Abstract
A 2-year-old presented with lethargy, acute visual loss, fixed dilated pupils and severe bilateral retinal haemorrhages. The retinal findings raised concerns about abusive head trauma, but subsequent investigations confirmed the diagnosis of bilateral optic neuritis associated with acute disseminated encephalomyelitis.
Keywords: retina, child abuse, visual pathway, neuroopthalmology
Background
Paediatricians and ophthalmologists should be aware that severe retinal haemorrhages may rarely be caused by acute disseminated encephalomyelitis in a young child.
Case presentation
A 2-year-old presented to a district hospital. He had been well except for mild coryza. On the evening before the presentation, he went to sleep early. The next morning, he was difficult to rouse, irritable and had dilated pupils. He vomited three times.
There were no other symptoms. Developmental, medical and family medical history was normal. He had recently stayed on a farm. There was no history of toxic ingestion or trauma.
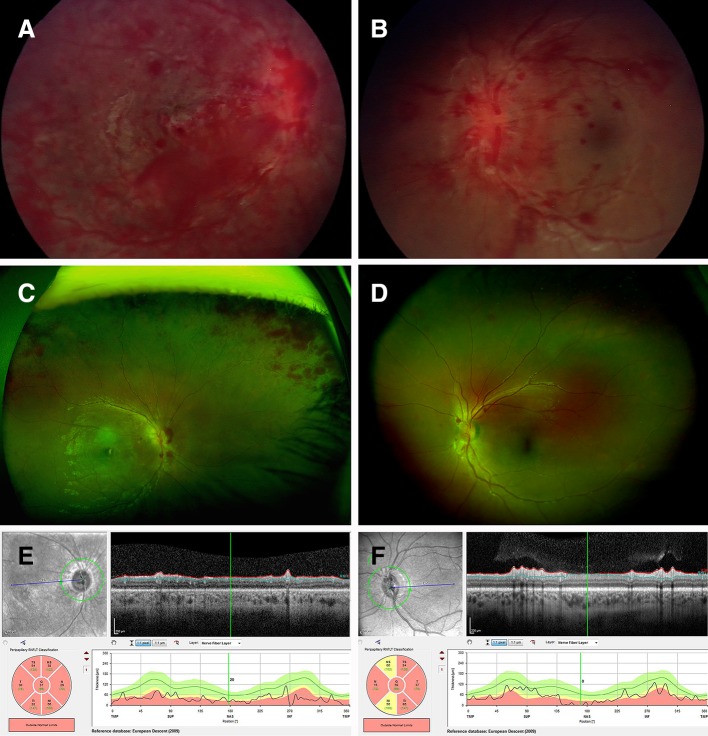
On examination, he was afebrile. He was miserable and drowsy, but his Glasgow Coma Score was 15. Both pupils were 8 mm, fixed and dilated. He had no blink reflex and could not fix or follow. He was unsteady but physical examination was otherwise normal. On indirect ophthalmoscopy, it was reported that he had bilateral intraretinal and preretinal haemorrhages. These were confluent and widespread (especially in the right eye) and extended to the far periphery (figure 1A, B).
Figure 1.
Ocular images of child with ADEM. (A,B) Retinal images on presentation right (A) and left (B), demonstrating extensive retinal haemorrhages involving multiple layers of the retina, right eye greater than left, dilated and tortuous retinal venules, and optic nerve head swelling left eye, but not visible in the right eye. (C,D) Optos ultra-widefield retinal photos day 10 postpresentation, showing improvement, but persistent haemorrhages particularly peripapillary and in the peripheral retina, with optic nerve head swelling and cotton wool spots. (E,F) Retinal nerve fibre layer 18 months’ postpresentation demonstrating significant thinning both eyes, right >left. ADEM, acute disseminated encephalomyelitis.
Because of the retinal haemorrhages, background checks were carried out with statutory child welfare and the police. These were normal. The child was transferred on day 5 for further management in a tertiary paediatric centre.
Investigations
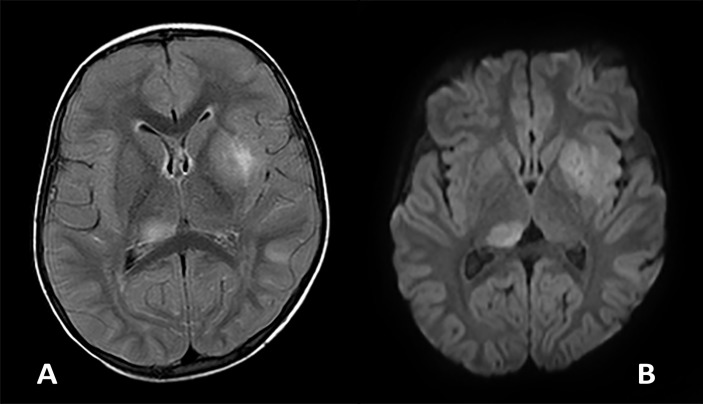
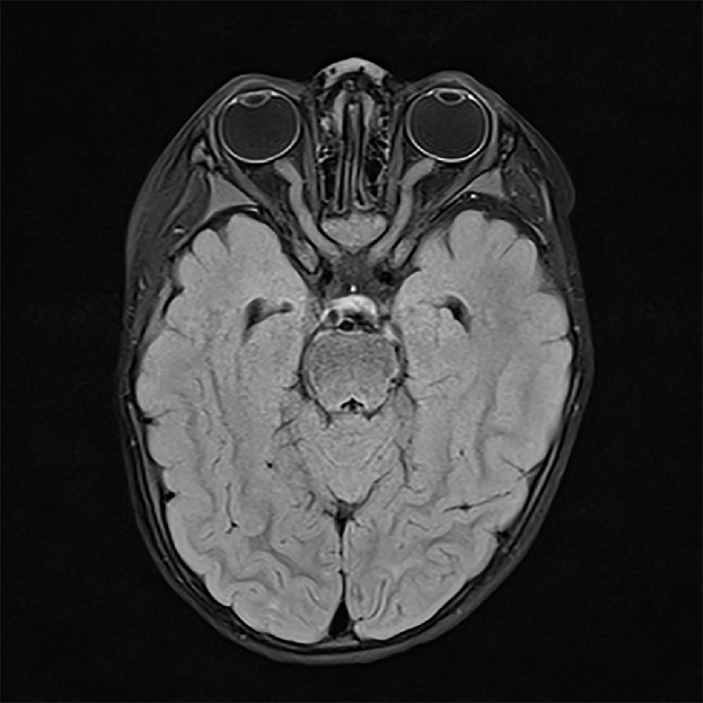
CT head and skeletal survey were normal. MRI showed asymmetric multifocal patches of a bright signal on the T2 imaging involving the left insula and external capsule, left lentiform nucleus and the right thalamus (figure 2). There were also patches of subcortical white matter and cortical T2 hyperintensity involving the left and right frontal cortex and the periventricular white matter of both parietal lobes. There were diffuse enlargement, tortuosity and abnormal signal of both prechiasmatic optic nerves and chiasm (figure 3). There was an abnormal diffuse enhancement of the nerves and restricted diffusion involving the nerves, chiasm and left postchiasmal nerve. The brainstem and spinal cord were normal.
Figure 2.
Axial FLAIR (A) and diffusion (B) images demonstrating two representative lesions within the right thalamus and left lentiform nucleus and external capsule at day 2 of presentation. The lesions are asymmetrical, ill-defined and T2 hyperintense on the FLAIR and have increased diffusivity (bright signal) on the diffusion. FLAIR, fluid-attenuated inversion recovery.
Figure 3.
Axial FLAIR image demonstrating bilateral enlarged, tortuous and increased T2 signal of the optic nerves on day 2 of presentation. FLAIR, fluid-attenuated inversion recovery.
On admission, there was a raised blood white cell count of 21.9×109/L (14.1 neutrophils), and a cerebrospinal fluid (CSF) white cell count of 17 (89% neutrophils) which was normal when repeated on day 10. The opening pressure at lumbar puncture was not obtained, but there were no clinical or radiological indicators of raised intracranial pressure. There was a positive nasopharyngeal aspirate for rhinovirus and bocavirus and a very low level of human herpesvirus 6 DNA in one blood sample. Extensive investigations were negative for indicators of: other infection (C-reactive protein; erythrocyte sedimentation rate; CSF protein and glucose; CSF culture; CSF, blood and urine panels for a wide range of viral or bacterial pathogens); haematological disorders; systemic lupus erythematosus; neuromyelitis optica (aquaporin-4 immunoglobulin (Ig)G); multiple sclerosis (oligoclonal bands); one subtype of acute disseminated encephalomyelitis associated with optic neuritis (myelin oligodendrocyte glycoprotein IgG); neoplastic disease (CSF cytology); metabolic disease; renal or liver dysfunction; B12 deficiency and toxic ingestion. CSF analysis for myelin basic protein was not available in our healthcare setting at the time of this child’s presentation.
Differential diagnosis
The retinal haemorrhages at first raised concern for abusive head trauma. Although this may present with encephalopathy and no external signs of trauma, fixed dilated pupils in abusive head trauma occur only in comatose children with imminent tonsillar herniation or global brain injury. On CT and MRI, there was no intracranial trauma, and there was no other evidence of trauma on clinical examination or skeletal survey. Abusive head trauma could therefore be confidently excluded and required no further investigation.1
Radiology excluded a ruptured aneurysm or arteriovenous malformation as causes of retinal haemorrhage (Terson syndrome)2 and there was no evidence of raised intracranial pressure.3 Examination and investigations excluded rare causes of retinal haemorrhage such as coagulation disorders, hypertension, hyperviscosity, infection, leukaemia and retinal dysplasia or telangiectasia,4 which, in any event, would not explain multifocal central nervous system (CNS) inflammation of subcortical white matter, thalamus, basal ganglia and the optic nerves.
The likely diagnosis was a multifocal acquired demyelinating syndrome. Investigations did not support other differentials such as vasculitis, systemic lupus erythematosus, other autoantibody-mediated diseases, haemophagocytic lymphohistiocytosis or neurometabolic disease. The differential was between acute disseminated encephalomyelitis (ADEM), neuromyelitis optica and multiple sclerosis.5
The diagnosis was made of ADEM, an immune-mediated disease possibly triggered by a viral infection. This has a multifocal onset characterised by encephalopathy, often including optic neuritis.6 It is usually a single episode but may represent the first attack of multiple sclerosis, which is only excluded by follow-up over time.
Treatment
He received three doses of high-dose intravenous methylprednisolone (20 mg/kg) followed by 5 days of intravenous Ig(400 mg/kg/day) and a reducing course of oral prednisone.
Outcome and follow-up
He became less drowsy after 48 hours. By day 6, he could detect bright light. On day 6, indirect ophthalmoscopy by a paediatric ophthalmologist showed profound optic nerve swelling with a dilated tortuous venous system. There were scattered cotton wool spots (yellow-white lesions representing focal ischaemic damage) and superficial retinal haemorrhages, far fewer than before. There were no preretinal haemorrhages or retinoschisis. By day 10, his pupils were 5 mm and reactive. On day 10, optos ultra-widefield retinal images (Optos plc, Dumferline, UK) showed relatively few remaining haemorrhages (figure 1C,D). By day 14, his mother felt his vision was about 80% of normal.
On follow-up at 4 and 7 months, the optic nerves were pale but there was no retinal haemorrhage. Serial MRI showed persistent swelling and abnormal signal in both optic nerves and the optic chiasm and residual signal abnormality in subcortical white matter. At 7 months, visual acuity was measured with both eyes open using Cardiff cards at 6/12.
By 18 months, it was clear there was permanent visual impairment. Visual acuity was 3/30 in the right eye (0.900 logMAR) and 3/6 in the left eye (0.325 logMAR). He had a right afferent pupillary defect (consistent with worse visual function in the right eye) and bilateral pale optic nerves. Optical coherence tomography showed profound thinning of the retinal nerve fibre layer (figure 1E,F). However, his mother described him as being quite capable in the vision required for most activities of daily living. MRI showed subtle enhancement of both optic nerves after gadolinium, but the normal calibre and no significant brightness. Subcortical white matter was unchanged.
He is now early school age, with no evidence to date of recurrence
Discussion
ADEM is a clinical diagnosis, and there was strong clinical evidence for that diagnosis in this case: monophasic but multifocal inflammation of the central nervous system involving not only the optic nerve but also widespread areas of white and deep grey matter. Other causes were excluded both on extensive initial investigation and by follow-up over time.
Haemorrhages of the optic disc or surrounding retina are rare in optic neuritis.7 Although swelling of the optic disc is more common in children,7 haemorrhages are usually few and small.8 There is one previous report of a 6-year-old boy with acute disseminated encephalomyelitis and marked peripapillary haemorrhages,9 but this is the first report of confluent and multilayer haemorrhages extending to the far retinal periphery.
The typical retinal haemorrhages of abusive head trauma are too numerous to count, involve multiple layers of the retina and extend to the far retinal periphery. Although this child did not have retinoschisis, that does not exclude the possibility of abusive head trauma. However, swelling of the optic nerves is uncommon10 and a clinical presentation with acute blindness, fixed dilated pupils and a GCS of 15 has never been described. Even before the radiological findings, this was unlikely to represent a case of abusive head trauma.
The most likely mechanism of retinal haemorrhage in our case appeared to be a bilateral vaso-occlusive event similar to central retinal vein occlusion. Although other causes of central retinal vein occlusion (such as infiltrative or primary neoplastic disease) were considered, there was no evidence of such conditions at the time of presentation or subsequently. The onset of symptoms and the severity of haemorrhage suggest a rapid and profound event. A similar but unilateral presentation has been described in an adolescent with systemic lupus erythematosus.11 It is uncertain how much of the permanent visual impairment is due to damage to the optic nerves from optic neuritis, how much to retinal damage from ischaemic vascular occlusion and how much to amblyopia from suppression of the image from his worse eye, but his overall visual outcome (like most children with optic neuritis from ADEM)12 has been relatively good.
Patient’s perspective.
It was a very difficult time for our family. My son’s vision has improved so much since this incident back in 2015. He can see much more but sometimes suffers from anxiety issues as well as temper tantrums. Now that he is 5, he is at the correct learning stage for his age. Also, he can see things as small as a grain of rice. He struggles sometimes to see transparent objects.
Learning points.
Acute disseminated encephalomyelitis in childhood may cause bilateral, severe, diffuse retinal haemorrhages by a vaso-occlusive mechanism.
Interpreting the significance of retinal haemorrhages should not be driven solely by the characteristics of those haemorrhages, but by the complete clinical picture.
The medical investigation of suspected abusive head trauma requires a meticulous multidisciplinary approach.
Footnotes
Contributors: PK: cared for the patient and conceptualised the case report, obtained consent, reviewed the data, wrote and revised the manuscript and approved the final manuscript as submitted. AV: cared for the patient and reviewed the data, contributed the ophthalmological images, revised the manuscript and approved the final manuscript as submitted. MN: cared for the patient and reviewed the data, revised the manuscript and approved the final manuscript as submitted. SB: cared for the patient and reviewed the data, contributed the radiological images, revised the manuscript and approved the final manuscript as submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Kemp AM. Abusive head trauma: recognition and the essential investigation. Arch Dis Child Educ Pract Ed 2011;96:202–8. 10.1136/adc.2009.170449 [DOI] [PubMed] [Google Scholar]
- 2. Bhardwaj G, Jacobs MB, Moran KT, et al. . Terson syndrome with ipsilateral severe hemorrhagic retinopathy in a 7-month-old child. J Aapos 2010;14:441–3. 10.1016/j.jaapos.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 3. Binenbaum G, Rogers DL, Forbes BJ, et al. . Patterns of retinal hemorrhage associated with increased intracranial pressure in children. Pediatrics 2013;132:e430–4. 10.1542/peds.2013-0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aryan HE, Ghosheh FR, Jandial R, et al. . Retinal hemorrhage and pediatric brain injury: etiology and review of the literature. J Clin Neurosci 2005;12:624–31. 10.1016/j.jocn.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 5. Rostasy K, Bajer-Kornek B, Venkateswaran S, et al. . Differential diagnosis and evaluation in pediatric inflammatory demyelinating disorders. Neurology 2016;87(9 Suppl 2):S28–37. 10.1212/WNL.0000000000002878 [DOI] [PubMed] [Google Scholar]
- 6. Pohl D, Alper G, Van Haren K, et al. . Acute disseminated encephalomyelitis: Updates on an inflammatory CNS syndrome. Neurology 2016;87(9 Suppl 2):S38–45. 10.1212/WNL.0000000000002825 [DOI] [PubMed] [Google Scholar]
- 7. Balcer LJ. Clinical practice. Optic neuritis. N Engl J Med 2006;354:1273–80. 10.1056/NEJMcp053247 [DOI] [PubMed] [Google Scholar]
- 8. Cassidy L, Taylor D. Pediatric optic neuritis. J Aapos 1999;3:68–9. 10.1016/S1091-8531(99)70072-8 [DOI] [PubMed] [Google Scholar]
- 9. Chan WH, Lloyd IC, Ashworth JL, et al. . Acute disseminated encephalomyelitis associated with optic neuritis and marked peri-papillary hemorrhages. Eye 2011;25:1658–9. 10.1038/eye.2011.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Binenbaum G, Chen W, Huang J, et al. . The natural history of retinal hemorrhage in pediatric head trauma. J Aapos 2016;20:131–5. 10.1016/j.jaapos.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korematsu S, Goto H, Gotoh C, et al. . Central retinal vein occlusion in a pediatric patient with SLE and antiphospholipid antibodies without anti-cardiolipin or anti-β2 glycoprotein I antibodies. BMC Pediatr 2014;14:116 10.1186/1471-2431-14-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kotlus BS, Slavin ML, Guthrie DS, et al. . Ophthalmologic manifestations in pediatric patients with acute disseminated encephalomyelitis. J Aapos 2005;9:179–83. 10.1016/j.jaapos.2004.10.009 [DOI] [PubMed] [Google Scholar]