Abstract
Marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) lymphomas of the breast with mammary amyloidosis are exceedingly rare entities. This report describes the case of women with long-standing Sjögren’s syndrome presenting with breast MALT lymphoma and amyloïd light-chain (AL) amyloidosis. Breast microcalcification needle biopsy made the positive diagnosis. This unusual finding should be kept in mind. It emphasises the need for careful clinical examination of nodes and extranodal organs supposedly affected in patients with autoimmune disease.
Keywords: haematology (incl blood transfusion), immunology, breast cancer
Background
Marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) lymphomas involving the breast are rare. Patients with MALT lymphomas have a history of chronic inflammatory or autoimmune diseases.1 Sjögren’s syndrome (SS) is an autoimmune disease of the exocrine gland, involving in particular the salivary and lacrimal glands. An association with lymphoproliferation appears to be one of the major complications for SS patients.2 A study from the Mayo Clinic reviewed 40 cases of amyloidosis of the breast, and demonstrated that systemic amyloidosis accounted for nearly 50% of all cases.3 An unusual mammary MALT lymphoma with AL amyloidosis in the setting of SS is described.
Case presentation
A 65-year-old woman with a 5-year history of primary SS presented with recent fatigue, decreased appetite and unintentional weight loss. SS, first manifested by arthralgias, xerostomia and xerophtalmia, was confirmed by lymphocytic Chisholm grade III infiltration of the accessory salivary gland and positive antinuclear (1/1280) and anti-SSa antibodies. The patient had remained asymptomatic during 5 years of follow-up. In March 2017, she complained of general symptoms. Physical examination revealed parotid and submandibular gland enlargement and dacryoadenitis. Breast palpation showed a 3 cm diameter mass on the upper quadrant of the right breast.
Investigations
Biological investigations showed that cell blood count, liver and renal tests, inflammatory and hemostatic parameters were normal. Besides, tumour serum markers (CA 125, CA 15.3, ACE and NSE) and viral serologies (HIV, HBV, HCV and HTLV1) were negative. Serum protein electrophoresis showed mild elevated gammaglobulinemia and immunofixation electrophoresis detected an IgG k monoclonal gammopathy. The free light channel serum dosage showed elevated free light kappa (267 mg/L, normal range 3.3–19.4 mg/L) and elevated kappa/lambda ratio (8.2, normal range 0.2–1.6). The controlled antinuclear antibodies were negative and a mild cryoglobulinemia level was detected. Complement factor dosage revealed low C3 and C4 factors. Mammography visualised a focus of microcalcifications within the right breast (figure 1). Breast echography showed a 39 mm diameter hyper echogenic mass with irregular borders.
Figure 1.

Focus microcalcifications on mammography.
Breast biopsy concluded to a neoplastic infiltrate composed of by monocytoid medium size lymphoid cells and plasma cells associated with hyperplastic B-cell follicles which occupied the marginal zone at the periphery of B-cell follicle mantle zone (figure 2). Immunohistochemistry markers showed positivity for pancytokeratine staining, CD5, CD20 and CD138. Congo red staining showed reddish orange amyloid material in the interstitium (figure 3), with apple-green birefringence under polarised light (figure 4). Submandibular biopsy confirmed tumorous lymphoid infiltration and AL amyloid deposits. Bone marrow biopsy was normal. Positron emission tomography (PET) CT scan did not show internal lymph node or spleen enlargement.
Figure 2.

Lymphoid cells and plasma cells associated with hyperplastic B-cell follicles in the marginal zone of B-cell follicle mantle zone on breast biopsy.
Figure 3.
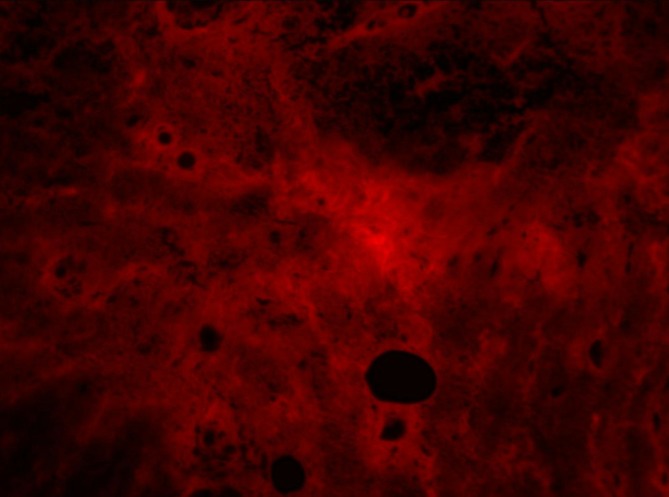
Marked interstitial expansion with amyloid deposits on breast biopsy.
Figure 4.
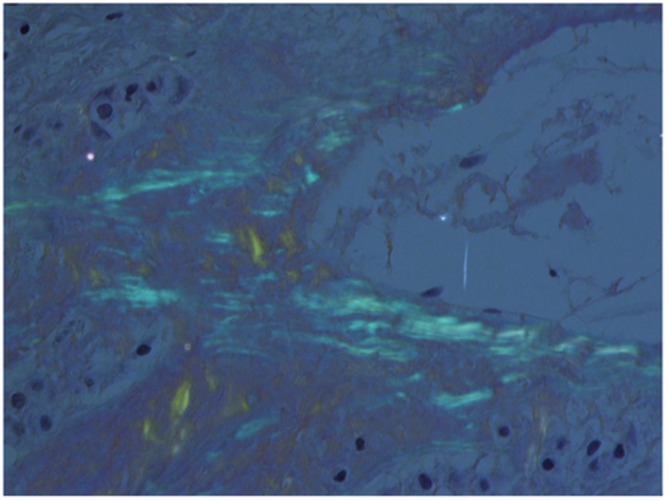
Amyloid material showing apple-green birefringence under polarised light.
Differential diagnosis
The differential diagnoses were as follows:
Breast solid cancer associated with autoimmune disease.
Benign breast tumour, for example, fibroadenoma.
Mammary tuberculosis with pseudoneoplastic presentation.
Treatment
The patient was referred to a haematologist for specific treatment.
Outcome and follow-up
The diagnosis of extra nodular MALT lymphoma associated to systemic AL amyloidosis complicating SS was retained.
Discussion
MALT lymphomas are extranodal B-cell lymphomas and a type of marginal zone lymphoma. The most common sites of MALT lymphomas are the stomach, spleen and the eye/adnexa. MALT lymphomas involving the breast are rare—<0.5% of all breast malignancies—because of the scarcity of mucosa associated lymphoid tissue in the breast. Breast MALT lymphoma is reported in 1.7%–2.2% of extranodal breast lymphoma.4 In our case, MALT lymphoma coexisted in breast and submandibular and parotid glands. Most cases arise primarily in the breast, but secondary involvement of the breast by MALT lymphomas arising at other sites can be seen.5 SS patients have a 1000-fold increased risk of MALT lymphoma of the parotid gland. Other sites of MALT lymphoma involvement in SS include the orbital adnexa, nasopharynx, thyroid, stomach and lung.2 6 The originality of this observation is to describe a patient with SS presenting with breast microcalcifications revealing MALT lymphoma as well as amyloid deposits found in submandibular gland. The MALT lymphoma in patients with SS patients is exceedingly rare and thus its incidence is not established. There is a progressive polyclonal B and T cell infiltration of the exocrine glands in SS patients. It is demonstrated that the progression of this pathologic lesion to MALT lymphoma is characterised by the expansion of the population of centrocyte-like B-cells with an aberrant expression of CCD5 and CD43 by the infiltrating B-cell population. The underlying mechanisms by which SS predisposes some patients to develop lymphomas are matters of ongoing study.7 Several studies focused on predictors of lymphoproliferation in SS. Positive cryoglobulinemia, hypocomplementemia, autoantibodies negativation and CD4+ lymphocytopenia were found to be a strong risk factor for developing lymphomas in two distinct register study.8 9 These parameters were screened in our patient and predicted the underlined lymphoma. In the current case, systematic nodal and extranodal physical examination showed a 3 cm diameter mass on the upper quadrant of the right breast and biopsy led to the diagnosis. It is largely known that patients with SS are highly exposed to develop B-cell lymphomas, but recent register showed that these patients are also at risk of developing some non-haematological cancers (thyroid, oral cavity and stomach).9 The amyloid deposits can either be isolated (amyloid tumour) or associated with plasma cell dyscrasia, connective tissue diseases, carcinoma or lymphoma as illustrated in our case.9 10 In a retrospective study of 40 cases of breast amyloidosis, Said et al indicated that it was of AL type in the majority of cases and associated with hematologic malignancy.3 Thus, a special awareness is needed in case of fortuitous pathological findings of amyloidosis of the breast to rule out lymphoma.
Learning points.
This is the first report of a case of mucosa-associated lymphoid tissue lymphoma with systemic amyloidosis with occurred in a women with long standing Sjögren’s syndrome.
Regular physical assessment is required with particular attention to lymph nodes and extra nodal organs supposedly affected in patients with long-standing autoimmune disorders.
Persistent salivary gland enlargement, particularly when unilateral or asymmetric, should be investigated for lymphomas.
Footnotes
Contributors: NB, SB and AB: discussed this complex clinical case. We illustrated its complexity in the investigation part and discussed its scarcity in another part. NB: wrote the case with input of all authors. SB: performed the interpretation of the different biopsies. AB: supervised the management of the patient and the redaction of the report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Kambouchner M, et al. Low grade marginal zone B cell lymphoma of the breast associated with localised amyloidosis and corpora amylacea in a woman with long standing primary Sjogren’s syndrome. J Clin Pathol 2003;56:74–7. 10.1136/jcp.56.1.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood 2008;111:4029–38. 10.1182/blood-2007-10-119974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Said SM, Reynolds C, Jimenez RE, et al. Amyloidosis of the breast: predominantly AL type and over half have concurrent breast hematologic disorders. Mod Pathol 2013;26:232–8. 10.1038/modpathol.2012.167 [DOI] [PubMed] [Google Scholar]
- 4. Hissourou Iii M, Zia SY, Alqatari M, et al. Primary MALT lymphoma of the breast treated with definitive radiation. Case Rep Hematol 2016;2016:1–6. 10.1155/2016/1831792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jain MF, Khandekar SL, Mahadani JW, et al. Report of a case of primary breast lymphoma highlighting the importance of fine needle aspiration cytology as an initial diagnostic tool. J Cytol 2015;32:127–9. 10.4103/0970-9371.160570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Theander E, Henriksson G, Ljungberg O, et al. Lymphoma and other malignancies in primary Sjögren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis 2006;65:796–803. 10.1136/ard.2005.041186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nocturne G, Mariette X. Sjögren Syndrome-associated lymphomas: an update on pathogenesis and management. Br J Haematol 2015;168:317–27. 10.1111/bjh.13192 [DOI] [PubMed] [Google Scholar]
- 8. Nishishinya MB, Pereda CA, Muñoz-Fernández S, et al. Identification of lymphoma predictors in patients with primary Sjögren’s syndrome: a systematic literature review and meta-analysis. Rheumatol Int 2015;35:17–26. 10.1007/s00296-014-3051-x [DOI] [PubMed] [Google Scholar]
- 9. Fernandez BB, Hernandez FJ. Amyloid tumor of the breast. Arch Pathol 1973;95:102–5. [PubMed] [Google Scholar]
- 10. Brito-Zerón P, Kostov B, Fraile G, et al. Characterization and risk estimate of cancer in patients with primary Sjögren syndrome. J Hematol Oncol 2017;10:90 10.1186/s13045-017-0464-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


