Abstract
A 47-year-old previously healthy man presented with acute moderate flank pain. Evaluation revealed left renal cell carcinoma, with inferior vena cava tumour thrombus invasion. Patient had no significant history or risk factors to pre-dispose him to genitourinary cancers. Surgery was deemed to not be appropriate due to distant metastases, but patient received targeted molecular therapy and immunotherapy with striking regression of the thrombus.
Keywords: cancer intervention, immunological products and vaccines, urology, urological surgery
Background
Renal cell carcinoma (RCC) is a common cancer, believed to make up 2%–3% of all cancers worldwide.1Inferior vena cava (IVC) tumour thrombus formation is unique to RCC and radical surgery for those with symptomatic thrombi or no distant metastasis has been commonplace since 1970.2 The recent arrival of new immunologic agents has shown promise for patients with RCC, as adjuvant, neoadjuvant, and first line treatment. This case report describes a patient with an extensive IVC tumour thrombus who had unprecedented positive results with immunotherapy.
Case presentation
The 47-year-old man presented himself in May 2016 to his local emergency room (ER) with acute flank pain. The pateint had no prior medical or surgical history. He had no history of smoking or chemical exposure. His physical examination was negative for any findings of palpable flank mass or varicoceles. On undergoing CT imaging, he was found to have a large left kidney mass, consisting of two confluent masses measuring 9.1 and 14.2 cm, respectively, five lung nodules bilaterally (the largest on the right measuring 2.5 cm) and a 2.7 cm retroperitoneal lymph node (figure 1). After referral to Medical Oncology, the patient underwent bronchial biopsy of the right hilar mass which revealed high-grade clear cell RCC.
Figure 1.
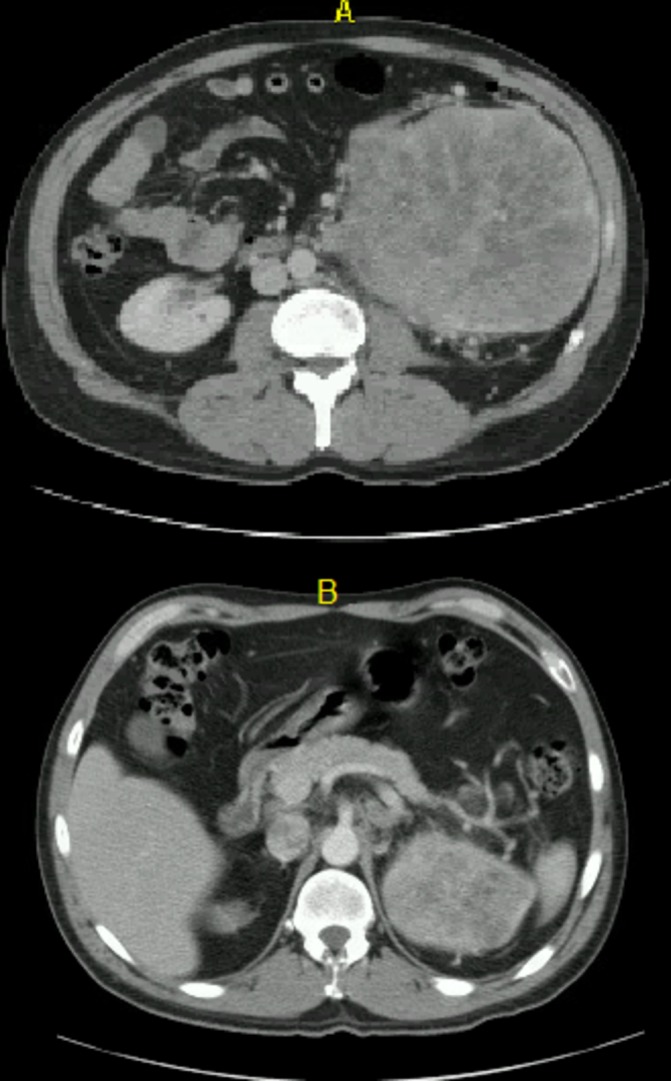
Axial views of left renal mass in May 2016 at L3–L4 spinal level (A) and T11–T12 (B).
Positron Emission Tomography (PET) CT scan in June 2016 demonstrated an IVC tumour thrombus that extended just distal to the intrahepatic IVC. The patient was evaluated by Urology and determined to be an appropriate surgical candidate at that time, but he refused surgical intervention. Urology discussed the role of cytoreductive nephrectomy in the setting of IVC tumour thrombus. The patient’s continued flank pain was well controlled with oral medications and he only otherwise reported a recent occurrence of intermittent haematuria. Sunitinib (50 mg dose 4 weeks on/2 weeks off) was started in July 2016 and the patient tolerated a single cycle of therapy moderately well, with some associated dehydration. He was admitted to the hospital for fluid repletion and re-staging of his disease in August 2016, after his first cycle of targeted therapy (figures 2 and 3).
Figure 2.
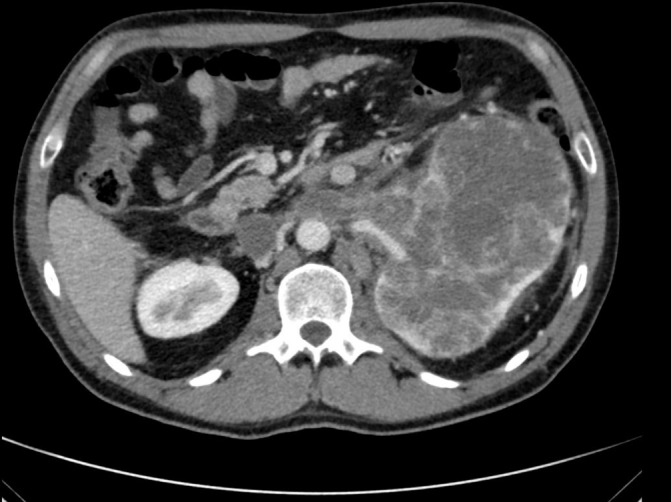
Axial view of left renal mass and tumour thrombus at L2–L3 spinal level in August 2016.
Figure 3.
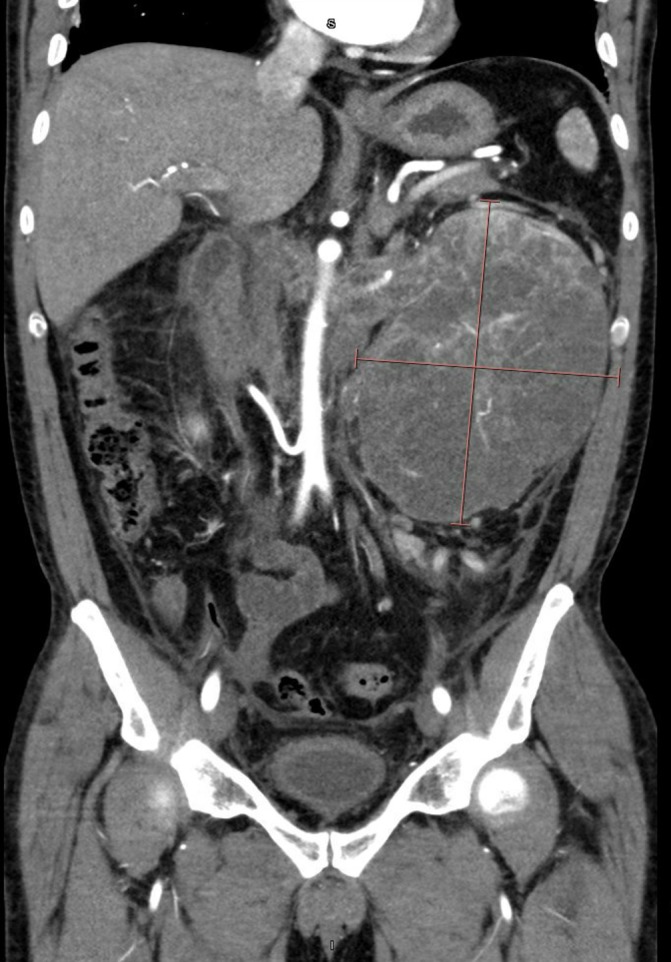
Coronal view of left renal mass and tumour thrombus in August 2016.
His case was discussed at a multidisciplinary genitourinary tumour board and a consensus was reached that he would not benefit from surgery, due to his unresectable lung
metastases. The consensus at tumour board was progression of his lung metastases while on Sunitinib indicated rapid progressive disease; therefore, he would not benefit from surgery. The patient was discharged with anticoagulation, and an IVC filter was later placed. Additionally, the patient underwent angioembolisation of left kidney by interventional radiology at an outside facility close to his hometown. Unfortunately, the patient subsequently suffered a loss of insurance coverage and was lost to follow-up until mid-2017.
Investigations
It is important to note that the patient underwent extensive imaging to exclude the possibility of venous thromboembolism, including Doppler bilateral leg ultrasound, CT with pulmonary embolism protocol, and transthoracic echocardiogram, both at time of diagnosis and immediately before planned surgery. No evidence of embolisation was found at any time.
Treatment
In April 2017, the patient was able to regain insurance coverage and began therapy with nivolumab. He received three cycles over the following year. A CT scan in December 2017 revealed that his renal mass was 11.4 cm, with interval change of the 1.7 cm lymph node abutting the left common iliac vessel. In April 2018, the patient was preadmitted to the hospital to prepare for a planned open left cytoreductive nephrectomy with IVC thrombectomy and further workup of his tumour thrombus. However, following new CT imaging just prior to his surgery, his case was cancelled due to the discovery of thromboses throughout his portal venous system. The diffuse distribution of possible thromboses in the superior and inferior mesenteric veins, splenic vein and portal vein (all presenting over a short period of time) showed evidence of a hyper-coagulable state of which immunotherapy might have contributed.3 Following discussion with vascular surgery, the risk of intra-operative embolisation was judged to be too high and the patient was again discharged with anti-coagulation to continue nivolumab immunotherapy (figures 4–6). In August 2018, with no evidence of thromboses and significant decrease in size and number of pulmonary nodules, the patient was able to undergo cytoreductive nephrectomy.
Figure 4.
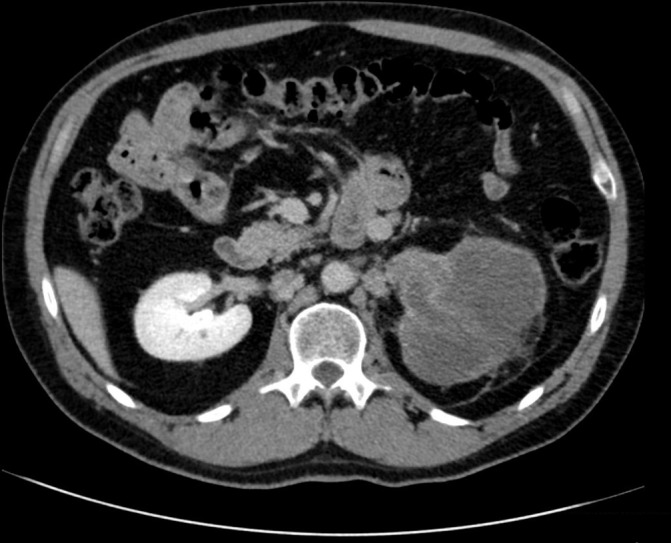
Axial view of left renal mass at L2–L3 spinal level in April 2018.
Figure 5.
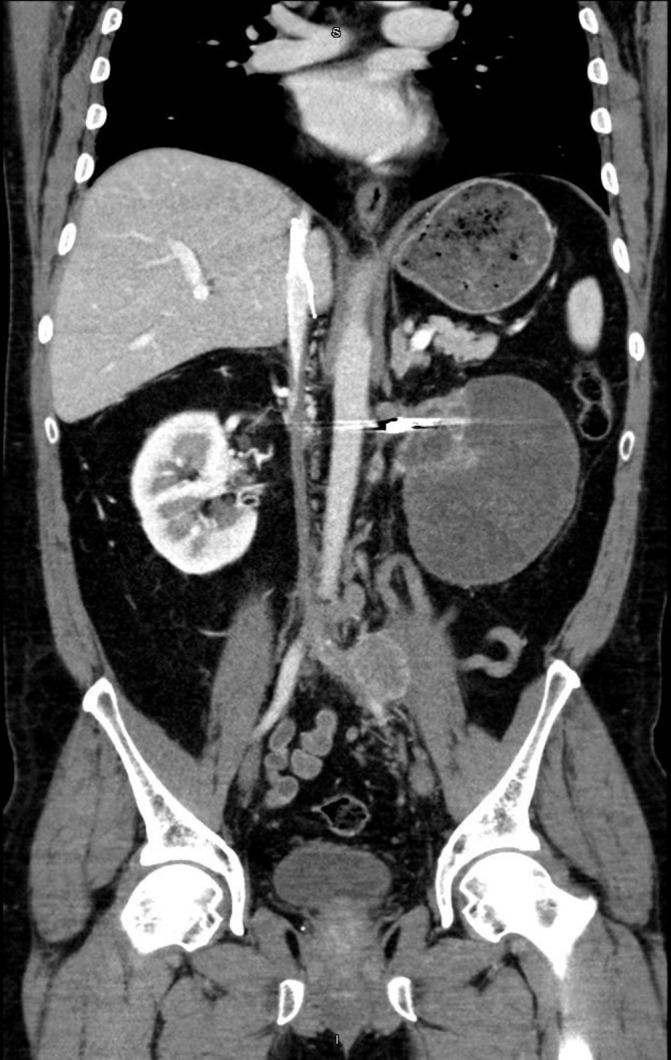
Coronal view of left renal mass in April 2018. Artefact depicts renal artery embolisation.
Figure 6.
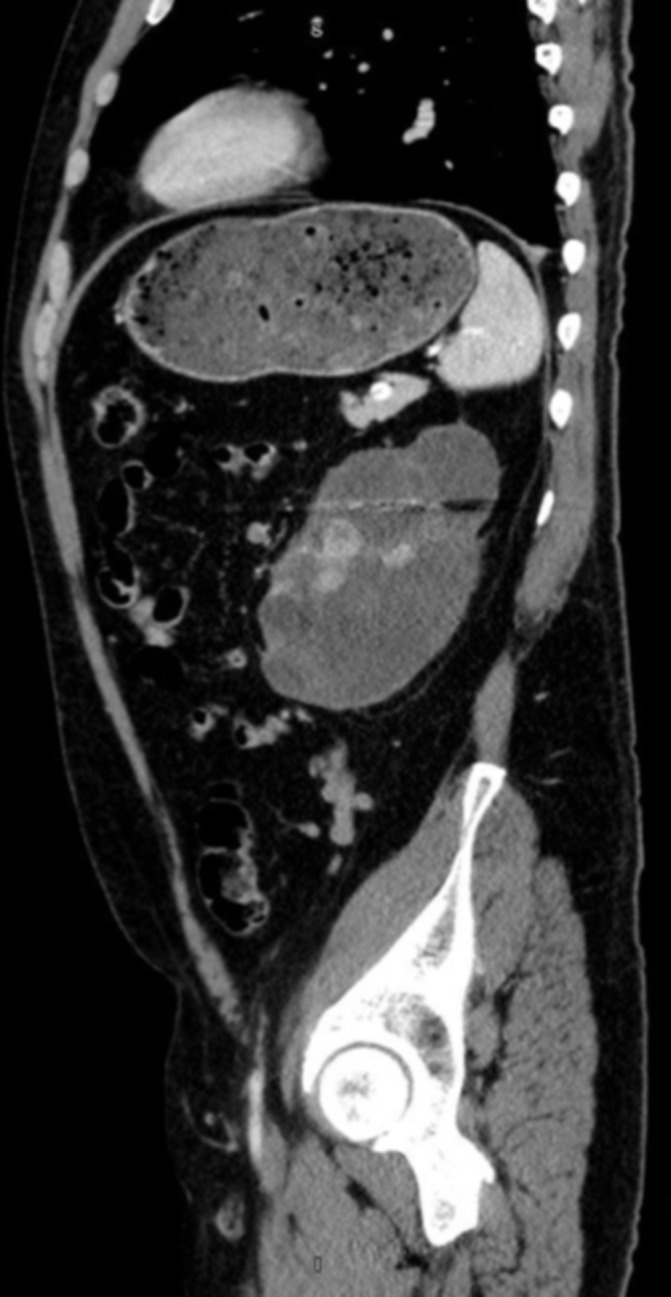
Sagittal view of left renal mass in April 2018.
Outcome and follow-up
Of note, the final pathology on the iliac lymph node was metastatic clear cell RCC. The previously mentioned retroperitoneal lymph node did not show any evidence of metastatic RCC. Imaging during the patient’s April 2018 admission was significant for the aforementioned portal vein thromboses and interval decrease in the left renal mass and his lung nodules. However, the absence of the previous infra-hepatic location of the IVC thrombus was curious. Repeated CT imaging exhibited the renal tumour thrombus to extend only to the junction of the IVC. Interestingly, it is likely that this regression was secondary to the nivolumab treatment the patient received. To our knowledge, this is the first time that an IVC tumour thrombus has responded in this fashion to immunotherapy. At present, the patient continues with close follow-up to monitor for tumour recurrence.
Discussion
RCC incidence in the USA is close to 65 000 cases per year and the American Cancer Society predicts that 15 000 will die from the disease in 2018.4 This is similar to the global incidence of 337 000 cases with 143 000 deaths.5 The Czech Republic has the highest incidence of RCC worldwide.1 Some have suggested that arsenic exposure or higher rates of smoking and obesity (smoking increases the risk of RCC by 50% in male and 20% in female smokers, and 5 kg/m2 increments in body mass index (BMI) increase the risk of RCC by 24% in men and 34% in women) are to blame, but surrounding countries such as Slovakia do not have a large enough difference in risk factors for this to be the case.6 Our patient did not have any identifiable risk factors, save for male gender.
IVC tumour thrombus has historically been classified using the Mayo system: level I is defined as <2 cm from the confluence of the renal vein and IVC; level II indicates >2 cm extension, but below hepatic veins; level III indicates intrahepatic involvement and level IV indicates that the thrombus extends supra-diaphragmatic or involves the right atrium.2 At presentation, our patient had an extensive level II thrombus, almost extending to level III. After therapy, his thrombus had almost completely regressed, barely classifying as a level I thrombi. To ensure optimal patient surgical outcomes, levels III or IV thrombi usually require a multidisciplinary surgical team and, even with these measures, the 5 year overall survival remains only between 32% and 69%.7 Though our patient was unable to undergo surgery, our institution’s vascular surgery service was involved in the patient’s case early in the planning stages. Interestingly, recent studies have shown that thrombus volume may have more prognostic value than thrombus level.8
The 2018 National Comprehensive Cancer Network Guidelines for Kidney Cancer now list adjuvant sunitinib as the first adjuvant therapy for patients with stages II and III clear cell RCC.9 Sunitinib is a small-molecule, multitargeted receptor tyrosine kinase inhibitor (TKI) that was approved by the Food and Drug Administration (FDA) for RCC in 2006. It targets receptors for platelet-derived growth factor and vascular endothelial growth factor to prevent tumour angiogenesis. However, in RCC patients with IVC tumour thrombi, neoadjuvant treatment with targeted molecular therapy like sunitinib have proved to have minimal to limited effects.10–13 Even when sunitinib has shown small regressions in tumour thrombi, it is not clear if this has an added survival benefit.14 15 Of note, pazopanib, another TKI, has shown to have a greater impact on tumour thrombi.16 It is highly unlikely that our patient’s single cycle of sunitinib effected a change in his tumour thrombus.
It must be noted that the angioembolisation of the primary tumour could have conceivably contributed to the regression of the IVC tumour thrombus. One of the chief benefits of preoperative renal artery embolisation (RAE) is to cause a reduction in tumour bulk to include extent of vascular thrombi.17 There is evidence to suggest that RAE produces a change in the immune system that is dependent on the malignancy’s isolation from the systemic vasculature.18 Due to patient compliance and loss of insurance, there unfortunately was no imaging immediately prior to the patient beginning nivolumab. This undeniably impacts the strength of the claim that nivolumab was the primary driver behind tumour thrombus regression. However, nivolumab has already shown impressive results in a similar case of RCC with intracardiac metastases.19
Immunotherapy is ideal for RCC as it is an ‘immunogenic’ tumour, based on several characteristics: incidence of spontaneous tumour regression, high level of tumour T-cell infiltration, and responsiveness to immunotherapies such as interleukin-2 and interferon alpha.20 Nivolumab, a fully human monoclonal IgG4 anti-programmed death (PD)-1 antibody, selectively blocks the interaction between PD-1 and its ligands PD-L1 and PD-L2 and provides a novel therapy option for patients with advanced RCC.5
CheckMate 025, the prospective randomised trial which led to FDA approval of nivolumab demonstrated improved overall survival (26 month vs 19.7 month; HR 0.73; p=0.0006) and response rate (26% vs 5%) as well as a favourable toxicity profile compared with Everolimus.21 The European Association of Urology guidelines were updated in 2017 to reflect the results of Checkmate 214. This trial showed superior survival for a combination of ipilimumab (an immunotherapy that targets CTLA-4 to activate cytotoxic T lymphocytes) and nivolumab, when compared with sunitinib, in patients with metastatic clear cell RCC and a poor prognosis.22 The 18-month overall survival rate was 75% with nivolumab plus ipilimumab and 60% with sunitinib.23
At this time, no clinical trial has assessed the efficacy of nivolumab on IVC tumour thrombi, either as neoadjuvant therapy or standalone.
Learning points.
Renal cell carcinoma (RCC can present with only moderate flank pain, eschewing the typical triad of haematuria, flank pain, and palpable flank mass accompanied by scrotal varicocele.
Inferior vena cava (IVC) tumour thrombi are a unique disease complication of RCC and are associated with markedly increased morbidity and mortality.
Surgery for IVC tumour thrombi is technically challenging, often requires a multidisciplinary surgical team, and patient surgical outcomes may benefit from neoadjuvant immunotherapy.
Patients with extensive IVC tumour thrombi secondary to RCC may benefit from immunotherapy if thrombectomy is not clinically indicated or if associated conditions preclude surgery.
Footnotes
Contributors: JB did background research on the case. He contributed to the conception, design of the case report. He drafted the initial project and wrote out the discussion. EG helped review the draft, reviewed imaging and proofread the discussion. He also was in contact with the patient and helped review the outcome and follow up for the patient. DK was the lead on this patient and was lead physician for discussion and recommendations for this patient’s care. He reviewed and approved the final copy for the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Richter I, Dvořák J. [Treatment of Metastatic Renal Cell Carcinoma]. Klin Onkol 2018;31:110–6. 10.14735/amko2018110 [DOI] [PubMed] [Google Scholar]
- 2. Kulkarni J, Jadhav Y, Valsangkar RS. IVC Thrombectomy in Renal Cell Carcinoma-Analysis of Out Come Data of 100 Patients and Review of Literature. Indian J Surg Oncol 2012;3:107–13. 10.1007/s13193-011-0114-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roopkumar J, Kim AS, Bicky T, et al. Venous Thromboembolism in Cancer Patients Receiving Immunotherapy. Blood 2018;132:2510. [Google Scholar]
- 4. Alsharedi M, Katz H. Check point inhibitors a new era in renal cell carcinoma treatment. Med Oncol 2018;35:85 10.1007/s12032-018-1147-y [DOI] [PubMed] [Google Scholar]
- 5. Ochoa CE, Joseph RW. Nivolumab in Renal Cell Carcinoma: Current Trends and Future Perspectives. J Kidney Cancer VHL 2018;5:15–18. 10.15586/jkcvhl.2018.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519–30. 10.1016/j.eururo.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 7. Lawindy SM, Kurian T, Kim T, et al. Important surgical considerations in the management of renal cell carcinoma (RCC) with inferior vena cava (IVC) tumour thrombus. BJU Int 2012;110:926–39. 10.1111/j.1464-410X.2012.11174.x [DOI] [PubMed] [Google Scholar]
- 8. Zargar-Shoshtari K, Sharma P, Espiritu P, et al. Caval tumor thrombus volume influences outcomes in renal cell carcinoma with venous extension. Urol Oncol 2015;33:112.e23–112.e29. 10.1016/j.urolonc.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 9. Jonasch E. Updates to the Management of Kidney Cancer. J Natl Compr Canc Netw 2018;16(5S):639–41. 10.6004/jnccn.2018.0039 [DOI] [PubMed] [Google Scholar]
- 10. Cost NG, Delacroix SE, Sleeper JP, et al. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol 2011;59:912–8. 10.1016/j.eururo.2011.02.032 [DOI] [PubMed] [Google Scholar]
- 11. Bigot P, Fardoun T, Bernhard JC, et al. Neoadjuvant targeted molecular therapies in patients undergoing nephrectomy and inferior vena cava thrombectomy: is it useful? World J Urol 2014;32:109–14. 10.1007/s00345-013-1088-1 [DOI] [PubMed] [Google Scholar]
- 12. Fukuda H, Kondo T, Takagi T, et al. Limited benefit of targeted molecular therapy for inferior vena cava thrombus associated with renal cell carcinoma. Int J Clin Oncol 2017;22:767–73. 10.1007/s10147-017-1119-9 [DOI] [PubMed] [Google Scholar]
- 13. Peng C, Gu L, Wang L, et al. Role of presurgical targeted molecular therapy in renal cell carcinoma with an inferior vena cava tumor thrombus. Onco Targets Ther 2018;11:1997–2005. 10.2147/OTT.S158114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Field CA, Cotta BH, Jimenez J, et al. Neoadjuvant sunitinib decreases inferior vena caval thrombus size and is associated with improved oncologic outcomes: a multicenter comparative analysis. Clin Genitourin Cancer 2019. [Epub ahead of print 30 Jan 2019]. 10.1016/j.clgc.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 15. Cai W, Huang J, Yuan Y, et al. Sunitinib or Sorafenib as Neoadjuvant Therapy May not Improve the Survival Outcomes of Renal Cell Carcinoma with Tumor Thrombus. Urol Int 2018;101:391–9. 10.1159/000492723 [DOI] [PubMed] [Google Scholar]
- 16. Terakawa T, Hussein AA, Bando Y, et al. Presurgical pazopanib for renal cell carcinoma with inferior vena caval thrombus. Anticancer Drugs 2018;29:565–71. 10.1097/CAD.0000000000000627 [DOI] [PubMed] [Google Scholar]
- 17. Li D, Pua BB, Madoff DC. Role of embolization in the treatment of renal masses. Semin Intervent Radiol 2014;31:070–81. 10.1055/s-0033-1363845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zielinski H, Syrylo T, Szmigielski S. Chen J, Renal Artery Embolization in Treatment of Renal Cancer with Emphasis on Response of Immune System: Renal Tumor. InTech, 2013:95–108. [Google Scholar]
- 19. Ansari J, Alhelali S, Albinmousa Z, et al. Rare case of intracardiac renal cell carcinoma metastasis with response to nivolumab: case report and literature review. Case Rep Oncol 2018;11:861–70. 10.1159/000495459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mazza C, Escudier B, Albiges L. Nivolumab in renal cell carcinoma: latest evidence and clinical potential. Ther Adv Med Oncol 2017;9:171–81. 10.1177/1758834016679942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grimm MO, Foller S. [Immunotherapy for renal cell carcinoma - current status]. Aktuelle Urol 2018;49:187–91. 10.1055/a-0581-4451 [DOI] [PubMed] [Google Scholar]
- 22. Powles T, Albiges L, Staehler M, et al. Updated European Association of Urology Guidelines Recommendations for the Treatment of First-line Metastatic Clear Cell Renal Cancer. Eur Urol 2017. 10.1016/j.eururo.2017.11.016 [DOI] [PubMed] [Google Scholar]
- 23. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018;378:1277–90. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]


