Abstract
We report a potential association between an abnormally raised pregnancy level of alkaline phosphatase (ALP) and intrauterine growth restriction (IUGR). There are few reports of women with abnormally high ALP during pregnancy. However, there is work to suggest an association with placental insufficiency, low birth weight and preterm delivery. In conjunction with a rising ALP, fetal IUGR and intermittent absence of umbilical artery end diastolic flow had evolved. A greatly elevated ALP may be a marker for placental insufficiency and IUGR.
Keywords: pregnancy, materno-fetal medicine
Background
The enzyme, alkaline phosphatase (ALP), is derived mostly from liver and bone but is also found in the intestine, kidney and placenta.1 An increase in ALP production occurs when tissues are functionally disturbed, for example, hepatic obstruction or greatly stimulated, for example, the placenta in pregnancy. During pregnancy, levels may reach three times the upper limit of normal (30–130 IU/L).2 We present a case of a 31-year-old woman who had a significantly elevated ALP of 1259 IU/L at 26 weeks gestation. At 33 weeks gestation, she underwent caesarean section because of evolving intra uterine growth restriction (IUGR) and abnormal Doppler studies. This case report was prepared following the CAse REport guidelines and written informed consent was obtained from the patient.3
Case presentation
This 31-year-old female is gravid 2 para 1 with an uncomplicated first pregnancy. She had documented normal liver function tests preconception and a background of iron deficiency anaemia. She had an uncomplicated antenatal course until she self-referred at 26+5 weeks gestation with a headache. She had a 1-day history of frontal headache, which eased with paracetamol. Clinical examination findings were normal, as were laboratory indices apart from a markedly raised ALP: 1259 IU/L (normal range for pregnancy: 32–418 IU/L).2 Liver ultrasound and full bone profile were reported as normal.
ALP isoenzymes showed a normal pattern and so we hypothesised that the additional level of enzyme was of placental origin.
The ALP level continued to rise (figure 1). Serial fetal growth had been measuring along the 40–50th centile but at 33+3 weeks, it was noted that fetal growth had dropped to the fifth centile, together with a high resistance index on umbilical artery Doppler ultrasound and demonstrable intermittent absent end diastolic flow. Caesarean section was performed and a live female infant weighing 1515 g delivered. The liquor was clear at delivery. Her first baby had weighed 3275 g and she had no additional risk factors for IUGR in the index pregnancy.
Figure 1.
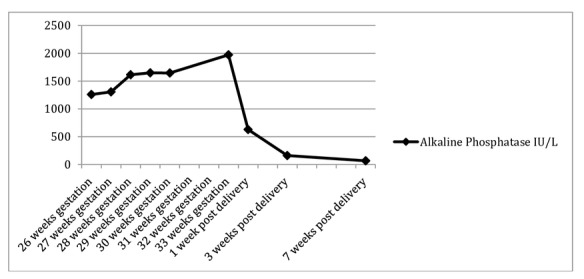
Rising alkaline phosphatase (IU/L) versus time.
The placenta weighed 372 g and histology showed villous maturity in keeping with third trimester gestation. There was no villitis, funisitis, acute chorioamnionitis, associated infarction, thrombosis, haemosiderin deposition, meconium staining or other abnormality. There was a small chorioangioma thought unlikely to be of clinical significance.
The ALP level had returned to normal 7 weeks post partum.
Outcome and follow-up
The female infant in this case is doing well at 1 year of age and has met all developmental milestones.
Discussion
Despite the fact that there have been few reported cases of markedly raised ALP during pregnancy, it has been suggested that it could be a marker for placental insufficiency,4–8 low birth weight9 10 and preterm delivery.4
It is almost three decades since the publication of Brock,9 Best10 and Meyer’s4 work on maternal serum ALP and its association with placental-mediated fetal problems. Little has appeared in the interim apart from sporadic and isolated case reports.6–8 11
Cellular enzymes and proteins are typically released from damaged muscle tissue and organs. Circulating levels of these proteins and enzymes are measured and used to aid diagnosis, for example, myocardial infarction.4 5 7 Similarly, elevated circulating ALP may be a marker of placental injury.4 5 7 Cases of elevated ALP in pregnancy have demonstrated placental infarction7 8 and microscopic damage to the villous syncytiotrophoblast.6 A high or acutely rising ALP could be a useful tool to identify high-risk pregnancies and underlying placental damage.7
The placenta plays a crucial role in fetal development and IUGR can be considered a placentation disorder.12 Recent evidence suggests that microRNAs (miRNAs), which regulate gene expression and are mostly expressed in the placenta, have a key role in the pathogenesis of IUGR.12 13 Higher levels of circulating extracellular miRNA has been associated with pregnanices which progress normally.13 14 Other studies have shown a varied expression of miRNAs in IUGR.12 Epigenetics may be the reason for selective miRNA expression and account for these variations.12 Future research may be able to identify a panel of maternal biomarkers that will enable early detection of IUGR.12
Our patient was fortuitous in presenting with an unrelated issue (headache) and in undergoing baseline laboratory screening investigations. The isolated finding of a markedly raised ALP provoked closer monitoring, which allowed for early detection of fetal growth restriction and intervention.
This case report supports the previously noted association of an abnormally raised ALP and IUGR. However, a limitation of this case report is that this association may not be applicable in patients in general.3
In conclusion, we believe that an abnormally elevated and rising ALP may be a potential marker for placental dysfunction and that indeed it may pose a problem for the fetus.
Learning points.
During pregnancy, alkaline phosphatase may reach three times the upper normal limit.
An abnormally elevated or rising alkaline phosphatase may be a marker of placental dysfunction.
In cases with an abnormally elevated alkaline phosphatase more frequent monitoring may be indicated.
Footnotes
Contributors: SM was directly involved in the care of this patient, obtained informed consent and wrote up the main body of the case report. CK was the supervising consultant who made key clinical decisions and edited the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 2017;112:18–35. 10.1038/ajg.2016.517 [DOI] [PubMed] [Google Scholar]
- 2. Walker I, Chappell LC, Williamson C. Abnormal liver function tests in pregnancy. BMJ 2013;347:f6055 10.1136/bmj.f6055 [DOI] [PubMed] [Google Scholar]
- 3. Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218–35. 10.1016/j.jclinepi.2017.04.026 [DOI] [PubMed] [Google Scholar]
- 4. Meyer RE, Thompson SJ, Addy CL, et al. Maternal serum placental alkaline phosphatase level and risk for preterm delivery. Am J Obstet Gynecol 1995;173:181–6. 10.1016/0002-9378(95)90187-6 [DOI] [PubMed] [Google Scholar]
- 5. Moawad AH, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: the value of serum alkaline phosphatase, alpha-fetoprotein, plasma corticotropin-releasing hormone, and other serum markers for the prediction of spontaneous preterm birth. Am J Obstet Gynecol 2002;186:990–6. 10.1067/mob.2002.121727 [DOI] [PubMed] [Google Scholar]
- 6. Boronkai A, Than NG, Magenheim R, et al. Extremely high maternal alkaline phosphatase serum concentration with syncytiotrophoblastic origin. J Clin Pathol 2005;58:72–6. 10.1136/jcp.2003.015362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ranganath L, Taylor W, John L, et al. Biochemical diagnosis of placental infarction/damage: acutely rising alkaline phosphatase. Ann Clin Biochem 2008;45:335–8. 10.1258/acb.2007.007098 [DOI] [PubMed] [Google Scholar]
- 8. Vongthavaravat V, Nurnberger MM, Balodimos N, et al. Isolated elevation of serum alkaline phosphatase level in an uncomplicated pregnancy: a case report. Am J Obstet Gynecol 2000;183:505–6. 10.1067/mob.2000.105841 [DOI] [PubMed] [Google Scholar]
- 9. Brock DJ, Barron L. Measurement of placental alkaline phosphatase in maternal plasma as an indicator of subsequent low birthweight outcome. Br J Obstet Gynaecol 1988;95:79–83. 10.1111/j.1471-0528.1988.tb06484.x [DOI] [PubMed] [Google Scholar]
- 10. Best RG, Meyer RE, Shipley CF. Maternal serum placental alkaline phosphatase as a marker for low birth weight: results of a pilot study. South Med J 1991;84:740–2. 10.1097/00007611-199106000-00016 [DOI] [PubMed] [Google Scholar]
- 11. Lozo S, Atabeygi A, Healey M. Extreme elevation of alkaline phosphatase in a pregnancy complicated by gestational diabetes and infant with neonatal alloimmune thrombocytopenia. Case Rep Obstet Gynecol 2016;2016:1–3. 10.1155/2016/4896487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiofalo B, Laganà AS, Vaiarelli A, et al. Do miRNAs play a role in fetal growth restriction? A fresh look to a busy corner. Biomed Res Int 2017;2017:1–8. 10.1155/2017/6073167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higashijima A, Miura K, Mishima H, et al. Characterization of placenta-specific microRNAs in fetal growth restriction pregnancy. Prenat Diagn 2013;33:214–22. 10.1002/pd.4045 [DOI] [PubMed] [Google Scholar]
- 14. Hromadnikova I, Kotlabova K, Doucha J, et al. Absolute and relative quantification of placenta-specific micrornas in maternal circulation with placental insufficiency-related complications. J Mol Diagn 2012;14:160–7. 10.1016/j.jmoldx.2011.11.003 [DOI] [PubMed] [Google Scholar]


