Abstract
Recurrence of herpes simplex virus (HSV) keratitis is a problem of keratoplasty and the prognosis is often poor in spite of oral acyclovir (ACV) prophylaxis. This 64-year-old woman was a known case of recurrent HSV endotheliitis with irreversible corneal oedema in the left eye for 2 years. She underwent Descemet membrane endothelial keratoplasty with intraocular lens implantation under perioperative oral ACV and prednisolone. After 4 weeks, her cornea cleared with the best-corrected vision of 6/9. After 2.5 months, she presented with sudden photophobia and visual loss. An increasing focal endothelial lesion was noticed even after oral ACV. Suspecting fungal interface infection, anterior chamber tap was done for PCR for panfungal and viruses. It was only positive for HSV. Oral ACV was changed to oral valacyclovir. The patient responded dramatically within 2 weeks, and after 12 weeks, the lesion disappeared completely, leaving behind a faint scar with 6/9 p vision. Oral valacyclovir, a prodrug of ACV, may work better than oral ACV.
Keywords: anterior chamber, herpes simplex virus, transplantation
Background
There have been increasing evidence of viral corneal endotheliitis from the Asian countries including India, mainly due to herpes simplex virus (HSV) and cytomegalovirus (CMV).1 2 HSV endotheliitis is more common and the medical management remains the mainstay of treatment. However, in some eyes, persistent irreversible corneal oedema often ensues because of the cytopathic effect of inflammation on corneal endothelial cells.3 Thereby, these cases require corneal transplantations. Currently, there is limited information regarding the outcomes of endothelial keratoplasty in eyes with irreversible corneal decompensation secondary to viral endotheliitis.4 5 Descemet membrane endothelial keratoplasty (DMEK) has recently been considered as a promising and evolving endothelial keratoplasty technique for the management of corneal oedema due to endothelial failure.6 7 Recently, there is a case report which describes the outcomes of DMEK-triple in corneal decompensation secondary to HSV endotheliitis. The patient was treated with perioperative oral acyclovir (ACV) in full doses and then twice daily for 1 year, and without any recurrence till 1 year follow-up.8
Here, we report a recurrence of HSV endotheliitis after DMEK-triple procedure in a patient with HSV endotheliitis-induced cornea oedema that was mimicking fungal interface infection.
Case presentation
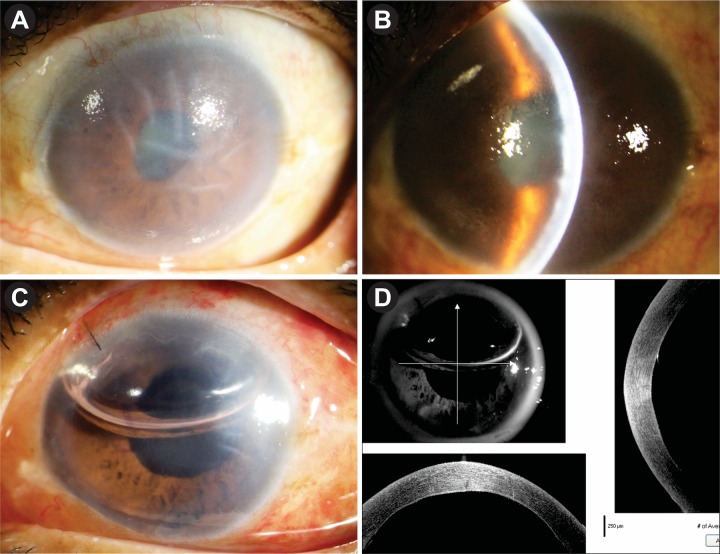
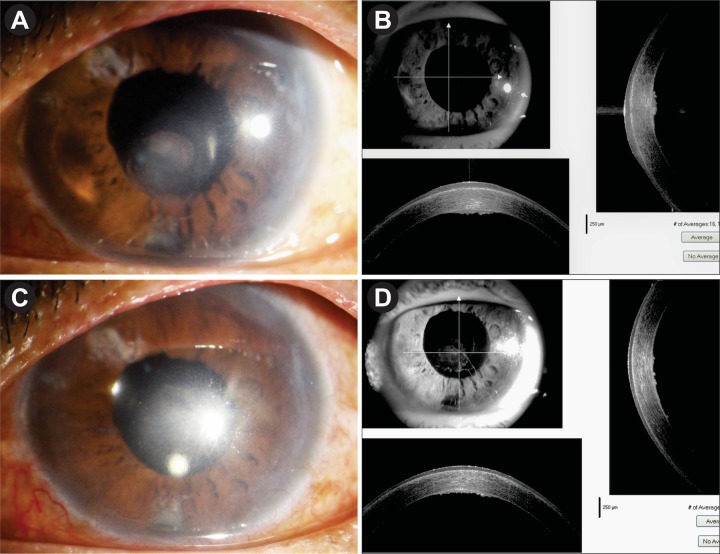
A 64-year-old one-eyed woman was under our treatment for the last 2 years with the diagnosis of recurrent herpes simplex endotheliitis in the left eye (LE) which was confirmed by aqueous polymerase chain reaction (PCR) for HSV. She was treated intermittently with topical ACV and topical prednisolone acetate. In the past, she had a similar attack of recurrent corneal oedema and anterior uveitis for 3 years and treated elsewhere in a similar way. Her intraocular pressure (IOP) in the LE was within normal limits in all past records. She lost her right eye (RE) in early childhood due to injury and was phthisical. During the course of treatment, her corneal oedema increased and did not clear with medical management. After a quiescent period of 6 months, we planned for DMEK-triple procedure. Aqueous tap for viral PCR was done prior to surgery and it was negative. The preoperative clinical picture is shown in figure 1A and B. Her best-corrected spectacle visual acuity (BSCVA) in the LE was only hand movement close to face. There was gross corneal oedema, shallow anterior chamber, old keratic precipitates, small pupil, posterior synechia, moderate cataract which was difficult to assess, and IOP was normal. We investigated her for anterior segment optical coherence tomography (ASOCT) and biometry for IOL power calculation. Ultrasonography B scan was normal. She was a non-hypertensive, non-diabetic, otherwise healthy immune-competent individual. There was no other systemic problem.
Figure 1.
(A,B) Preoperative appearance. (C) Postoperative appearance on day 1. (D) ASOCT appearance on day 1. ASOCT, anterior segment optical coherence tomography.
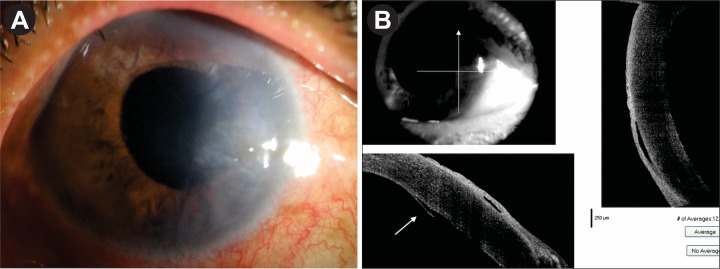
She underwent DMEK-triple procedure with explained prognosis under cover of oral ACV 400 mg five times daily and oral prednisolone 40 mg/day for 7 days. Postoperatively, she received oral prednisolone 40 mg in tapering doses for 4 weeks; oral ACV 400 mg five times for 1 week, then twice daily to continue; prednisolone eye drop six times daily to continue; moxifloxacin eye drop four times daily for 10 days and atropine eye drop two times daily for 10 days. On day 1, she was doing fine with well-attached graft, BSCVA was 6/36, and ASOCT confirmed the attachment of DM graft (figure 1C,D). After 1 week, her BSCVA was 6/18; cornea was clear except peripheral detachment on the temporal side as evident by ASOCT (figure 2A,B). After 1 month, the graft was attached completely with the clear cornea. BSCVA was 6/9 and IOP was 18 mm Hg. She was continued with oral ACV 400 mg twice daily for the next 6 months. Prednisolone eye drop was continued four times daily and she was asked to report after 2 months.
Figure 2.
(A) Postoperative appearance on day 7. Mild corneal oedema on the temporal side. (B) ASOCT image on day 7. Corresponding small peripheral DM detachment (white arrow). ASOCT, anterior segment optical coherence tomography; DM, Descemet membrane.
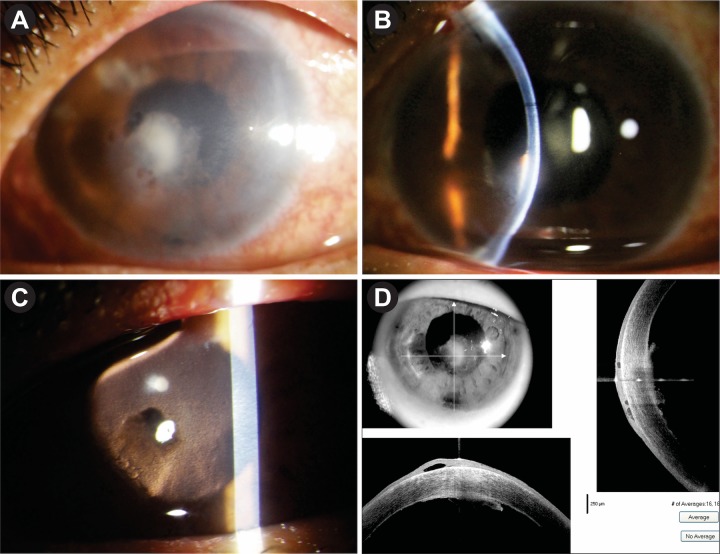
After 2.5 months, before her scheduled visit, she presented with sudden dimness of vision in the LE with photophobia. On examination, her BSCVA was 6/18. Slit-lamp examination showed a small round focal infiltrate in the paracentral region at the level of DM. ASOCT showed focal elevated lesion with epithelial hypertrophy at the corresponding area (figure 3A,B). ACV eye ointment five times daily was added, and prednisolone eye drop was increased to eight times. After 2 weeks, the lesion increased in size (figure 3C,D); and as the picture was then confusing with fungal interface infection, we planned for immediate aqueous tap for PCR for HSV and CMV and also for panfungal. ACV eye ointment was stopped. PCR report was negative for panfungal but positive for HSV. Oral ACV was restarted 400 mg five times daily for 14 days and oral prednisolone was added 1 mg/kg/day; prednisolone eye drop was increased to one hourly dose.
Figure 3.
(A,B) Focal endothelial lesion with corresponding ASOCT image after 2.5 months. (C,D) Focal lesion increasing in size and density with ASOCT image after 2 weeks. ASOCT, anterior segment optical coherence tomography
After 2 weeks, there was worsening of the lesion with yellowish appearance, and the clinical picture looked like fungal interface infection (figure 4A–D). At this point, we stopped oral ACV and started her on oral valacyclovir (VCV) 500 mg twice daily along with oral and topical prednisolone. After 2 weeks, the picture improved dramatically with a trace of endothelial haze which was also evident with ASOCT picture (figure 5A,B). Oral VCV (500 mg) once daily dose was continued along with tapering doses of topical prednisolone. Oral prednisolone was stopped after 4 weeks. After 3 months of oral VCV, her BSCVA improved to 6/12. Slit-lamp examination showed cornea much clear with localised stromal haze, but without any endothelial plaque (figure 5C,D), IOP was normal. Oral VCV was continued until 12 months. Prednisolone eye drop was continued as a once daily dose.
Figure 4.
(A) Focal lesion increasing more. (B) In slit section. (C) In retroillumination. (D) In ASOCT image, all mimicking fungal interface infection. ASOCT, anterior segment optical coherence tomography.
Figure 5.
(A,B) Image and ASOCT appearance 1 week after administration of tab valacyclovir. (C,D) Appearance of the cornea and ASOCT image, 3 months after tab valacyclovir therapy. ASOCT, anterior segment optical coherence tomography.
Investigations
AC tap for PCR: though HSV endotheliitis is a clinical diagnosis, aqueous tap PCR for the virus was the most important investigation in this case as she is one-eyed, and we wanted to treat her by DMEK-triple procedure. Second, when the recurrence happened and it was mimicking like fungal interface infection, PCR of aqueous was important to exclude fungal infection and to confirm that the lesion was indeed due to HSV recurrence.
ASOCT: it is important in this case preoperatively to check corneal oedema and postoperatively to check graft attachment, and also helped in this case to see the level of the lesion during recurrence and its resolution.
Kidney function and liver function tests: repeated every 3 months to exclude renal or hepatic toxicity.
Differential diagnosis
During recurrence, the most important differential diagnosis in this case was fungal interface infection following DMEK-triple. Primarily, because there are reports of fungal interface infection following DMEK procedure9 and fungal infections are very common in Gangetic West Bengal, India.10
Outcome and follow-up
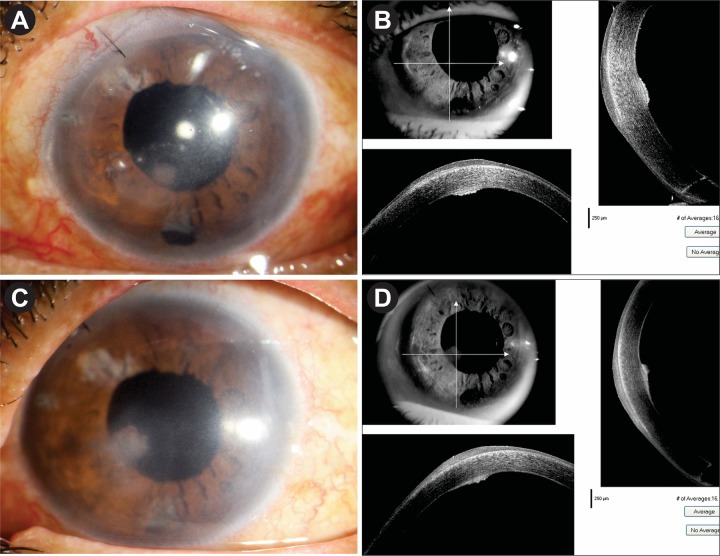
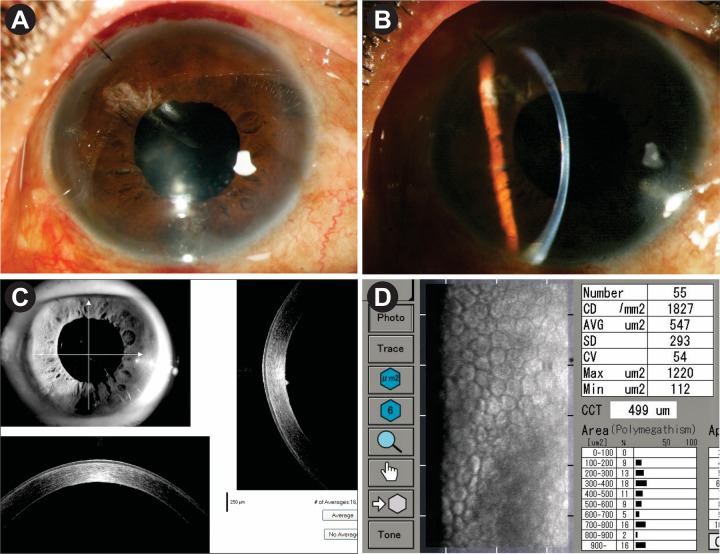
The patient was followed up after 3 months, then at 6 monthly intervals. After 18 months, her BSCVA is 6/9 p. The cornea is clear with small localised area of scarring at the level of DM which is shown in ASOCT image (figure 6A–C). Specular microscopic appearance after 18 months showed endothelial cell density of 1827/ mm2 with patchy areas of cell loss (figure 6D). She did not have a further recurrence during this period.
Figure 6.
(A–C) Appearance after 1.5 years. Mild paracentral haze at the level of Descemet membrane as evident by slit section and ASOCT. (D) Specular microscopic appearance after 1 year. Endothelial cell density: 1827/mm2 with patchy areas of cell loss. ASOCT, anterior segment optical coherence tomography.
Discussion
The overall recurrence of HSV keratitis following penetrating keratoplasty is 25% of the transplants in the first year and in 44% during the first 2 years.11 Ghosh et al reported a recurrence of herpetic keratitis after penetrating keratoplasty was 12% when the patients received prophylactic oral ACV for 6 months.12 Recent Cochrane review showed that compared with placebo or to no treatment, oral ACV may reduce the risk of recurrence of herpetic keratitis in the first 12 months in eyes that have undergone corneal graft surgery.13 Ang et al reported a recurrence of corneal endotheliitis in 5 eyes (out of 32 eyes) following endothelial keratoplasty (Descemet stripping automated endothelial keratoplasty (DSAEK) or DMEK), and CMV-positive patients had a higher rate of recurrence of endotheliitis within 1 year after corneal transplantation, compared with CMV-negative eyes (60% vs 7.4%).4 Recently, there is one case report of HSV endotheliitis recurrence following DMEK-triple procedure and it appeared in the early postoperative period and the patient presented with classical signs. But, this patient did not receive oral antivirals perioperatively. Confirmation of HSV-1 was done by aqueous PCR and the patient responded with oral ACV therapy.14
In our case, the recurrence of endotheliitis happened in spite of perioperative coverage with oral ACV in appropriate doses. It presented after 2.5 months while the patient was on a maintenance dose of oral ACV, and the clinical picture was atypical and was mimicking fungal interface infection of DMEK graft. AC tap for viral and panfungal PCR was done to establish recurrence of HSV endotheliitis and to exclude fungal aetiology. In our case, PCR confirmed the presence of HSV in the aqueous. And in spite of increasing dose of ACV, the clinical course was worsening. At that point, we changed oral antiviral from ACV to VCV and the response was dramatic within 2 weeks, and we continued the same treatment for 12 months. VCV, a prodrug, is the l-valyl ester of ACV, and currently thought to be more effective than ACV.3 The conversion of VCV to ACV leads to an increased ACV bioavailability by three-fold to five-fold compared with oral ACV.15 Oral VCV 500 mg once daily as a maintenance dose is well tolerated and without much systemic toxicity. Our patient did not develop any systemic toxicity.
In conclusion, HSV endotheliitis recurrence following DMEK may happen even after perioperative oral ACV prophylaxis. That may present the atypical clinical picture as focal endotheliitis, which may mimic as fungal interface infection. Viral PCR of aqueous is helpful to confirm the diagnosis. Instead of oral ACV, oral VCV must be used both prophylactically and therapeutically.
Patient’s perspective.
My one-eyed auntie was doing fine after this difficult surgery. The doctor told earlier that she might have a similar recurrence in the future. But, when the recurrence happened our doctor told that this is a different type of recurrence, and she needs more test to confirm the diagnose. The picture was worsening and the doctor was more worried as she is one-eyed. The doctor changed the medicine which was little costlier, and we cooperated fully with the treating doctor. After one and half years my auntie is doing fine even after stopping the costlier medicine.
Learning points.
HSV endotheliitis may recur in Descemet membrane endothelial keratoplasty graft in spite of perioperative oral acyclovir (ACV) prophylaxis.
It may present as atypical focal endotheliitis which may mimic fungal interface infection.
Early anterior chamber aqueous tap for viral and fungal PCR is important to confirm the diagnosis.
Valacyclovir, a prodrug of ACV, may work better than oral ACV because of increased bioavailability.
Footnotes
Contributors: SKB: surgery and medical management part of this patient. SB: manuscript preparation and literature search.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Next of kin consent obtained.
References
- 1. Suzuki T, Ohashi Y. Corneal endotheliitis. Semin Ophthalmol 2008;23:235–40. 10.1080/08820530802111010 [DOI] [PubMed] [Google Scholar]
- 2. Inoue Y. Review of clinical and basic approaches to corneal endotheliitis. Cornea 2014;33(Suppl):S3–S8. 10.1097/ICO.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 3. Tsatsos M, MacGregor C, Athanasiadis I, et al. . Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin Exp Ophthalmol 2016;44:824–37. 10.1111/ceo.12785 [DOI] [PubMed] [Google Scholar]
- 4. Ang M, Sng CC, Chee SP, et al. . Outcomes of corneal transplantation for irreversible corneal decompensation secondary to corneal endotheliitis in Asian eyes. Am J Ophthalmol 2013;156:260–6. 10.1016/j.ajo.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 5. Fernández López E, Chan E. Descemet Stripping Automated Endothelial Keratoplasty Outcomes in Patients with Cytomegalovirus Endotheliitis. Cornea 2017;36:108–12. 10.1097/ICO.0000000000001028 [DOI] [PubMed] [Google Scholar]
- 6. Melles GR, Ong TS, Ververs B, et al. . Preliminary clinical results of Descemet membrane endothelial keratoplasty. Am J Ophthalmol 2008;145:222–7. 10.1016/j.ajo.2007.09.021 [DOI] [PubMed] [Google Scholar]
- 7. Tourtas T, Laaser K, Bachmann BO, et al. . Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty. Am J Ophthalmol 2012;153:1082–90. 10.1016/j.ajo.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 8. Asi F, Milioti G, Seitz B. Descemet membrane endothelial keratoplasty for corneal decompensation caused by herpes simplex virus endotheliitis. J Cat Refract Surg 2018;44:106–8. 10.1016/j.jcrs.2017.10.046 [DOI] [PubMed] [Google Scholar]
- 9. Augustin VA, Weller JM, Kruse FE, et al. . Fungal Interface Keratitis After Descemet Membrane Endothelial Keratoplasty. Cornea 2018;37:1366–9. 10.1097/ICO.0000000000001727 [DOI] [PubMed] [Google Scholar]
- 10. Basak SK, Basak S, Mohanta A, et al. . Epidemiological and microbiological diagnosis of suppurative keratitis in Gangetic West Bengal, Eastern India. Indian J Ophthalmol 2005;53:17–22. 10.4103/0301-4738.15280 [DOI] [PubMed] [Google Scholar]
- 11. Lomholt JA, Baggesen K, Ehlers N. Recurrence and rejection rates following corneal transplantation for herpes simplex keratitis. Acta Ophthalmol Scand 1995;73:29–32. 10.1111/j.1600-0420.1995.tb00008.x [DOI] [PubMed] [Google Scholar]
- 12. Ghosh S, Jhanji V, Lamoureux E, et al. . Acyclovir therapy in prevention of recurrent herpetic keratitis following penetrating keratoplasty. Am J Ophthalmol 2008;145:198–202. 10.1016/j.ajo.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 13. Bhatt UK, Abdul Karim MN, Prydal JI, et al. . Oral antivirals for preventing recurrent herpes simplex keratitis in people with corneal grafts. Cochrane Database Syst Rev 2016;11:CD007824 10.1002/14651858.CD007824.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zarei-Ghanavati S, Alizadeh R, Yoo SH. Herpes Simplex Virus Endotheliitis following Descemet’s Membrane Endothelial Keratoplasty. J Ophthalmic Vis Res 2015;10:184–6. 10.4103/2008-322X.163764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higaki S, Itahashi M, Deai T, et al. . Effect of oral valaciclovir on herpetic keratitis. Cornea 2006;25:S64–S67. 10.1097/01.ico.0000247216.08886.9d [DOI] [PubMed] [Google Scholar]