Abstract
Coronary artery disease (CAD) due to Takayasu arteritis (TA) is rare. This article reports a case of severe involvement of multiple coronary arteries in a young woman. She was treated with coronary artery bypass grafting and had an early venous graft stenosis despite immunosuppressants. She became asymptomatic one year after a drug-eluting stent placement. This report shows the complexity of the diagnostic and therapeutic approach to TA with complex CAD.
Keywords: ischaemic heart disease, vasculitis
Background
Takayasu arteritis (TA) is a chronic granulomatous large vessel vasculitis, classically affecting the aorta and its large and medium branches. The inflammation may be localised to a portion of the thoracic or abdominal aorta and its branches, or may involve the entire vessel.1 Women are affected in 80%–90% of cases at an age of onset ranging from 10 to 40 years.2 The coronary arteries are seldom involved. Coronary arteries are involved in 6%–30% of the cases associated with aorta involvement, but it happens about 5% in isolation.3 This article proposes to present a case of TA with severe coronary artery involvement in a young patient without risk factors for coronary artery disease (CAD).
Case presentation
A 23-year-old woman was admitted to the cardiology department of the Universidade Federal do Ceara, with a history of intermittent chest pain for the past one and a half years. The pain was of moderate intensity and lasted 3–30 minutes per episode. She also complained about mild lower extremities claudication. No classic risk factors for CAD were present. She had several prior visits to the emergency department always with early discharges attributing her symptoms to anxiety. On this admission, physical examination was normal, including symmetrical peripheral pulses and four limbs blood pressure.
Investigations
Laboratory tests including cardiac biomarkers were within normal limits except for an elevated sedimentation rate of 35 mm/hour. Electrocardiogram showed repolarisation abnormalities (plus-minus type) in the anterior wall. Echocardiogram revealed hypokinesis of the apex of the left ventricle. The coronary angiography at admission (figure 1) demonstrated multiple vessel obstruction including total occlusion of the left anterior descending (LAD) and left circumflex artery. CT of the aorta exhibited signs of inflammation in the aorta and its branches (figure 2). Clinical diagnosis of TA was made based on 1990 American College of Rheumatology criteria. She met three out six criteria.
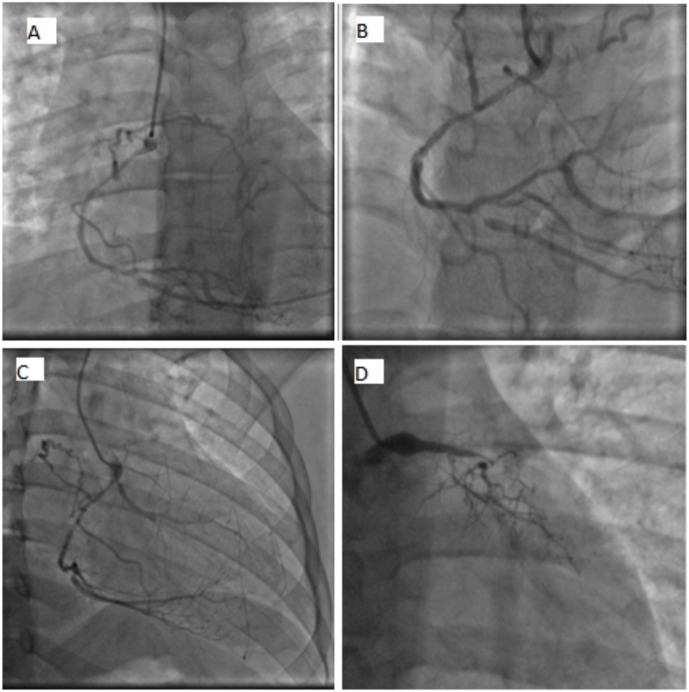
Figure 1.
Coronary angiography: (A) right coronary artery with moderate segmental obstruction sending a collateral to the left anterior descending (LAD). (B) Posterior descending artery with severe ostial lesion. (C) Marginal of circumflex sends a collateral to the LAD. (D) Left main with a severe obstruction. Proximal LAD and left circumflex occluded.
Figure 2.
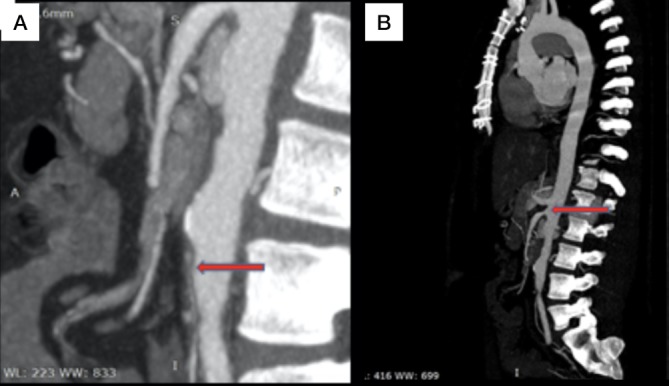
Aorta CT angiography. Red arrows point to inferior mesenteric stenosis (A) and superior mesenteric stenosis (B).
Treatment
The patient underwent an urgent coronary artery bypass grafting (CABG) using a saphenous vein to the posterior descending artery and left internal mammary artery to the LAD due to intractable pain. She was started on prednisone and methotrexate after the CABG.
Outcome and follow-up
Myocardial scintigraphy performed 8 months later due to atypical chest pain demonstrated severe myocardial ischaemia and new angiographic evidence of total occlusion of the left main coronary, patent arterial graft (figure 3A and B) and a 70% stenosis of the venous graft (figure 3C) to the posterior descending artery. A drug-eluting stent was placed in the venous graft to the posterior descending artery (figure 3C). Patient has been asymptomatic since then.
Figure 3.
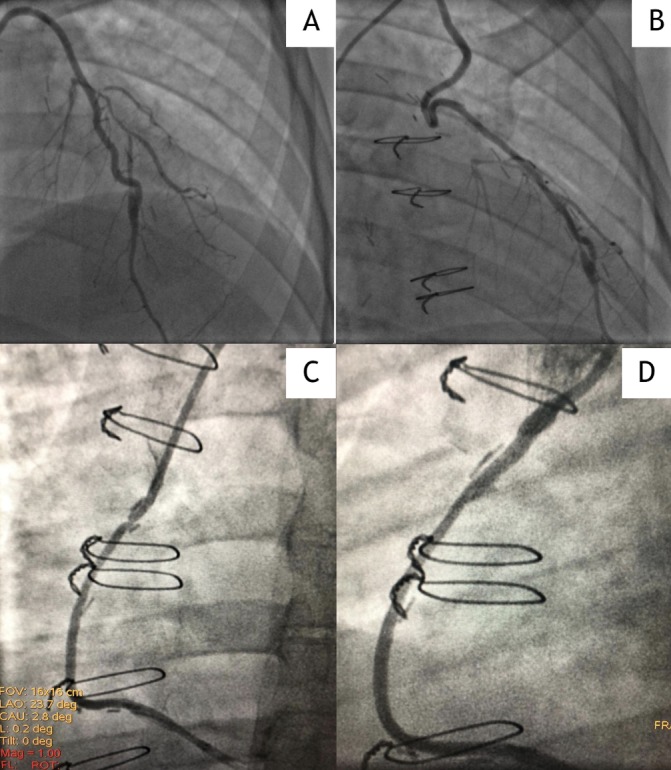
Coronary angiography: (A, B) patent arterial graft. (C) About 70% stenosis of the venous graft to the posterior descending artery. (D) Post-stent placement.
DISCUSSION
CAD is an uncommon entity in young women, however, it constitutes an important problem for patients and physicians due to devastating effects on the more active lifestyle of young patients.4 Our patient presented to the emergency department several times for chest pain, but due to her low predictive risk scores, she was discharged without any further investigation. Misdiagnosis is common in patients without atherosclerotic-related CAD, such as in autoimmune diseases.
TA is characterised as pan-arteritis causing ischaemic symptoms related to stenotic lesions.5 This patient had aortic and coronary artery involvement, but presented with only CAD-related symptoms without any constitutional symptom that made the diagnosis more challenging.
TA is often progressive and recurrent and may involve subclavian arteries, the aorta and the coronary arteries, leading to arterial graft compromise and disastrous consequences in the long term.6
CT, MRI, positron emission tomography (PET)/CT and PET/MRI are still the standard imaging methods to evaluate vasculitis.7 New imaging modalities can identify coronary arteritis, such as cardiac multi-detector CT that can detect various disease manifestations, including wall thickening, dissection, aneurysm, stenosis and abnormal periarterial soft tissue structures.8 Even though new intracoronary techniques such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT) have not been initially studied for vasculitis, there is a report of OCT supporting a TA diagnosis9 and the IVUS can help in complexes percutaneous coronary interventions (PCIs) when indicated.10
In cases of coronary involvement, ideally the clinical treatment with glucocorticoids (prednisone 40–60 mg/day or equivalent) should be started before cardiac intervention when possible.2 Our patient underwent an emergency revascularisation due to persistent chest pain, but the immunosuppressants were started just after the procedure.
In the stable stages of disease, the prognosis of PCI is similar to cardiac bypass surgery. For patients with active disease, if emergency revascularisation is necessary, CABG is ideal. If not, receiving medical therapy until disease remission and then undergoing PCI may be an alternative choice of CABG.11 Recent data suggest that both types of interventions are associated with a high failure rates12 with reported mortality ranging between 3% and 21.0%.13 Our patient had three vessel disease with left main involvement, signs of active disease and a high SyntaxScore II. Although PCI could be tried in our case we decided for CABG after a discussion with our Heart Team and family.
As TA damages medium arterial vessels, the use of arterial grafts is controversial due to their potential impairment by the disease, therefore the use of venous grafts is preferred with a survival of up to 80% in 10 years.11 Our patient had recurrence on her venous graft which is unusual.
This report shows the complexity of the diagnostic and therapeutic approach to TA with CAD. TA should be considered in young women without classical risk factors. The optimal treatment should be driven by a number of factors including coronary anatomy, age and available resources. Adequate follow-up and immunosuppressive therapy should be maintained to prevent early undesirable outcomes.
Learning points.
American College of Rheumatology Criteria for Takayasu Arteritis. It needs to satisfy three out six of the following: (1) Age at disease onset ≤40 years. (2) Claudication of the extremities. (3) Decreased pulsation of one or both brachial arteries. (4) Difference of at least 10 mm Hg in systolic blood pressure between the arms. (5) Bruit over one or both subclavian arteries or the abdominal aorta. (6) Arteriographic narrowing or occlusion of the entire aorta, its primary branches, or large arteries in the proximal upper or lower extremities, not due to arteriosclerosis, fibromuscular dysplasia or other causes.
The coronary involvement should be assessed as early as possible and treated according to its clinical manifestation.
The best graft for coronary artery bypass grafting in patients with TA cannot be definitive. Indeed if the arteritis can affect the arterial grafts, venous grafts can be affected by both, early and late failure, especially in the young.
A multidisciplinary team with rheumatology, cardiology, cardiothoracic surgery and interventional cardiology should be involved in cases of TA and coronary involvement.
Footnotes
Contributors: LMM: conception of the work, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published. NdAL: conception of the work, data analysis and interpretation, drafting the article, critical revision of the article and final approval of the version to be published. RLdC Jr: data collection, critical revision of the article and final approval of the version to be published. SFB: drafting the article, critical revision of the article and final approval of the version to be published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Seyahi E. Takayasu arteritis: an update. Curr Opin Rheumatol 2017;29:51–6. 10.1097/BOR.0000000000000343 [DOI] [PubMed] [Google Scholar]
- 2. Chatterjee S, Flamm SD, Tan CD, et al. Clinical diagnosis and management of large vessel vasculitis: Takayasu arteritis. Curr Cardiol Rep 2014;16:499 10.1007/s11886-014-0499-y [DOI] [PubMed] [Google Scholar]
- 3. Lie JT. Pathology of isolated nonclassical and catastrophic manifestations of Takayasu arteritis. Int J Cardiol 1998;66 Suppl 1(1Suppl):S11–S21. 10.1016/S0167-5273(98)00144-2 [DOI] [PubMed] [Google Scholar]
- 4. Cole JH, Miller JI, Sperling LS, et al. Long-term follow-up of coronary artery disease presenting in young adults. J Am Coll Cardiol 2003;41:521–8. 10.1016/S0735-1097(02)02862-0 [DOI] [PubMed] [Google Scholar]
- 5. Lee GY, Jang SY, Ko SM, et al. Cardiovascular manifestations of Takayasu arteritis and their relationship to the disease activity: analysis of 204 Korean patients at a single center. Int J Cardiol 2012;159:14–20. 10.1016/j.ijcard.2011.01.094 [DOI] [PubMed] [Google Scholar]
- 6. Reddy SM, Byrapaneni RB, Rangappa C, et al. Surgical revascularization for premature coronary artery disease in second and third decade of life. Interact Cardiovasc Thorac Surg 2017;24:99–101. 10.1093/icvts/ivw295 [DOI] [PubMed] [Google Scholar]
- 7. Ikonomidis I, Makavos G, Katsimbri P, et al. Imaging risk in multisystem inflammatory diseases. JACC Cardiovasc Imaging 2019;1936-878X:30179–2. 10.1016/j.jcmg.2018.06.033 [DOI] [PubMed] [Google Scholar]
- 8. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 9. Amsallem M, Henry P, Manzo Silberman S. Optical coherence tomography imaging of Takayasu coronary arteritis. Can J Cardiol 2014;30:464.e13–464.e14. 10.1016/j.cjca.2013.12.019 [DOI] [PubMed] [Google Scholar]
- 10. Ochijewicz D, Tomaniak M, Koltowski L, et al. Intravascular imaging of coronary artery disease: recent progress and future directions. J Cardiovasc Med 2017;18:733–41. 10.2459/JCM.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 11. Wang X, Dang A, Lv N, et al. Long-term outcomes of coronary artery bypass grafting versus percutaneous coronary intervention for Takayasu arteritis patients with coronary artery involvement. Semin Arthritis Rheum 2017;47:247–52. 10.1016/j.semarthrit.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 12. Labarca C, Makol A, Crowson CS, et al. Retrospective comparison of open versus endovascular procedures for takayasu arteritis. J Rheumatol 2016;43:427–32. 10.3899/jrheum.150447 [DOI] [PubMed] [Google Scholar]
- 13. Li J, Zhu M, Li M, et al. Cause of death in chinese takayasu arteritis patients. Medicine 2016;95:e4069 10.1097/MD.0000000000004069 [DOI] [PMC free article] [PubMed] [Google Scholar]