Abstract
The main aim of origins of life research is to find a plausible sequence of transitions from prebiotic chemistry to nascent biology. In this context, understanding how and when phospholipid membranes appeared on early Earth is critical to elucidating the prebiotic pathways that led to the emergence of primitive cells. Here we show that exposing glycerol-2-phosphate to acylating agents leads to the formation of a library of acylglycerol-phosphates. Medium-chain acylglycerol-phosphates were found to self-assemble into vesicles stable across a wide range of conditions and capable of retaining mono- and oligonucleotides. Starting with a mixture of activated carboxylic acids of different lengths, iterative cycling of acylation and hydrolysis steps allowed for the selection of longer-chain acylglycerol-phosphates. Our results suggest that a selection pathway based on energy-dissipative cycling could have driven the selective synthesis of phospholipids on early Earth.
Introduction
Phospholipids are among the major components of all modern membranes.1 Their predominance, together with the fundamental role of compartmentalization, which allows the genetic and metabolic systems to cooperate and Darwinian evolution to occur, might suggest that such amphiphiles appeared at an early stage on the evolutionary timeline.2 Yet, how and when phospholipids started to play such a crucial role for life still represent intriguing open questions.
A wide variety of single-chain amphiphiles, considered to have been available on early Earth, could have formed cell-like compartments under prebiotically plausible conditions.3−5 For example, fatty acids and alcohols can be abiotically produced via Fischer–Tropsch synthesis6,7 and readily self-assemble into vesicles.2,3 Since membrane permeability is inversely proportional to the length of the aliphatic chains, membranes formed of medium-chain (between 6 and 12 carbon atoms) fatty acids and alcohols would have potentially been advantageous for prebiotic systems.3,8 Primordial membranes would have required a certain degree of permeability to facilitate the diffusion of polar and ionic solutes between the prebiotic cell-like structure’s (or protocell’s) interior and the environment. Non-enzymatic RNA polymerization reactions in vesicles,8,9 as well as complete cycles of growth and division of fatty acid membranes,10,11 have already been shown to occur under prebiotically plausible conditions. However, the formation of such prebiotic membranes requires extremely high concentrations of medium-chain amphiphiles or the presence of cosurfactants,8,12 and the stability of such primitive cells is tremendously affected by environmental conditions such as divalent cations, pH, salinity, and ionic strength.2,13 Thus, in order to reduce the amphiphile critical aggregation concentration, to prevent the salt-induced disruption of fatty acid vesicles, and to generate and sustain chemical gradients across membranes, more complex phospholipids must have emerged to give rise to more advanced protocells.4,14−16
The identification of primitive selection processes is a long-standing challenge in prebiotic chemistry, including lipid self-assembly.17,18 Although model systems are useful for characterizing the properties of potentially primordial membranes,2 these models do not consider the heterogeneity of prebiotically available molecules. The demonstration of selective accumulation and self-assembly of primordial amphiphiles into vesicles among non-assembling species could represent an important advance toward the emergence of primitive cells.
A recent report from our group describes a non-enzymatic proofreading mechanism that progressively converts RNA 2′,5′-linkages into 3′,5′-linkages through iterative rounds of degradation and repair.19 The combination of selective hydrolysis of 2′,5′-linked duplex RNA with templated 3′,5′-selective ligation chemistry led to the development of a model in which repeated cycles of acetylation, ligation, and hydrolysis progressively increase the ratio of RNA 3′,5′-linkages. Thinking along these lines, we investigated a similar scheme whereby iterative rounds of acylation and hydrolysis could efficiently allow for the selection of self-assembling amphiphiles through energy-dissipative cycling. Considering the prevalence of ester-based phospholipids in the extant cellular world,20 and the extensive endeavors spent in the past years in achieving and optimizing RNA acylation chemistry,19,21−23 we investigated the synthesis of prebiotic acylglycerol phospholipids and their selective self-assembly properties. Here we show that repeated cycles of acylation of the phospholipid precursor glycerol-2-phosphate, followed by hydrolysis of the products, lead to the selective accumulation of longer-chain amphiphiles capable of self-assembly into cell-like structures, suggesting a plausible pathway toward the emergence of biologically relevant membranes.
Results and Discussion
Non-enzymatic Acylation of Glycerol-Phosphates
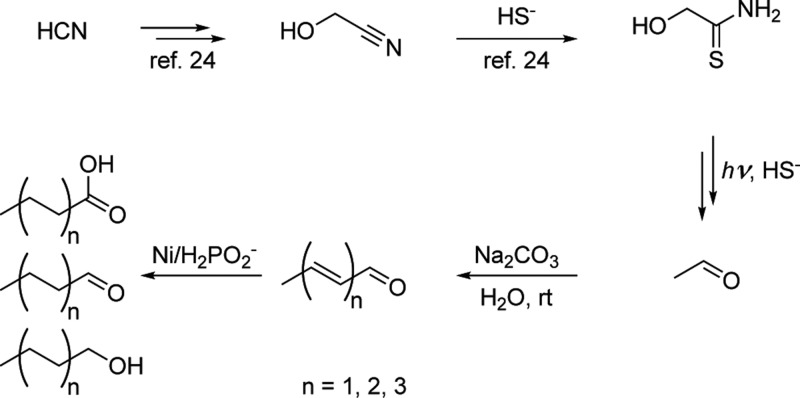
The major membrane-forming amphiphiles of all three kingdoms of life are esters or ethers of glycerol-1-phosphate.1 Several pathways for the prebiotic phosphorylation of glycerol have recently been published.24−26 For example, it has been reported that schreibersite (40g/L) reacts with aqueous glycerol (0.5 M) to give glycerol-1-phosphate and glycerol-2-phosphate in 2.5% combined yield (presumably based on total mobilization of phosphate).25 These authors suggest a meteoritic origin of glycerol citing the presence of the latter in the Murchison meteorite (where it is found at 160 nmol/g alongside dihydroxyacetone27 and likely millions of other compounds28).
We showed that glycerol-phosphates can be efficiently synthesized from the RNA precursor glyceraldehyde in 14% overall yield. Specifically, the photoreduction of glyceraldehyde’s 1 more stable isomer dihydroxyacetone 2 by hydrosulfide affords acetone 3 and glycerol 4. When heated with phosphate in urea, glycerol is efficiently converted into a mixture that contains predominantly glycerol-1,2-cyclic phosphate 5. Metal-based catalysis can be exploited to favor the hydrolytic ring-opening, which leads to the formation of the isomeric products glycerol-1-phosphate 6 and glycerol-2-phosphate 7 (Figure S1). This suggests that other chemistry in addition to Fischer–Tropsch processes can contribute to the inventory of lipid building blocks on early Earth. Moreover, we note that this chemistry would likely leave a 13C/12C isotopic distribution pattern similar to that left by fatty acid biosynthesis but distinct to that left by Fischer–Tropsch processes. Accordingly, we considered acylation of 7 with carboxylic acid moieties in the C2–C10 length range.
Acetaldehyde is a major product of the same cyanosulfidic chemistry that generates glyceraldehyde, and we have found that reduction of acetaldehyde homoenolization products generates C2, C4, C6, and C8 saturated alcohols and acids (see Scheme 1, Supporting Information (SI), and Figures S2–S10).
Scheme 1. Reaction Pathway That Leads to Synthesis of C2–C10 Alcohols, Aldehydes, and Carboxylic Acids.
For details, see SI.
The acetylation of nucleoside-3′-phosphates proceeds via attack of the phosphate dianion on the activated acetyl group. The acyl group then migrates from the phosphate to the 2′-hydroxyl group to give an ester.21 We envisioned a similar mechanism for the reaction of 6 and 7 with N-acetyl (C2) imidazolide 8a. The formation of the desired acetylated products was followed by 1H- and 31P NMR spectroscopy and was confirmed by LC-MS (Figures S11–S14). The reaction of 7 with 8a efficiently leads to the formation of two products, monoacetylglycerol-2-phosphate 9a and bis-acetylglycerol-2-phosphate 10a (Figure 1).
Figure 1.
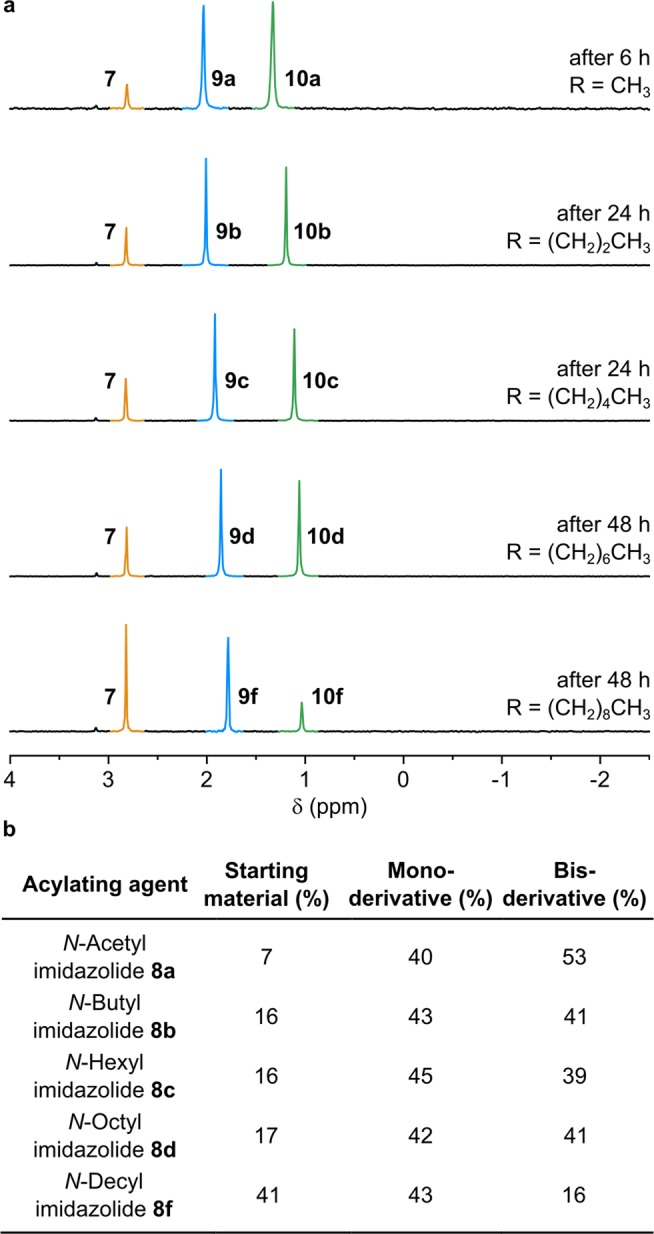
Synthesis of acylglycerol-2-phosphates. (a) 31P NMR Spectra of the products of the described reactions. Glycerol-2-phosphate 7 is highlighted in orange, monoacylated species 9a–d and 9f are highlighted in blue, and bis-acylated species 10a–d and 10f are highlighted in green. (b) Reaction yields for the synthesis of mono- and bis-acylglycerol-2-phosphate derivatives.
We suggest that the reaction proceeds via the initial formation of an intermediate mixed acetyl-phosphate anhydride, followed by subsequent migration of the acetyl group from the phosphate to the 1-hydroxyl group to afford the monoacetylated product 9a (Scheme 2). The second acetylation step would proceed in a similar manner with migration to the 3-hydroxyl group.
Scheme 2. Proposed Mechanism for the Synthesis of Acylglycerol-Phosphates.

In contrast, in the case of glycerol-1-phosphate 6, the migration of the acetyl group from the phosphate to the 2-hydroxyl group is disfavored due to it being secondary (cf. (2×) primary in the case of 7) and hydrolysis outcompetes migration. 31P NMR spectroscopy studies were undertaken to support this mechanistic assertion (Figure S15). One strong signal (−7.36 ppm) and two weaker signals (−8.12 and −8.56 ppm), characteristic of the mixed anhydride intermediates, were observed after 1 h when 6 and 7 were chosen as substrates, respectively.
We then investigated the reaction of 7 with a variety of acyl imidazolides 8b–f (with C4, C6, C8, C9, and C10 acyl chains, respectively) in a range of plausibly prebiotic solvent mixtures including water-formamide,30 taking into consideration the reduced aqueous solubility of longer-chain acyl imidazolides (Figures 1 and S16, Table S1). All of the new mono- and bis-acylglycerol-2-phosphate products formed efficiently and were characterized by 1H-, 13C-, and 31P NMR spectroscopy and LC-MS (Figures S17–S24).
Acylglycerol-Phosphates Are Capable of Membrane Self-Assembly
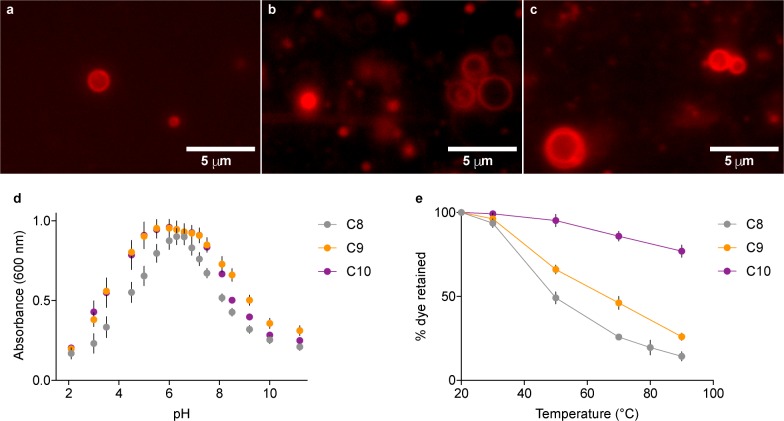
Amphiphiles more complex than fatty acids would have been required on early Earth to build up primordial cells capable of hosting nucleic acid replication, sustaining metabolic networks, and, ultimately, supporting Darwinian evolution.14 Membranes made of mono- and bis-acylglycerol-phosphates could thus represent a potential intermediate state in protocellular evolution. We therefore investigated the propensity of 9a–f and 10a–f to undergo membrane self-assembly (Figure S25). None of the monoacylated glycerol-2-phosphates (9d–f) were observed to form vesicles. Conversely, vesicles made of longer-chain bis-acylated glycerol-2-phosphates (10d–f) could be detected. Focusing our attention on the C8, C9, and C10 derivatives, we found that crude mixtures of mono- and bis-acylglycerol-2-phosphates synthesized as previously described also formed cell-like structures (Figure 2a–c). Specifically, when 9f was mixed with 10f in a 1:1 ratio, the critical aggregation concentration of the mixed decanoylglycerol-2-phosphate derivatives was found to be 3- and 100-fold lower than pure bis-decanoylglycerol-2-phosphate 10f and decanoic acid,8 respectively (Table S2). Vesicles made of either pure 10d–f or mixtures of 9d–f and 10d–f were stable to a wide range of pH (Figure 2d) and temperature values (Figures 2e and S26) and, furthermore, were able to retain small dyes, mono- and trinucleotides effectively over a 2 week period (Figure S27).
Figure 2.
Membrane self-assembly of acylglycerol-2-phosphates. (a–c) Vesicles were observed in crude mixtures of mono- and bis-acylglycerol-2-phosphates (pH 7.4, estimated lipid concentration: ∼40 mM): (a) 9d and 10d; (b) 9e and 10e; (c) 9f and 10f. (d) pH Stability range for vesicles made of pure 10d–f. (e) Temperature stability of vesicles made of 1:1 mixtures of 9d–f and 10d–f.
However, the incompatibility of fatty acid–based vesicles and the chemical conditions required for both ribozyme catalysis and non-enzymatic RNA polymerization remains problematic. Specifically, the high Mg2+ concentrations needed for non-enzymatic RNA replication chemistry cause lipid precipitation and membrane disruption. We thus examined the stability of acylglycerol-2-phosphate membranes upon addition of different amounts of Mg2+ ions (Figure S28).
Unsurprisingly, due to the high affinity of Mg2+ for dianionic phosphates,31 large lipid-magnesium crystals were observed in the presence of 10–40 mM Mg2+, suggesting that more advanced amphiphiles than acylglycerol-phosphates may be required to allow non-enzymatic RNA replication chemistry to take place within prebiotic protocells. Nevertheless, the equimolar addition of a chelating agent, such as EDTA or the more prebiotically plausible citrate,9 allowed for the efficient recovery of fully stable acylglycerol-2-phosphate vesicles.
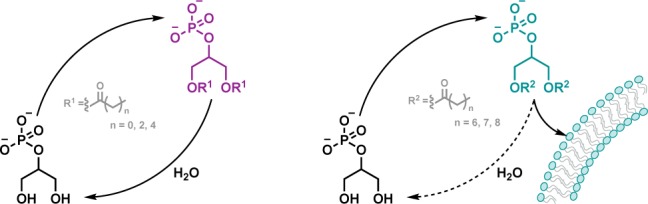
Alternatively, intrigued by the possibility to synthesize potentially Mg2+-stable lipids such as acylglycerol-2,3-cyclic phosphates,32 we explored isonitrile-driven phosphate activation chemistry33 on acylglycerol-2-phosphates. In the presence of methyl isonitrile and acetaldehyde, mono-octanoylglycerol-2-phosphate was shown to efficiently convert to the corresponding mono-octanoylglycerol-2,3-cyclic phosphate (Figure S29). A chemically derived set of prebiotic lipids could thus be generated and employed for the assembly of primordial membranes, complementary to previous studies on the non-enzymatic spontaneous remodeling of phospholipids, triggering changes in vesicle composition and stability.34 Our results are suggestive of a common chemical pathway that might have joined together the prebiotic activation of nucleotides, required for non-enzymatic Mg2+-driven RNA polymerization, and the synthesis of potentially Mg2+-resistant amphiphiles.
Selective Accumulation of Self-Assembling Amphiphiles through Recycling
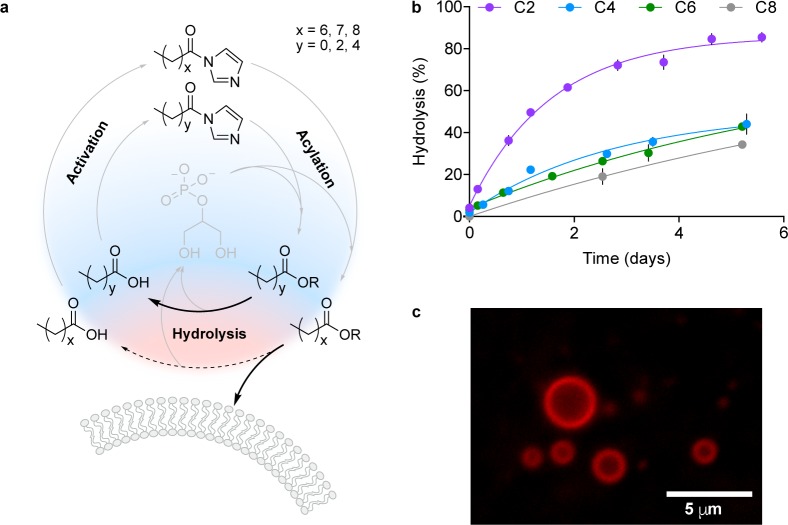
Due to the molecular heterogeneity of the prebiotic building blocks present on early Earth, an efficient mechanism for the selection of relevant biomolecules would have been required. Accordingly, we explored the ability of glycerol-2-phosphate 7 to undergo iterative rounds of competitive acylation and hydrolysis reactions, aiming to identify a selection process for self-assembling products from a heterogeneous library of amphiphiles (Figures S30–S35 and Table S3). When 7 was exposed to an equimolar mixture of 8a and 8b, C2 and C4 esters were observed by 1H NMR spectroscopy in a 1:1 ratio. However, when longer-chain imidazolides were tested in conjunction with short ones, 8a was found to outcompete 8d in the acylation process, leading to a higher yield of acetylated products (C2:C8 esters in a 2.6:1 ratio). We reasoned that the lower solubility and the greater steric hindrance of longer-chain imidazolides slowed down the formation of the mixed anhydride intermediate and the subsequent migration step, thus reducing the yield of the corresponding acylated product. We next focused on the ability of the acylated glycerol-2-phosphate products to undergo competitive hydrolysis (Figure S36). We observed that the shorter-chain derivatives 10a–c hydrolyzed faster than the longer-chain analogue 10d, yielding 7 and the corresponding carboxylic acid 11a–d.
Encouraged by these results, we performed sequential rounds of acylation and hydrolysis wondering if longer-chain amphiphiles capable of membrane self-assembly might be selected (Figures 3 and S37–S38). A solution containing 7 was exposed to our library of acylating agents (8a–d) for 22 h such that the resulting acylglycerol-2-phosphate derivatives were initially formed at concentrations lower than their critical aggregation concentrations. As expected, membrane formation was not observed in this initial phase. The solution was then subjected to hydrolysis conditions (for 24 h) to effect the selective degradation of the shorter-chain products. Repeated rounds (up to 6) of one-pot acylation with 8a–d and hydrolysis were performed. Accumulation of longer-chain glycerol-2-phosphate derivatives (9d and 10d) and membrane formation were monitored through 1H- and 31P NMR spectroscopy and fluorescence microscopy, respectively.
Figure 3.
Selective accumulation of prebiotic phospholipids via energy-dissipative cycling. (a) Scheme of the acylation–hydrolysis cycles. (b) Stability of 10a–d toward hydrolysis. (c) Membranes made of mixed acylglycerol-2-phosphates were observed from the second acylation–hydrolysis round.
In accordance with the cycling model proposed herein (Figure 3a), when 7 was subjected to multiple rounds of acylation with 8a–d and hydrolysis, the mixture became progressively enriched in the longer-chain amphiphiles (9d and 10d), at the expense of the shorter-chain species (9a–c and 10a–c), with concomitant formation of vesicles.
Conclusions
The non-enzymatic energy-dissipative conversion of non-assembling species into membrane self-assembling amphiphiles could represent a plausible pathway to drive the evolution of phospholipids toward those used by biology.35,36 Here we have presented an effective pathway for the selection of self-assembling amphiphiles from a library of diverse chain-length acylating reactants. Motivated by our observation of the prebiotic formation of glycerol phosphates, we investigated the acylation chemistry of these phospholipid precursors. We showed that C8–C10 acylglycerol-2-phosphates form stable membranes, which exhibit enhanced encapsulation efficiency and stability toward pH and temperature with respect to membranes made solely of fatty acids of analogous chain length. Phospholipid selection is achieved through multiple rounds of acylation and hydrolysis, which allow for membrane formation as the self-assembling species accumulate.
We have previously observed that backbone-heterogeneous RNA oligomers might have undergone proofreading to overcome the inherent lack of regiocontrol in the oligomerization step through energy-dissipative recycling.19 Similarly, iterative rounds of acylation and hydrolysis could be exploited to directly select for self-assembling phospholipids from a heterogeneous mixture of amphiphiles. The energetic cost of this optimization process is met by the hydrolytic turnover of acylating agents. The ideal scenario to promote acylation and hydrolysis processes might have emerged from a set of closely related geochemical settings29 or geophysical scenarios involving fluctuations in bulk conditions, for example, pH, temperature, and salt concentrations, such as those described in open rock pores.37,38
Although the identification of prebiotically plausible acylating agents for the formation of acylglycerol-phosphates still represents an open challenge in prebiotic chemistry, our findings delineate a potential route that could have driven the transition toward biological phospholipids on early Earth.
Acknowledgments
This work was supported by the Medical Research Council (grant no. MC_UP_A024_1009) and a grant from the Simons Foundation (grant no. 290362 to J.D.S.). We thank A. N. Albertsen, D. J. Ritson, D. A. Russell, and other J.D.S. group members for fruitful discussions.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b12331.
Materials, methods, compound characterization, supplementary figures and tables (PDF)
Author Contributions
† These authors contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Lombard J.; López-García P.; Moreira D. The early evolution of lipid membranes and the three domains of life. Nat. Rev. Microbiol. 2012, 10, 507. 10.1038/nrmicro2815. [DOI] [PubMed] [Google Scholar]
- Blain J. C.; Szostak J. W. Progress Toward Synthetic Cells. Annu. Rev. Biochem. 2014, 83, 615. 10.1146/annurev-biochem-080411-124036. [DOI] [PubMed] [Google Scholar]
- Monnard P. A.; Deamer D. W. Membrane self-assembly processes: Steps toward the first cellular life. Anat. Rec. 2002, 268, 196. 10.1002/ar.10154. [DOI] [PubMed] [Google Scholar]
- Chen I. A.; Walde P. From self-assembled vesicles to protocells. Cold Spring Harbor Perspect. Biol. 2010, 2, a002170. 10.1101/cshperspect.a002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansy S. S.; Szostak J. W. Reconstructing the emergence of cellular life through the synthesis of model protocells. Cold Spring Harbor Symp. Quant. Biol. 2009, 74, 47. 10.1101/sqb.2009.74.014. [DOI] [PubMed] [Google Scholar]
- McCollom T. M.; Ritter G.; Simoneit B. R. T. Lipid Synthesis Under Hydrothermal Conditions by Fischer–Tropsch-Type Reactions. Origins Life Evol. Biospheres 1999, 29, 153. 10.1023/A:1006592502746. [DOI] [PubMed] [Google Scholar]
- Mißbach H.; Schmidt B. C.; Duda J. P.; Lünsdorf N. K.; Goetz W.; Thiel V. Assessing the diversity of lipids formed via Fischer–Tropsch-type reactions. Org. Geochem. 2018, 119, 110. 10.1016/j.orggeochem.2018.02.012. [DOI] [Google Scholar]
- Mansy S. S.; Schrum J. P.; Krishnamurthy M.; Tobé S.; Treco D. A.; Szostak J. W. Template-directed synthesis of a genetic polymer in a model protocell. Nature 2008, 454, 122. 10.1038/nature07018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamala K.; Szostak J. W. Nonenzymatic template-directed RNA synthesis inside model protocells. Science 2013, 342, 1098. 10.1126/science.1241888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budin I.; Debnath A.; Szostak J. W. Concentration-driven growth of model protocell membranes. J. Am. Chem. Soc. 2012, 134, 20812. 10.1021/ja310382d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T. F.; Szostak J. W. Coupled growth and division of model protocell membranes. J. Am. Chem. Soc. 2009, 131, 5705. 10.1021/ja900919c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel C. L.; Deamer D. W. The formation of glycerol monodecanoate by a dehydration/condensation reaction: Increasing the chemical complexity of amphiphiles on the early earth. Origins Life Evol. Biospheres 2005, 35 (4), 323. 10.1007/s11084-005-2046-8. [DOI] [PubMed] [Google Scholar]
- Maurer S. E.; Nguyen G. Prebiotic Vesicle Formation and the Necessity of Salts. Origins Life Evol. Biospheres 2016, 46 (2–3), 215. 10.1007/s11084-015-9476-8. [DOI] [PubMed] [Google Scholar]
- Joyce G. F.; Szostak J. W. Protocells and RNA Self-Replication. Cold Spring Harbor Perspect. Biol. 2018, 10, a034801. 10.1101/cshperspect.a034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfio C.; Godino E.; Corsini M.; Fabrizi de Biani F.; Guella G.; Mansy S. S. Prebiotic iron–sulfur peptide catalysts generate a pH gradient across model membranes of late protocells. Nat. Catal. 2018, 1, 616. 10.1038/s41929-018-0116-3. [DOI] [Google Scholar]
- Jin L.; Kamat N. P.; Jena S.; Szostak J. W. Fatty Acid/Phospholipid Blended Membranes: A Potential Intermediate State in Protocellular Evolution. Small 2018, 14, 1704077. 10.1002/smll.201704077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budin I.; Prywes N.; Zhang N.; Szostak J. W. Chain-length heterogeneity allows for the assembly of fatty acid vesicles in dilute solutions. Biophys. J. 2014, 107, 1582. 10.1016/j.bpj.2014.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Mirazo K.; Briones C.; de la Escosura A. Prebiotic Systems Chemistry: New Perspectives for the Origins of Life. Chem. Rev. 2014, 114, 285. 10.1021/cr2004844. [DOI] [PubMed] [Google Scholar]
- Mariani A.; Sutherland J. D. Non-Enzymatic RNA Backbone Proofreading through Energy-Dissipative Recycling. Angew. Chem., Int. Ed. 2017, 56, 6563. 10.1002/anie.201703169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama T.; Riezman H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281. 10.1038/nrm.2017.138. [DOI] [PubMed] [Google Scholar]
- Bowler F. R.; Chan C. K. W.; Duffy C. D.; Gerland B.; Islam S.; Powner M. W.; Sutherland J. D.; Xu J. Prebiotically plausible oligoribonucleotide ligation facilitated by chemoselective acetylation. Nat. Chem. 2013, 5, 383. 10.1038/nchem.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-García C.; Powner M. W. Selective Acylation of Nucleosides, Nucleotides, and Glycerol-3-phosphocholine in Water. Synlett 2016, 28, 78. 10.1055/s-0036-1588626. [DOI] [Google Scholar]
- Liu R.; Orgel L. E. Oxidative acylation using thioacids. Nature 1997, 389, 52. 10.1038/37944. [DOI] [PubMed] [Google Scholar]
- Burcar B.; Pasek M.; Gull M.; Cafferty B. J.; Velasco F.; Hud N. V.; Menor-Salvan C. Darwin’s Warm Little Pond: A One-Pot Reaction for Prebiotic Phosphorylation and the Mobilization of Phosphate from Minerals in a Urea-Based Solvent. Angew. Chem., Int. Ed. 2016, 55, 13249. 10.1002/anie.201606239. [DOI] [PubMed] [Google Scholar]
- Pasek M. A.; Harnmeijer J. P.; Buick R.; Gull M.; Atlas Z. Evidence for reactive reduced phosphorus species in the early Archean ocean. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (25), 10089. 10.1073/pnas.1303904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B. H.; Percivalle C.; Ritson D. J.; Duffy C. D.; Sutherland J. D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301. 10.1038/nchem.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G.; Kimmich N.; Belisle W.; Sarinana J.; Brabham K.; Garrel L. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 2001, 414, 879. 10.1038/414879a. [DOI] [PubMed] [Google Scholar]
- Schmitt-Kopplin P.; Gabelica Z.; Gougeon R. D.; Fekete A.; Kanawati B.; Harir M.; Istvan G.; Eckel G.; Hertkorn N. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 2763. 10.1073/pnas.0912157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritson D. J.; Battilocchio C.; Ley S. V.; Sutherland J. D. Mimicking the surface and prebiotic chemistry of early Earth using flow chemistry. Nat. Commun. 2018, 9, 1821. 10.1038/s41467-018-04147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powner M. W.; Sutherland J. D. Prebiotic chemistry: a new modus operandi. Philos. Trans. R. Soc., B 2011, 366, 2870. 10.1098/rstb.2011.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. B.; Huang H. W.; Cowan J. A. Biological Coordination Chemistry of Magnesium, Sodium, and Potassium-Ions - Protein and Nucleotide-Binding Sites. Coord. Chem. Rev. 1994, 135, 165. 10.1016/0010-8545(94)80068-5. [DOI] [Google Scholar]
- Gibard C.; Bhowmik S.; Karki M.; Kim E.-K.; Krishnamurthy R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 2017, 10, 212. 10.1038/nchem.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani A.; Russell D. A.; Javelle T.; Sutherland J. D. A Light-Releasable Potentially Prebiotic Nucleotide Activating Agent. J. Am. Chem. Soc. 2018, 140, 8657. 10.1021/jacs.8b05189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brea R. J.; Rudd A. K.; Devaraj N. K. Nonenzymatic biomimetic remodeling of phospholipids in synthetic liposomes. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (31), 8589. 10.1073/pnas.1605541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena-Solsona M.; Wanzke C.; Riess B.; Bausch A. R.; Boekhoven J. Self-selection of dissipative assemblies driven by primitive chemical reaction networks. Nat. Commun. 2018, 9, 2044. 10.1038/s41467-018-04488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walde P.; Wick R.; Fresta M.; Mangone A.; Luisi L. P. Autopoietic Self-Reproduction of Fatty Acid Vesicles. J. Am. Chem. Soc. 1994, 116, 11649. 10.1021/ja00105a004. [DOI] [Google Scholar]
- Kreysing M.; Keil L.; Lanzmich S.; Braun D. Heat flux across an open pore enables the continuous replication and selection of oligonucleotides towards increasing length. Nat. Chem. 2015, 7, 203. 10.1038/nchem.2155. [DOI] [PubMed] [Google Scholar]
- Keil L. M. R.; Möller F. M.; Kieß M.; Kudella P. W.; Mast C. B. Proton gradients and pH oscillations emerge from heat flow at the microscale. Nat. Commun. 2017, 8, 1897. 10.1038/s41467-017-02065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.