 (139.5) |
| Diarrhoea duration in hours*, median (IQR) | 96 (41–186) | 77 (50–134) |
| Symptoms†, n (%) | ||
| Nausea | 21 (50) | 21 (49) |
| Vomiting | 10 (24) | 12 (28) |
| Fever (≥38°C) | 7 (16) | 21 (49) |
| Abdominal pain | 37 (86) | 30 (70) |
| Potential sources of infection, n (%) | ||
| Travel | 9 (21) | 6 (14) |
| Other contact | 6 (14) | 8 (19) |
| Food poisoning | 7 (17) | 4 (9) |
| Use of medication‡, n (%) | 9 (21) | 7 (16) |
Listing for each group: age, sex, ethnicity, diarrhoea duration prior to randomisation (hours), symptoms (experienced prior to randomisation), potential sources of infection and use of any medication/treatment for the diarrhoea episode prior to randomisation.
*Duration of diarrhoea symptoms prior to randomisation (hours).
†Experienced prior to randomisation.
‡Use of any medication/treatment for the diarrhoea episode prior to randomisation.
ITT, intention-to-treat.
Table 2.
Adjusted Cox regression models for the time (hours) from randomisation to first non-watery stool (soft or firm)
| Fully adjusted model | Reduced model | |||
| Variable | HR (95% CI) | P value | HR (95% CI) | P value |
| Group (Enterosgel) | 1.560 (0.881 to 2.764) | 0.127 | 1.737 (1.055 to 2.859) | 0.030 |
| Age | 1.016 (0.993 to 1.040) | 0.163 | ||
| Gender (female) | 0.756 (0.429 to 1.331) | 0.332 | ||
| Site (10 sites)* | 0.129 | |||
| Duration of diarrhoea† | 1.001 (0.999 to 1.003) | 0.352 | ||
| Use of medications at baseline (No) | 2.253 (1.036 to 4.897) | 0.040 | 1.797 (0.942 to 3.429) | 0.075 |
| Nausea at baseline (No) | 0.598 (0.322 to 1.109) | 0.103 | ||
| Vomiting at baseline (No) | 0.673 (0.351 to 1.291) | 0.234 | ||
| Fever at baseline (No) | 1.887 (0.968 to 3.68) | 0.062 | ||
| Abdominal pain at baseline (No) | 2.535 (1.135 to 5.661) | 0.023 | 1.799 (0.999 to 3.242) | 0.050 |
*Individual site data not shown.
†Duration of diarrhoea symptoms prior to randomisation.
For the analysis of detected stool pathogen, there was missing data for five subjects as they did not return their stool sample for analysis. Pathogen was detected in 20 subjects (7 Enterosgel and 13 control) and no pathogen was detected in 62 subjects (35 Enterosgel and 27 control).
Duration of diarrhoea (to first non-watery stool)
The primary outcome was the duration of diarrhoea defined as the time in hours from subject randomisation to first non-watery stool. It was not possible to derive the duration of diarrhoea for nine participants (nine control and seven Enterosgel) due to non-completion of the diary. Two patients had diary data missing and were censored at their last recorded stool. The mean time from randomisation to first non-watery stool (soft or firm) was shorter in the Enterosgel group 27.1 (se: 4.7) hours versus 38.9 (se: 6.3) hours in the control group (figure 2). The adjusted Cox regression models are presented in table 2 and retaining pain and use of medications at baseline gave an HR of 1.74 (95% CI 1.055 to 2.859, p=0.030), which was statistically significant. This corresponds to a 64% chance of the Enterosgel subject's diarrhoea resolving first.
Figure 2.
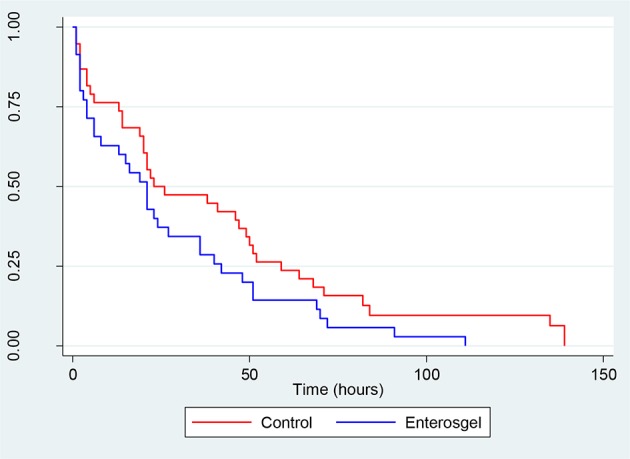
Survival curve showing the time in hours to first non-watery stool (soft or firm) from randomisation by treatment group.
The percentage of subjects in the control group whose diarrhoea had resolved at 12, 24 and 36 hours was 29%, 55% and 57%, compared with Enterosgel group of 39%, 64% and 72%, respectively. The number needed to treat, so that diarrhoea was resolved at 12 hours was estimated at 4.98 (2.79–47.43), at 24 hours as 6.26 (3.80 to 52.69) and at 36 hours as 6.51 (3.97 to 54.34).
Duration of diarrhoea (to last liquid stool)
As a secondary outcome, we analysed the duration of diarrhoea defined as the time in hours from subject randomisation to the last liquid stool. For 77 patients with data, 44 subjects (57%) reported having at least one liquid stool after randomisation; 24/41 (59%) controls and 20/36 (56%) in the Enterosgel group. There was a non-significant shorter diarrhoea duration until last liquid stool in the Enterosgel group (25.3 hours vs 38.4 hours) than the control group (p=0.20). The ANCOVA model, to adjust for baseline characteristics (age and gender), duration of diarrhoea, symptoms and use of medication at baseline and study site, revealed no significant differences between groups (p=0.08). The beta value was 19.44 (SE 10.70), which indicates that the control group had on average 19.44 hours longer until last liquid stool.
Stool frequency
There was no significant difference between groups in the total number of stools until diarrhoea resolved (MW, p=0.84), within the Enterosgel group, the mean number of stools was 3.3 (SD 6.2) and in the control group 3.5 (SD 5.6). Likewise, the mean number of stools per day from randomisation to first soft or firm stool showed no significant difference between groups (MW, p=0.83). The Enterosgel group had 1.8 (SD 2.3) stools per day and the control group 1.3 (1.6) (p=0.83).
Duration of other symptoms
The duration of other symptoms is summarised in table 3. Only subjects who reported to have experienced the symptom prior to randomisation on the day of screening visit were included in this data set. There were no significant differences between groups for the duration of nausea (MW, p=0.85), vomiting (MW, p=0.89), fever (MW, p=0.15) or abdominal pain (MW, p=0.80), where the duration was determined based on the day on which the symptom was last recorded in the patient diary.
Table 3.
Summary statistics for duration (days) from randomisation to the last patient reported symptoms of nausea, vomiting, fever, defined as ≥38°C, and abdominal pain, for each treatment group (n, mean [SD], median [IQR] and min–max)
| Group | N | Mean (SD) | Median (IQR) | Min–Max |
| Nausea | ||||
| Control | 13 | 2.1 (2.5) | 1 (0–3) | 0–8 |
| Enterosgel | 12 | 2.2 (2.4) | 1 (1–4) | 0–8 |
| Vomiting | ||||
| Control | 4 | 0.8 (1.0) | 1 (0–2) | 0–2 |
| Enterosgel | 4 | 0.5 (0.6) | 1 (0–1) | 0–1 |
| Fever | ||||
| Control | 21 | 3.8 (2.0) | 4 (3–5) | 0–7 |
| Enterosgel | 26 | 3.0 (2.6) | 2 (1–5) | 0–8 |
| Abdominal pain | ||||
| Control | 9 | 0.2 (0.4) | 0 (0–0) | 0–1 |
| Enterosgel | 1 | 0.0 (0.0) | 0 (0-0) | 0 |
Stool sample pathogens
A total of 20 subjects had a pathogen detected (7 Enterosgel and 13 control), the HR for the primary outcome was 1.67 (95% CI 0.63 to 4.35, p=0.30) but the sample size was underpowered to derive any clinically meaningful interpretation. The most common pathogen detected was Campylobacter, which was detected in the stools of sixteen subjects, norovirus was detected in four subjects, Giardia lamblia in two subjects and there was one case of Salmonella enterica and Cryptosporidium.
Analysis of AEs
Enterosgel treatment was well tolerated, the total number of AEs reported was higher in the control group (n=18) than in the Enterosgel group (n=13). AEs were reported by 8 control subjects and 10 Enterosgel subjects. Most AEs were mild and similar in the two treatment groups, but the control group reported more moderate AE (3 vs 1). In the Enterosgel group, subjects reported headache (four cases), mucous in nose and throat, constipation (two cases), nausea (two cases), bloating, burping, powdery taste in mouth and indigestion. The control group reported similar AEs, headache (three cases), abdominal pain (four cases), flatulence (one case), fatigue, back pain, fever, dry tongue, and constipation. All AEs could be related to the underlining condition.
One serious AE (SAE) was recorded, this was a diarrhoea-related complication (sepsis) in a control subject, which resulted in hospitalisation (1.9% of patients). No SAEs or diarrhoea-related complications resulting in hospitalisation were reported in the Enterosgel group. No patients in either group had an unscheduled visit to their GP where the reason for visit was an intestinal AE.
Discussion
The study provides evidence that Enterosgel reduces diarrhoea duration in patients with acute diarrhoea of infectious or non-infectious aetiology. Primary outcome of duration of diarrhoea to first non-watery stool showed a statistically significant decrease (p=0.03) in the Enterosgel group, which corresponds to a 64% chance of the Enterosgel subject's diarrhoea resolving first compared with standard therapy alone. The proportion whose diarrhoea had resolved at 12, 24 and 36 hours was comparatively higher in the Enterosgel group at each time interval. As all efficacy and safety data were recorded in patient diaries, the study outcomes were not subject to assessor bias and outcomes were standardised to minimise any bias resulting from the subjects being unblinded. The number needed to treat, for resolution of diarrhoea at 12 hours, was estimated at 4.98, which equates to one extra patient with diarrhoea resolved at 12 hours for every 4.98 patients treated with Enterosgel.
These findings are consistent with previously published data from three studies of acute intestinal infection in children.8–10 Diarrhoea resolved more rapidly in patients treated with Enterosgel compared with standard care8 or antibiotic and standard care.9 In a randomised prospective open comparative study of the intestinal adsorbents, Enterosgel, Diosmectite and Kaolin, there were no statistically significant differences between the groups for the duration of diarrhoea. Other studies have shown that Enterosgel use in newborns with rotavirus infection can reduce the duration of diarrhoea by 1.4 times,11 and in antibiotic-associated diarrhoea in adults, result in a decrease in length of hospital stay.12
The secondary outcome, safety and tolerability, of Enterosgel was confirmed, as there were no SAE or serious adverse device effect in the Enterosgel arm. Furthermore, none of the patients had an unscheduled visit to their General Practitioner (GP) where the reason for visit was an AE related to intestinal infection. There was one control group patient that had a serious adverse diarrhoea-related complication that resulted in hospitalisation. There were five transient mild AEs possibly relating to the investigational device. These included constipation and nausea, known infrequent possible side effects which are currently listed in the Instruction for Use, whereas burping and indigestion have not previously been recorded as side effects and could be linked to the underlying condition.
There were no statistically significant differences between groups for secondary outcomes, that is, the duration of diarrhoea defined as the time until the last liquid stool, percentage of patients with diarrhoea resolved from 12 hours to day 3, stool frequency or duration of nausea, vomiting, fever or abdominal pain. However, there was a trend towards shorter duration in the resolution of diarrhoea in the Enterosgel group (25.3 hours vs 38.4 hours), although this was not statistically significant (p=0.20), possibly due to being underpowered.
Enterosgel is an oral adsorbent, typical of several materials, such as charcoal, clay, silica-based materials and dietary fibres used to treat poisoning and different diseases, such as diarrhoea.15 The therapeutic mode of action of Enterosgel may occur through the adsorption of bacterial toxins known causes of diarrhoea followed by complete removal from the body. In-vitro studies have demonstrated Enterosgel’s capacity for Escherichia coli endotoxin, bacterial enterotoxins Clostridium difficile toxin A and B, and Shigella toxin16 and staphylococcal enterotoxins A and B.17 Other mechanisms may include creation of an adverse environment for pathogenic microorganisms,18 immunocorrection19 and inhibiting viral replication,20 primarily through physical adsorption. A possible benefit of Enterosgel use is in reducing the level of complications of diarrhoea-related bacterial infection. Prospective studies have shown that up to 36% of intestinal infections can be followed by prolonged post-infective irritable bowel syndrome.21 Thus, treatment with Enterosgel could offer a reduction in secondary care admissions, and financial burden on the health service. However, a large-scale study is recommended with health economics evaluation before introducing Enterosgel onto the UK’s drug tariff.
In young children with acute infectious diarrhoea, it is important to provide rehydration therapy. Although ORS can alleviate dehydration, it has no effect on the duration of diarrhoea or abdominal pain.5 Thus, Enterosgel may have an additional benefit, especially in vulnerable groups; for example, children and elderly or immunocompromised/renal dialysis/receiving chemotherapy where more rapid resolution of diarrhoeal symptoms could have a beneficial impact on health.
This study had limitations namely that the median duration of diarrhoea prior to randomisation was 86 hours (min–max: 7–690) as there was no upper limit in the exclusion criteria. The Department of Health guidance for adults with acute diarrhoea is to only visit their GP if they have diarrhoea for more than 7 days. The delay could have impacted on the measured efficacy of the treatment. The secondary outcome diarrhoea duration until the last liquid stool was only available for those patients who had a liquid stool after randomisation and resulted in an underpowered sample size, which may have contributed to the non-significance. These may have been avoided by capturing patients at an earlier stage; for example, from pharmacies, before patients made an appointment with their GP. This study was not placebo-controlled, although it was compared with current recommended standard of care, which can be justified for studies wanting to provide evidence of improved efficacy of a new therapy over an existing therapy.22 Moreover, outcomes were standardised to minimise any bias and based on patient-reported outcomes.
In conclusion, a course of Enterosgel treatment was associated with a significant reduction in the duration of diarrhoea in adults with a patient-reported episode of acute diarrhoea, compared with ORS alone. As these patients were mainly from the working population, the indirect benefit from this rapid resolution of symptoms could translate to prevention of further complications, less healthcare visits with reduced absence from work and a reduction in the economic burden on society. Future work will need to define Enterosgel’s efficacy in acute diarrhoea of infectious or non-infectious aetiology in different healthcare settings and patient populations. This will help to inform our understanding of oral adsorbents and may have wider implications regarding their use as an alternative to antibiotics and in conditions, such as chronic diarrhoea, and other diseases related to the gastrointestinal tract.
Footnotes
Contributors: CAH is the author responsible for drafting the manuscript. PP acted as the Chief Investigator for the study and provided support in the recruitment, assisted with the study design and contributed to the manuscript. EM, AVK and RA assisted with the study design and contributed to the manuscript. VA conducted the statistical analyses and data interpretation and contributed to the manuscript. AK was responsible for the management and coordination of the study, conducted the literature search and oversaw data collection and management. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: CAH and EM are employees of Enteromed Ltd, the sponsor of the study. PP, VA, AK and RA have no conflicts of interest to declare. AVK is an employee of TNK SILMA LLC, manufacturer of Enterosgel in Russia.
Patient consent for publication: Not required.
Ethics approval: The study obtained ethical approval from the North West—Lancaster Research Ethics Committee (16/NW/0818). Study was approved by the Health Research Authority (HRA), UK.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available upon request.
References
- 1.Tam CC, Rodrigues LC, Viviani L, et al. . Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut 2012;61:69–77. 10.1136/gut.2011.238386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones R, Rubin G. Acute diarrhoea in adults. BMJ 2009;338:b1877 10.1136/bmj.b1877 [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence (NICE) Diarrhoea and vomiting in children under 5: diagnosis and management. Clinical guideline [CG84]. Available: https://www.nice.org.uk/guidance/cg84 [Accessed Published date: April 2009. Accessed January 2019]. [PubMed]
- 4.Wheeler JG, Sethi D, Cowden JM, et al. . Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. the infectious intestinal disease Study executive. BMJ 1999;318:1046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh J-S, Hahn W-H, Cho B-S. Recent advances of oral rehydration therapy (ort). Electrolyte Blood Press 2010;8:82–6. 10.5049/EBP.2010.8.2.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopecna E, Mica M, Vlcek J, et al. . Use of medicines among students of high schools in the Czech Republic. Acta Pol Pharm 2015;72:389–96. [PubMed] [Google Scholar]
- 7.Das RR, Sankar J, Naik SS. Efficacy and safety of diosmectite in acute childhood diarrhoea: a meta-analysis. Arch Dis Child 2015;100:704–12. 10.1136/archdischild-2014-307632 [DOI] [PubMed] [Google Scholar]
- 8.Usenko DV, Gorelova EA, Rudyk AV. Application of enterosorbents in the treatment of intestinal infections in children with concomitant atopic dermatitis. Pharmateca 2015;N10:31–5. [Google Scholar]
- 9.Uchaykin VF, Novokshonov AA, Sokolova NV, et al. . Clinical report: study of clinical efficacy of gastrointestinal adsorbent Enterosgel in acute intestinal infections in children. Moscow 2001. [Google Scholar]
- 10.Ruzhentsova TA, Gorelov AV, Ploskireva AA. Choice of an adequate therapy regimen for acute enteric infections in children: results of a randomized trial. Epidemiology and Infectious Diseases 2016;№4:70–4. [Google Scholar]
- 11.Tunda IP. Rotavirus infection in newborns children: clinical performance, treatment and prophylaxy 2007;59:102–6. [Google Scholar]
- 12.Pavlov AI, Fadina Zh. Tactics of management of diarrhoea of non-infectious genesis in hospital. Military Medical Journal 2018;6:49–54. [Google Scholar]
- 13.Gurpreet K, Tee GH, Amal NM, et al. . Incidence and determinants of acute diarrhoea in Malaysia: a population-based study. J Health Popul Nutr 2011;29:103–12. 10.3329/jhpn.v29i2.7814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teran CG, Teran-Escalera CN, Villarroel P. Nitazoxanide vs. probiotics for the treatment of acute rotavirus diarrhea in children: a randomized, single-blind, controlled trial in Bolivian children. Int J Infect Dis 2009;13:518–23. 10.1016/j.ijid.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 15.Cooney DO. Activated Charcoal in Medical Applications. In: Press CRC, ed 2nd, 1995. [Google Scholar]
- 16.Howell CA, Mikhalovsky SV, Markaryan EN, et al. . Investigation of the adsorption capacity of the enterosorbent Enterosgel for a range of bacterial toxins, bile acids and pharmaceutical drugs. Sci Rep 2019;9:5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fluer FS, Kudryavtseva AV, Titarev SI, et al. . A remedy for inhibiting the growth of staphylococci, suppressing the staphylococcal enterotoxins production and removing them from biological substrates. Zhurnal mikrobiologii, epidemiologii I immunobiologii 2017;3:71–7. [Google Scholar]
- 18.Buccigrossi V, Russo C, Guarino A, et al. . Mechanisms of antidiarrhoeal effects by diosmectite in human intestinal cells. Gut Pathog 2017;9 10.1186/s13099-017-0172-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zemskov AM, Zemskov VM, Zoloedov VI. Non pharmacological Immunocorrection. Immunopathology, allergology, infectology 2003;4:12–16. [Google Scholar]
- 20.Konorev MR. Clinical pharmacology of enterosorbents of new generation. Vestnik Pharmacy 2013;4:79–85. [Google Scholar]
- 21.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology 2009;136:1979–88. 10.1053/j.gastro.2009.02.074 [DOI] [PubMed] [Google Scholar]
- 22.Castro M. Placebo versus best-available-therapy Control group in clinical trials for pharmacologic therapies: which is better? Proc Am Thorac Soc 2007;4:570–3. 10.1513/pats.200706-073JK [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2019-000287supp001.docx (12.2KB, docx)