Abstract
A patient with a diagnosis of myelodysplastic syndrome (MDS) with isolated 5q deletion underwent repeat bone marrow biopsy to assess haematological response after 6 months of initial lenalidomide therapy. Subsequent bone marrow biopsies revealed persistent MDS with del(5q) in addition to a small atypical mast cell population with >25% of mast cells with spindle-shaped morphology and immunohistochemistry characteristics consistent with mastocytosis. Molecular testing on the bone marrow was positive for cKIT D816V and the patient was diagnosed with systemic mastocytosis (SM) with an associated haematological neoplasm. MDS with SM is well known to be associated; however, to the best of our knowledge, only one prior case report identifies MDS with del(5q) and associated cKIT D816V positive mastocytosis. While the exact clonal origin of both chromosomal aberrations is unclear, this case illustrates the therapeutic efficacy of lenalidomide in a patient with MDS with del(5q) and rarely associated cKIT positive SM.
Keywords: malignant and benign haematology, haematology (incl blood transfusion), oncology
Background
Mastocytosis is frequently caused by a gain of function somatic D816V mutation in the KIT tyrosine kinase domain. Systemic mastocytosis with an associated haematological neoplasm (SM-AHN) is an abnormal proliferation of mast cells, usually involving the bone marrow or gastrointestinal tract with a concomitant HN.1 2 Common clinical manifestations of SM-AHN include constitutional symptoms, such as fatigue, weight loss, or nausea related to the underlying neoplastic process. Other symptoms, such as flushing, urticaria, pruritus, abdominal pain, diarrhoea and GERD, can less commonly present depending on tissue involvement associated with abnormal mast cell infiltration.
WHO requires the presence of either one major criterion plus one minor criterion or at least three minor criteria for the diagnosis of SM. The major criterion is the presence of multifocal, dense infiltrates of mast cells (≥15 mast cells in aggregates) detected in bone marrow and/or other extracutaneous organs. The four minor criteria include: (1) >25% of mast cells on bone marrow/tissue biopsy are spindle-shaped or have atypical morphology, (2) presence of cKIT D816V mutation in blood, bone marrow or extracutaneous organ, (3) mast cell expression of CD2 and/or CD25 in bone marrow, blood and extracutaneous tissues and (4) persistently elevated serum tryptase above 20 ng/mL. The diagnosis of SM-AHN requires diagnosis of SM based on WHO criteria with the presence of a non-mast cell HN. Of note, the fourth minor criterion involving serum tryptase does not have to be met for diagnosis of SM-AHN if the AHN is an underlying myeloid disorder.3
The pathogenesis and clonal origin of this disorder are currently unclear; however, two proposed mechanisms are prevalent.4 One theory is that the gain of function cKIT mutation and associated myeloid abnormality share a common clonal origin. These cKIT mutated myeloid progenitor stem cells would result in a proliferative advantage compared with other cells in the stem cell compartment and lead to proliferation and differentiation down the mast cell lineage. Evidence to support this theory is best exemplified in SM associated with t(8;21) acute myeloid leukaemia (AML) where investigators demonstrated the presence of the t(8;21) fusion product in both mast cell and AML bone marrow populations with targeted fluorescence in situ hybridisation.5 Another possible mechanism to explain the pathogenesis of SM-AHN involves transformation of a neoplastic progenitor stem cell or ‘multi-hit’ theory of pathogenesis with the cKIT mutation occurring after the original AHN mutation or vice versa. Recent molecular profiling by Jawhar et al 6 lends evidence to this proposed mechanism and that mutations commonly involved in MDS precede the acquisition of KIT D816V and that KIT D816V is acquired as a distinct and late event in multimutated neoplasms.
Myeloid disorders, such as chronic myelomonocytic leukaemia, MDS, myeloproliferative neoplasms and acute myeloid leukaemia are the most frequently associated haematological disorders found in SM-AHN. Despite the frequent association of MDS with SM, only one other case has reported MDS del(5q) with SM and positive cKIT D816V mutational status.7 Isolated 5q deletion is found in approximately 15% of cases of MDS and is associated with a favourable prognosis, with decreased risk of progression to AML compared with other cytogenetic variants of MDS, and treatment with lenalidomide results in complete cytogenetic response (CR) in 50%–60% of patients, with transfusion independence in 70% of cases.8 Gain of even one additional cytogenetic abnormality has been shown to decrease CR to lenalidomide therapy in patients with 5q deletion.9 While current guidelines suggest treatment of the underlying AHN in patients with SM-AHN, this case report identifies the prognostic and therapeutic challenge of SM-MDS with 5q deletion and shows the activity of lenalidomide therapy in this setting.
Case presentation
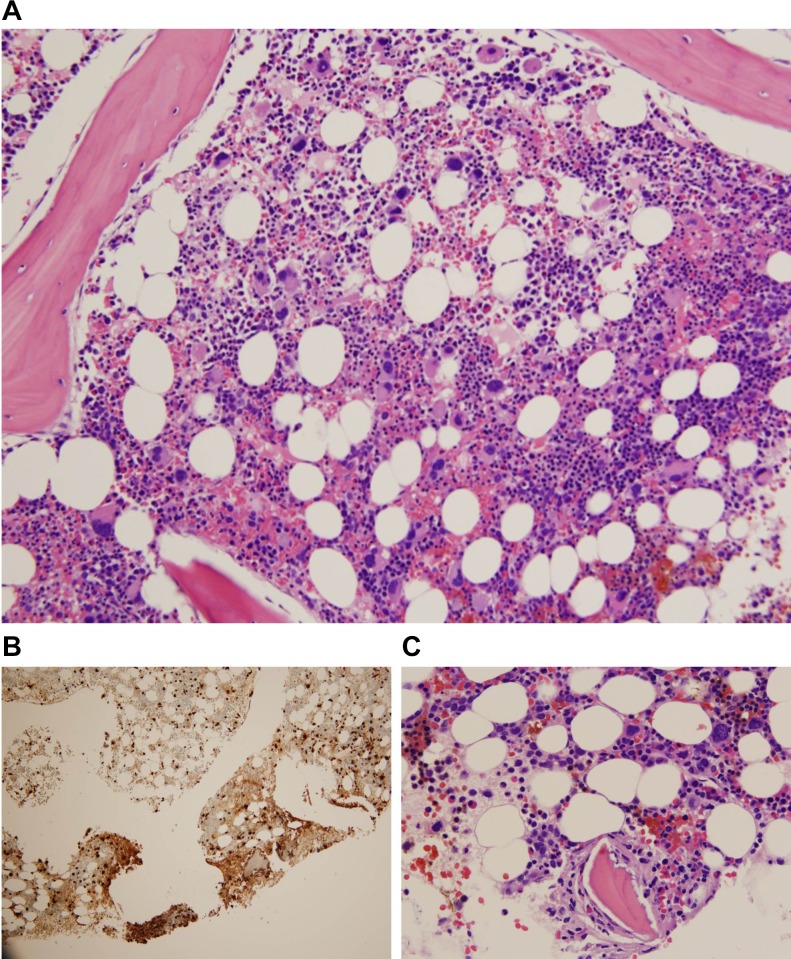
A 45-year-old man presented to our institution for investigation of macrocytic anaemia. Workup for nutritional deficiencies was negative and the patient underwent bone marrow biopsy for persistent macrocytic anaemia. Bone marrow aspirate was remarkable for approximately 2%–5% of blasts with variable morphology including small, agranular and medium-sized granular blasts identified, and an increased number of dysplastic hypolobulated megakaryocytes. Overall cellularity was approximately 30%–40%, M:E ratio 9:1 with reduced erythropoiesis and adequate granulopoiesis without dysplasia. MDS FISH panel was positive for 5q deletion in 136/200 nuclei (68%) as a single abnormality while FISH probes for trisomy 8, 20q12 del, monosomy 7/7 q del, inv(3)(q21q26), 13q del, RB1, MLL, p53 del and monosomy 13/13q del were negative; however, conventional karyotyping was remarkable for trisomy 8 in 2/20 metaphase cells, with 13/20 cells containing the del(5q) karyotype. According to WHO classification of myeloid neoplasms and acute leukaemia, the diagnosis of MDS with isolated 5q deletion is maintained with an additional cytogenetic abnormality, with the exception of monosomy 7 and del(7q).2 Concurrent flow cytometry of bone marrow aspirate was unremarkable for non-Hodgkin’s lymphoma, acute leukaemia or any other abnormalities and the patient was given an initial diagnosis of MDS with isolated 5q deletion. The patient was started on lenalidomide 10 mg daily for a period of 6 months. During the end of the patient’s 6-month period of lenalidomide treatment, a repeat bone marrow biopsy was done to assess the CR to treatment. The repeat bone marrow biopsy showed hypocellular bone marrow, 30% cellularity, with residual MDS with isolated del(5q). FISH analysis was negative for trisomy 8 and positive for 5q deletion in 41/200 (20.5%) of cells demonstrating a CR to lenalidomide; however, a new atypical mast cell population with aberrant CD25 expression on flow cytometry was also identified. cKIT mutation analysis from the bone marrow was sent to another institution and was suspicious for the presence of D816V KIT mutation; however, mutation-positive PCR product was detected at a very low level near the limit of the assay. The patient did not have any clinical manifestations of SM during this time, and the decision was made to monitor the patient clinically. Due to development of mild neutropenia (ANC 2.1>1.2) and thrombocytopenia (126>106), lenalidomide therapy was decreased from 10 mg daily to 10 mg for 3 weeks, followed by 1-week off therapy. A follow-up biopsy approximately 9 months later to re-assess the mast cell population demonstrated hypercellular marrow, persistent MDS with isolated del(5q) and the small aberrant mast cell population representing <1% of total bone marrow cells (figure 1A). This bone marrow aggregate identified atypical spindle-shaped mast cells representing >25% of the mast cell population and immunohistochemistry was remarkable for the expression of mast cell tryptase and CD117, with aberrant co-expression of CD2 and CD25 (figure 1B and C). As there was no multifocal dense mast cell infiltrates in the bone marrow, a definitive diagnosis of SM could not be met based on the major criterion of the WHO 2017 criteria. cKIT mutation analysis was re-sent to another institution and this time positive, meeting three out of four of the minor 2017 WHO criteria for the diagnosis of SM-AHN.
Figure 1.
(A) H&E of bone marrow with megakaryocytic dysplasia characteristic of del 5q. (B) Tryptase stain of bone marrow for mast cells. (C) Probable area of mast cells on H&E stain.
Outcome and follow-up
During subsequent office visits, the patient did not have any systemic symptoms of mastocytosis associated with mast cell mediator release, such as nausea, abdominal pain, diarrhoea, pruritus or anaphylaxis. The patient was referred for a second opinion at another institution and it was determined that due to low systemic involvement of mastocytosis, with lower qPCR levels of mutation-positive D816V KIT mutation, the treatment of the underlying haematological malignancy is appropriate. It was not recommended to start the patient on molecular therapy targeting the D816V KIT mutation. It is recommended that the patient receive anti-mast cell mediator medications if he develops symptoms of SM. Currently, the patient is transfusion independent and continues to be on lenalidomide 5 mg daily.
Discussion
Studies outlining the clinical characteristics and prognostic information of MDS with del(5q) and cKIT D816V mutation are limited. As mentioned previously, to the best of our knowledge there is only one other case report of MDS with del(5q) and cKIT positive SM.7 In a longitudinal cohort study examining prognostic factors in 123 patients’ with SM-AHN, one patient with SM-MDS was reported to have del(5q); however, the cKIT mutation status for this patient was not disclosed.10 In addition, this study reported that leukaemic transformation occurred more frequently in patients with SM-MDS (29%) compared with patients with SM-MPN (11%) and SM-CMML (6%), suggesting that there is an increased risk of leukaemic transformation associated with SM-MDS compared with patients in the other SM-AHN groups.
Current guidelines for the treatment of SM-AHN involve treatment of the underlying AHN as if no SM was present and treatment of the SM component of the disease as if AHN was not present.11 From these recommendations, treatment of the SM component of SM-AHN relies on the subtype of SM. The patient presented in this case would have a subtype of clinically indolent SM, due to the lack of C symptoms or organ dysfunction caused by organ infiltration by neoplastic mast cells. Patients’ with this clinical subclassification of indolent SM requires only mast cell mediator symptom-related treatment. We, therefore, decided to treat the patient with lenalidomide as the preferred treatment of choice for MDS with del(5q).
Management of MDS with del(5q) and cKIT associated mastocytosis presents a diagnostic, prognostic and therapeutic challenge. The different theories in regards to clonal origin of the del(5q) and cKIT D816V mutation brings in to question whether these abnormalities occur in a common myeloid progenitor stem cell, with both mutations contributing to a proliferative advantage in the original clone, or is there a transformation of the myeloid progenitor cell with cKIT mutation occurring after the 5q deletion, or vice versa, in a multimutated neoplastic process? Alternatively, could the SM and AHN components be independent of each other and represent a co-incidental finding? The clonal origin of both mutations carries clinical significance due to the poor prognostic factors associated with MDS del(5q) and additional cytogenetic abnormalities as well as increased risk of leukaemogenic transformation in patients with SM-MDS. Could molecular inhibition of cKIT, via tyrosine kinase inhibitors, such as midostaurin, provide therapeutic benefit if the del(5q) and D816V mutation do in fact share a common clonal origin? Further clinical characterisation including clinical trials and cohort studies of MDS patients with del(5q) and cKIT positive mastocytosis should be pursued in order to establish treatment guidelines and prognostic implications. This case illustrates the therapeutic efficacy of lenalidomide in a patient with MDS del(5q) and rarely associated cKIT positive SM. The patient has remained transfusion independent and monitored clinically for symptoms of SM. Long-term follow-up will be required for the patient presented in this case report to assess response to lenalidomide therapy and monitor for transformation to AML.
Learning points.
Myelodysplastic syndrome (MDS) with 5q deletion and cKIT positive mastocytosis is rarely reported in the medical literature.
Therapy directed at the underlying associated haematological neoplasm with lenalidomide shows efficacy in patients with MDS 5q deletion and cKIT positive systemic mastocytosis.
It is unclear whether the pathogenesis of systemic mastocytosis with an associated haematological neoplasm is a result of cKIT mutation co-occurring with other genetic mutations in a shared clonal cell of origin, transformation of an aberrant myeloid progenitor cell or a co-incidental finding independent of clonal origin.
Further studies are required to identify prognostic implications and therapeutic guidelines for MDS patients with 5q deletion and cKIT positive mastocytosis.
Footnotes
Contributors: DSS, TF and MMC were all involved equally in writing the manuscript. Each author has reviewed the final version of the manuscript and approves it for publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Horny HP, Akin C, Arber D, et al. Mastocytosis : Swerdlow SH, Campo E, Harris NL, et al, (). World Health Organization (WHO) Classification of Tumours. Pathology & Genetics. Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press, 2016. [Google Scholar]
- 2. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–405. 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 3. Pardanani A. Systemic mastocytosis in adults: 2017 update on diagnosis, risk stratification and management. Am J Hematol 2016;91:1146–59. 10.1002/ajh.24553 [DOI] [PubMed] [Google Scholar]
- 4. Stoecker MM, Wang E. Systemic mastocytosis with associated clonal hematologic nonmast cell lineage disease: a clinicopathologic review. Arch Pathol Lab Med 2012;136:832–8. 10.5858/arpa.2011-0325-RS [DOI] [PubMed] [Google Scholar]
- 5. Pullarkat V, Bedell V, Kim Y, et al. Neoplastic mast cells in systemic mastocytosis associated with t(8;21) acute myeloid leukemia are derived from the leukemic clone. Leuk Res 2007;31:261–5. 10.1016/j.leukres.2006.03.006 [DOI] [PubMed] [Google Scholar]
- 6. Jawhar M, Schwaab J, Schnittger S, et al. Molecular profiling of myeloid progenitor cells in multi-mutated advanced systemic mastocytosis identifies KIT D816V as a distinct and late event. Leukemia 2015;29:1115–22. 10.1038/leu.2015.4 [DOI] [PubMed] [Google Scholar]
- 7. Chan N, Tan SY, Opat S, et al. MDS with del(5q) and associated cKIT D816V positive mastocytosis. Pathology 2012;44:492–3. 10.1097/PAT.0b013e3283559d03 [DOI] [PubMed] [Google Scholar]
- 8. Zahid MF, Malik UA, Sohail M, et al. Cytogenetic abnormalities in myelodysplastic syndromes: an overview. Int J Hematol Oncol Stem Cell Res 2017;11:231–9. [PMC free article] [PubMed] [Google Scholar]
- 9. Adès L, Boehrer S, Prebet T, et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood 2009;113:3947–52. 10.1182/blood-2008-08-175778 [DOI] [PubMed] [Google Scholar]
- 10. Pardanani A, Lim KH, Lasho TL, et al. Prognostically relevant breakdown of 123 patients with systemic mastocytosis associated with other myeloid malignancies. Blood 2009;114:3769–72. 10.1182/blood-2009-05-220145 [DOI] [PubMed] [Google Scholar]
- 11. Vaes M, Benghiat FS, Hermine O. Targeted Treatment Options in Mastocytosis. Front Med 2017;4:110 10.3389/fmed.2017.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]