Abstract
Although accepted agents in chorioamnionitis and preterm birth, the role of Ureaplasma species (spp.) in inflammation-driven morbidities of prematurity, including the development of bronchopulmonary dysplasia, remains controversial. To add to scarce in vitro data addressing the pro-inflammatory capacity of Ureaplasma spp., pulmonary epithelial-like A549 cells and human pulmonary microvascular endothelial cells (HPMEC) were incubated with Ureaplasma (U.) urealyticum, U. parvum, and Escherichia coli lipopolysaccharide (LPS). Ureaplasma isolates down-regulated caspase mRNA levels in A549 cells (caspase 8: p<0.001, 9: p<0.001, vs. broth), while increasing caspase protein expression, enzyme activity, and cell death in HPMEC (active caspase 3: p<0.05, caspase 8: p<0.05, active caspase 9: p<0.05, viability: p<0.05). LPS, contrarily, induced caspase mRNA expression in HPMEC (caspase 3: p<0.01, 4: p<0.001, 5: p<0.001, 8: p<0.001, vs. control), but not in A549 cells, and did not affect enzyme activity or protein levels in either cell line. LPS, but neither Ureaplasma isolate, enhanced mRNA expression of pro-inflammatory interleukin (IL)-6 in both A549 (p<0.05, vs. control) and HPMEC (p<0.001) as well as tumor necrosis factor-α (p<0.01), IL-1β (p<0.001), and IL-8 (p<0.05) in HPMEC. We are therefore the first to demonstrate a differential modulation of pulmonary caspases by Ureaplasma spp. in vitro. Ureaplasma-driven enhanced protein expression and activity of caspases in pulmonary endothelial cells result in cell death and may cause structural damage. Down-regulated caspase mRNA in pulmonary epithelial cells, contrarily, may indicate Ureaplasma-induced inhibition of apoptosis and prevent effective immune responses. Both may ultimately contribute to chronic Ureaplasma colonization and long-term pulmonary inflammation.
Introduction
Ureaplasma species (spp.) commonly colonize the adult urogenital tract and are generally considered of low virulence [1]. Transmission from mother to infant is frequent and can occur in utero, intrapartum, or postpartum [1]. Intraamniotic Ureaplasma infection is an accepted risk factor for chorioamnionitis and premature birth [2–4], and Ureaplasma spp. are known to cause sepsis, meningitis, and pneumonia in neonates [5–8] as well as severe invasive infections in immunocompromised adults such as lung transplant patients [9]. Ureaplasma spp. can be detected in the respiratory tract in 65% of preterm infants < 26 weeks of gestation [10]. Fetal or neonatal respiratory tract colonization with Ureaplasma spp. has been associated with bronchopulmonary inflammation and altered lung development, which may ultimately culminate in chronic lung diseases such as bronchopulmonary dysplasia (BPD) in preterm infants [11–13]. Inflammation is considered a key factor in the multifactorial pathogenesis of BPD development [13, 14]. Animal models support a potential causality between Ureaplasma spp. and development of BPD, demonstrating pulmonary inflammation accompanied by structural lung tissue impairment upon fetal Ureaplasma exposure [15, 16]. Clinical studies, however, are contradictory [17], and in vitro data on the pro-inflammatory capacity of Ureaplasma spp. are generally scarce.
In pulmonary inflammation, lung epithelial and endothelial cells both deserve attention. They usually maintain intrapulmonary homeostasis as well as an immunological balance [18, 19]. Pulmonary epithelial cells maintain the air-blood barrier and comprise alveolar type I and II cells [20]. While type I cells primarily enable gas exchange, type II cells produce surfactant and are crucial for tissue repair [19]. Opposed to epithelial cells, lung endothelial cells are more permeable [21] and contribute to inflammatory processes by signal transduction and initiation of inflammatory cell migration into the alveolar space [19, 22].
Inflammation is classically understood to be initiated by early pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-8 [23]. However, recent studies have suggested an interlinkage of apoptosis and inflammation, proposing a crucial role for caspases in both [24, 25]. Whereas caspases 3, 8, and 9 are among those primarily involved in apoptosis, caspases 4 and 5 mediate inflammatory responses and pyroptosis as an inflammatory form of cell death [26, 27]. Regulation of caspase activity is complex and involves the transcription and translation processes as well as specific cleavage and activation of synthesized inactive caspase proenzymes [27].
We could recently demonstrate caspase modulation and a pro-apoptotic capacity of Ureaplasma spp. in human brain microvascular endothelial cells (HBMEC) [28]. In the present study, we addressed Ureaplasma-induced caspase expression as well as cytokine responses in the well described epithelial type II cell-like line A549 and human pulmonary microvascular endothelial cell line HPMEC-ST1.6R (HPMEC) [19, 29–31].
Materials and methods
Bacterial strains and culture conditions
Ureaplasma (U.) urealyticum serovar 8 and U. parvum serovar 3 were obtained from the American Tissue Culture Collection (ATCC, Manassas, VA; serovar 8: ATCC 27618, serovar 3: ATCC 27815). Isolates were cultured in in-house modified 10-B medium [32] (referred to as “broth”), containing 82% pleuropneumonia-like organism medium (Becton, Dickinson & Company, Franklin Lakes, NJ), 10% heat-inactivated horse serum (v/v), 1% urea (w/v) and 0.002% phenol red (w/v) (all: Sigma-Aldrich, St. Louis, CA), adjusted to pH 6.5. An endotoxin level < 0.06 EU/mL broth was verified using ToxinSensor Endotoxin Detection System (GenScript, Piscataway, NJ). Serial 10-fold dilutions were incubated for 18–20 h to achieve titers of 1×109−1×1010 color-changing units (CCU)/mL of viable cells.
Eukaryotic cells and culture conditions
A549 cells (ATCC CRM-CCL-185) were cultured in DMEM (Sigma-Aldrich) supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma-Aldrich). HPMEC-ST1.6R [31] were cultured in M199 Medium (Biochrom, Merck, Darmstadt, Germany) supplemented with 10% fetal bovine serum (Gibco), 2 mM L-glutamine (Biochrom), 5000 U/mL heparin (Biochrom), 5 μg/mL endothelial cell growth supplement (Omnilab, Bremen, Germany), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated in their respective growth media at 37°C in a humidified atmosphere with 5% CO2.
For stimulation assays, 3.5×105 A549 cells and 2.5×105 HPMEC per well were seeded in uncoated six well plates (Greiner, Frickenhausen, Germany). 24 h later, cells were stimulated as indicated in 1 mL fresh growth medium without antibiotics.
Stimulation assays
Ureaplasma suspensions of 109−1010 CCU/mL were concentrated by centrifugation, and 109−1010 CCU in 250 μl broth were added to each well. CCU were determined by 10-fold titration, as described elsewhere [33], and corresponding genome equivalents were identified, amounting to 5×107−6×108 copy numbers/mL (Institute of Medical Microbiology and Hospital Hygiene, Duesseldorf, Germany) [34]. Viability was verified by simultaneous culture on selective agar plates (medco Diagnostika GmbH, Ottobrunn, Germany). Lipopolysaccharide (LPS, Escherichia coli serotype 055:B5, Sigma-Aldrich) was added to a subgroup of cells at a concentration of 100 ng/mL. All doses had been determined by preliminary assays analogous to previous approaches [33, 35–40]. According to results of preliminary time kinetic experiments [35], exposure times of 4 and 30 h were chosen for RNA analysis, whereas flow cytometry was performed after 24 h. Experiments were repeated at least 3 times (n ≥ 3). Unstimulated cells served as negative controls. To adjust for potential confounding effects of Ureaplasma medium, cells exposed to Ureaplasma isolates were additionally compared to broth control and results were considered relevant if comparisons to both negative controls showed p values < 0.05.
RNA extraction and RT-PCR
For RNA extraction, cells were treated as indicated and total RNA was isolated using NucleoSpin RNA Kit (Macherey-Nagel, Dueren, Germany). For quantification of total RNA, a Qubit 2.0 Fluorometer (Thermo Fisher Scientific) was employed. Total RNA was eluted in 60 μL RNase-free H2O (Macherey-Nagel) and stored at -80°C until reverse transcription. For RT-PCR, 0.5–1 μg of total RNA was reverse transcribed using High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). First strand cDNA was diluted 1 to 10 with deionized, nuclease-free H2O (Sigma-Aldrich) and stored at -20°C upon analysis.
Quantitative real time RT-PCR (qRT-PCR)
For quantitative detection of mRNA, 10 μL of diluted first strand cDNA were analyzed in duplicates of 25 μL reactions using 12.5 μL iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA), 0.5 μL deionized H2O, and 1 μL of a 10 μM solution of forward and reverse primers (Sigma-Aldrich) as indicated in Table 1. Primers were designed using PerlPrimer software v1.1.20 [41]. Amplicons were designed to be 100–200 bp in size. A BLAST search was performed for every primer to confirm specificity with E values < 1. At least one primer of each pair spanned an intron/exon boundary to ensure mRNA-specific amplification. PCRs were performed on an Applied Biosystems 7500 Real-Time PCR System (Thermo Fisher Scientific), using a 2-step PCR protocol after an initial denaturation at 95°C for 10 min with 40 cycles of 95°C for 15 s and 60°C for 1 min. A melt curve analysis was performed at the end of every run to verify single PCR products. Levels of mRNA were normalized to those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Mean fold changes in mRNA expression were calculated by the ΔΔCT method by Livak and Schmittgen [42].
Table 1. Primers for qRT-PCR.
| Gene symbol | Sequence accession # | Orientation | Sequence [5’ to 3’] |
Amplicon length [bp] |
|---|---|---|---|---|
| CASP3 | NM_004346.3 | forward | CATTGAGACAGACAGTGG | 108 |
| reverse | TCGCCAAGAATAATAACCAG | |||
| CASP4 | NM_001225.3 | forward | GTTTGACCATCTGCCTCC | 126 |
| reverse | CGCTGACTCCATATCCCT | |||
| CASP5 | NM_004347.3 | forward | CTTTCTGTTCTTCAACACCA | 143 |
| reverse | ATGATTTCTGTACCTTCCGA | |||
| CASP8 | NM_001228.4 | forward | CTGATTCAGAGGAGCAACCC | 200 |
| reverse | GAATATCATCGCCTCGAGGAC | |||
| CASP9 | NM_001229.4 | forward | CCATATCTAGTTTGCCCACAC | 183 |
| reverse | GAAACAGCATTAGCGACCCT | |||
| CXCL8 | NM_000584.3 | forward | CAGTGCATAAAGACATACTCC | 198 |
| reverse | TTTATGAATTCTCAGCCCTC | |||
| GAPDH | NM_002046.5 | forward | CCATGGAGAAGGCTGGGG | 195 |
| reverse | CAAAGTTGTCATGGATGACC | |||
| IL1B | NM_000576.2 | forward | TTCATTGCTCAAGTGTCTG | 128 |
| reverse | GCACTTCATCTGTTTAGGG | |||
| IL6 | NM_000600.4 | forward | AACAAATTCGGTACATCCTC | 167 |
| reverse | AAGTCTCCTCATTGAATCCA | |||
| TNF | NM_000594.3 | forward | CAGCCTCTTCTCCTTCCT | 188 |
| reverse | GGGTTTGCTACAACATGG |
Flow cytometry
Harvested cells were separated by centrifugation and stained with Fixable Viability Dye eFluor 780 (eBioScience, San Diego, CA). Centrifugation and resuspension in Phosphate Buffered Saline (PBS, Sigma-Aldrich) were followed by fixation (fixation buffer, BioLegend). After another centrifugation step, cells were permeabilized (permeabilization wash buffer, BioLegend) and stained with antibodies to cleaved caspase 3 (Alexa Fluor 647 conjugated, rabbit, Cell Signaling Technology, Danvers, MA), caspase 8 (unconjugated, mouse, Abcam, Cambridge, UK), and cleaved caspase 9 (PE conjugated, rabbit, Cell Signaling Technology). To detect the unconjugated caspase 8 antibody, cells were once more separated by centrifugation and stained with an Alexa Fluor 405 conjugated secondary antibody (goat anti-mouse, Life Technologies, Thermo Fisher Scientific). After centrifugation and resuspension of the cells in PBS containing 1% human serum (Biochrom), samples were read on a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA). At least 10,000 events were acquired and analyzed with FACSDiva v6.1.3 software (BD Biosciences). Events were gated via forward and side scatter, and doublets were excluded employing a SSC-width versus FSC-area dot plot (the gating strategy is described in S1 Fig). For viability assessment, all events were included and the percentage of viability dye positive cells, considered dead, was determined.
Statistical analysis
Data were analyzed by a one way ANOVA with Tukey’s multiple comparisons test using Prism 6 software (GraphPad Software, San Diego, CA). The significance threshold for p values was set at < 0.05. Data are shown as means ± standard deviation (SD).
Results
Ureaplasma-driven cell death in pulmonary epithelial and endothelial cells
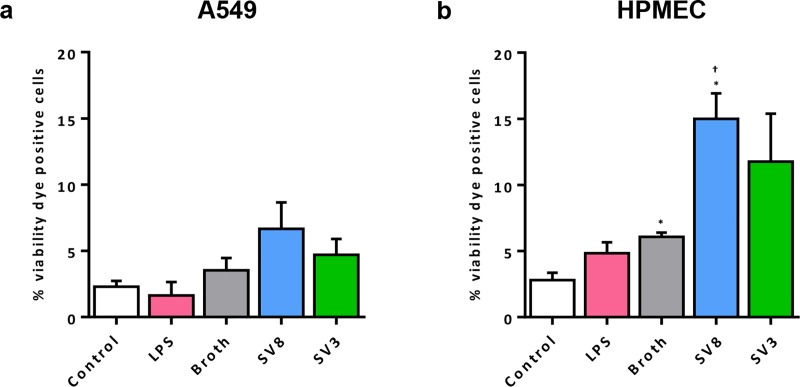
Flow cytometry was used to identify viability dye positive, dead cells. In A549 cells, we generally counted low numbers of dead cells, without any significant influences on cell viability yielded by Ureaplasma spp. (Fig 1A). Exposure of HPMEC to both Ureaplasma isolates, however, caused a distinct increase in numbers of dead cells after 24 h (Fig 1B), which was significant for serovar 8 (2.5 ± 0.3-fold, p = 0.0347, vs. broth). Broth itself induced a mild increase in dead cells. LPS did not affect viability of A549 cells or HPMEC (Fig 1).
Fig 1. Ureaplasma-driven cell death in pulmonary epithelial and endothelial cells.
Following a 24 h stimulation period, numbers of dead cells were determined for different conditions using flow cytometry and a viability dye staining dead cells. Results for A549 cells are shown in (a), whereas (b) depicts the percentage of dead HPMEC. Data are presented as means ± SD and were obtained from n = 3 individual experiments. Cells stimulated with LPS were compared vs. control, cells exposed to Ureaplasma spp. vs. control and vs. broth. * p < 0.05 compared to untreated controls; † p < 0.05 compared to cells treated with broth. SV8: Ureaplasma urealyticum serovar 8, SV3: Ureaplasma parvum serovar 3.
Ureaplasma-driven caspase responses in pulmonary epithelial cells
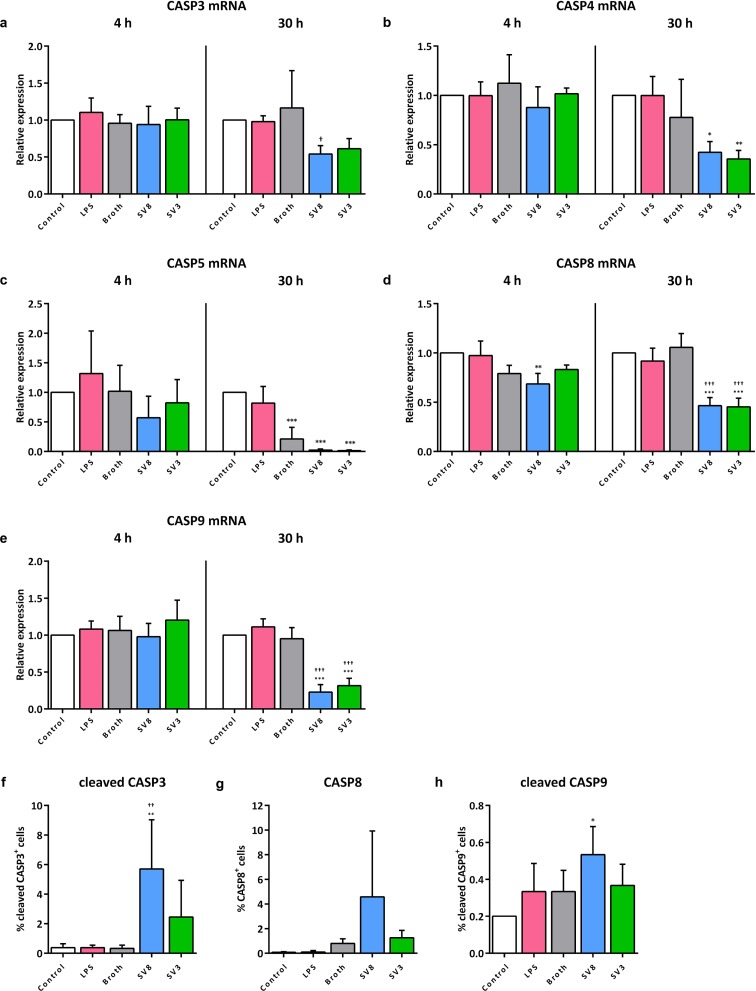
For caspases 3, 4, and 5, we observed a trend towards lower mRNA levels following 30 h Ureaplasma exposure of A549 cells, which mostly did not reach statistical significance (caspase 3, serovar 8: 0.5 ± 0.1-fold, p = 0.0442, serovar 3: 0.5 ± 0.1-fold, p = 0.0828; caspase 4, serovar 8: 0.5 ± 0.1-fold, p = 0.2332, serovar 3: 0.5 ± 0.1-fold, p = 0.1228; caspase 5, serovar 8: 0.1 ± 0.09-fold, p = 0.5481, serovar 3: 0.07 ± 0.06-fold, p = 0.5106, vs. broth) (Fig 2A–2C). Ureaplasma stimulation of A549 cells for 30 h resulted in a significant down-regulation of mRNA expression of caspase 8 (serovar 8: 0.4 ± 0.1-fold, p < 0.001, serovar 3: 0.4 ± 0.1-fold, p < 0.001, vs. broth) (Fig 2D). An even more distinct reduction was observable for caspase 9 mRNA upon a 30 h exposure to serovar 8 (0.2 ± 0.1-fold, p < 0.001) and serovar 3 (0.3 ± 0.1-fold, p < 0.001, vs. broth) (Fig 2E). Broth itself had mild suppressive effects on caspase 5 (Fig 2C). A short-term Ureaplasma exposure for 4 h as well as stimulation of A549 cells with LPS for 4 or 30 h did not significantly influence mRNA levels of caspases 3, 4, 5, 8, or 9 (Fig 2A–2E).
Fig 2. Ureaplasma-driven caspase mRNA and protein responses in A549 cells.
After 4 and 30 h stimulation of A549 cells, mRNA levels of caspase (CASP) 3 (a), caspase 4 (b), caspase 5 (c), caspase 8 (d), and caspase 9 (e) were assessed via qRT-PCR, and relative expression was calculated using the ΔΔCT method. After 24 h stimulation, the percentage of viable, active caspase 3 (f), caspase 8 (g), and active caspase 9 (h) positive A549 cells was determined by flow cytometry (the respective gating strategy is illustrated in S1 Fig). Data are shown as means ± SD and were obtained from n ≥ 3 independent experiments. Cells stimulated with LPS were compared vs. control, cells exposed to Ureaplasma isolates vs. control and vs. broth. * p < 0.05, ** p < 0.01, and *** p < 0.001 compared to untreated controls; † p < 0.05, †† p < 0.01, and ††† p < 0.001 compared to cells treated with broth. SV8: Ureaplasma urealyticum serovar 8, SV3: Ureaplasma parvum serovar 3.
Flow cytometry revealed slightly higher levels of active (cleaved) caspase 3 upon 24 h exposure to serovar 8 (17.5 ± 10.2-fold, p = 0.0075, vs. broth, Fig 2F). Ureaplasma exposure did not affect caspase 8 protein abundance or caspase 9 activity in A549 cells, and neither did LPS evoke any responses on caspase protein or activity levels (Fig 2F–2H).
Ureaplasma-driven caspase responses in pulmonary endothelial cells
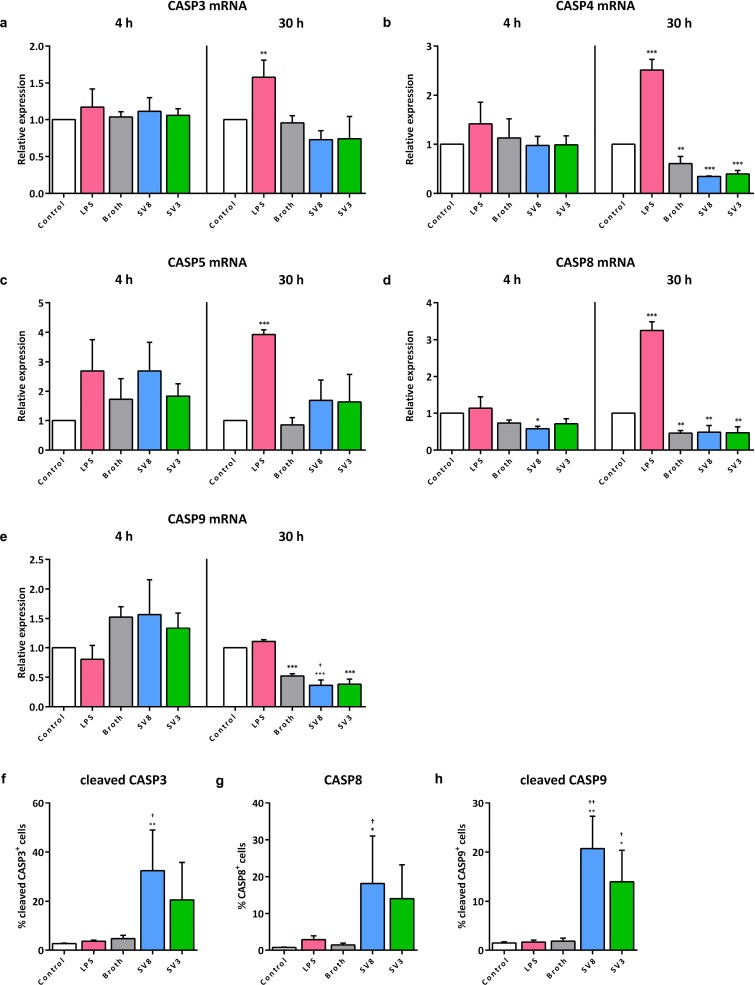
With the exception of a mild reduction of caspase 9 mRNA upon 30 h of serovar 8 exposure (0.7 ± 0.2-fold, p = 0.0374, vs. broth), Ureaplasma isolates did not influence caspase mRNA levels in HPMEC (Fig 3A–3E). Variations in comparison to unstimulated control cells were not significant compared to broth control, since broth itself had moderate and inconsistent effects on caspase mRNA expression. LPS stimulation of HPMEC for 4 h did not evoke any significant mRNA variances for caspases 3, 4, 5, 8, and 9 (Fig 3A–3E). After 30 h of LPS exposure, however, we could observe significantly increased mRNA levels for caspase 3 (1.6 ± 0.2-fold, p = 0.0086, vs. control, Fig 3A), caspase 4 (2.5 ± 0.2-fold, p < 0.001, Fig 3B), caspase 5 (3.9 ± 0.2-fold, p < 0.001, Fig 3C), and caspase 8 (3.3 ± 0.2-fold, p < 0.001, Fig 3D).
Fig 3. Ureaplasma-driven caspase mRNA and protein responses in HPMEC.
Following an incubation period of 4 and 30 h, mRNA levels of caspase (CASP) 3 (a), caspase 4 (b), caspase 5 (c), caspase 8 (d), and caspase 9 (e) were obtained via qRT-PCR, and relative expression was calculated using the ΔΔCT method. The percentage of viable, active caspase 3 (f), caspase 8 (g), and active caspase 9 (h) positive HPMEC was determined by flow cytometry after 24 h stimulation (the respective gating strategy is illustrated in S1 Fig). Data are presented as means ± SD from n ≥ 3 independent experiments. Cells stimulated with LPS were compared vs. control, Ureaplasma exposed cells vs. control and vs. broth. * p < 0.05, ** p < 0.01, and *** p < 0.001 compared to untreated controls; † p < 0.05, and †† p < 0.01 compared to cells treated with broth. SV8: Ureaplasma urealyticum serovar 8, SV3: Ureaplasma parvum serovar 3.
Flow cytometry revealed an Ureaplasma-induced significant increase of positive cells for cleaved caspase 3 (serovar 8: 6.9 ± 3.5-fold, p = 0.0104, vs. broth), caspase 8 (serovar 8: 12.9 ± 9.2-fold, p = 0.0315), and cleaved caspase 9 (serovar 8: 11.3 ± 3.6-fold, p = 0.0016; serovar 3: 7.6 ± 3.5-fold, p = 0.0303) (Fig 3F–3H), with serovar 8 tending to have slightly stronger effects than serovar 3. LPS had no significant influences on caspase 3, 8, or 9 protein expression or enzyme activity in HPMEC, respectively (Fig 3F–3H).
Ureaplasma-driven cytokine responses in pulmonary epithelial and endothelial cells
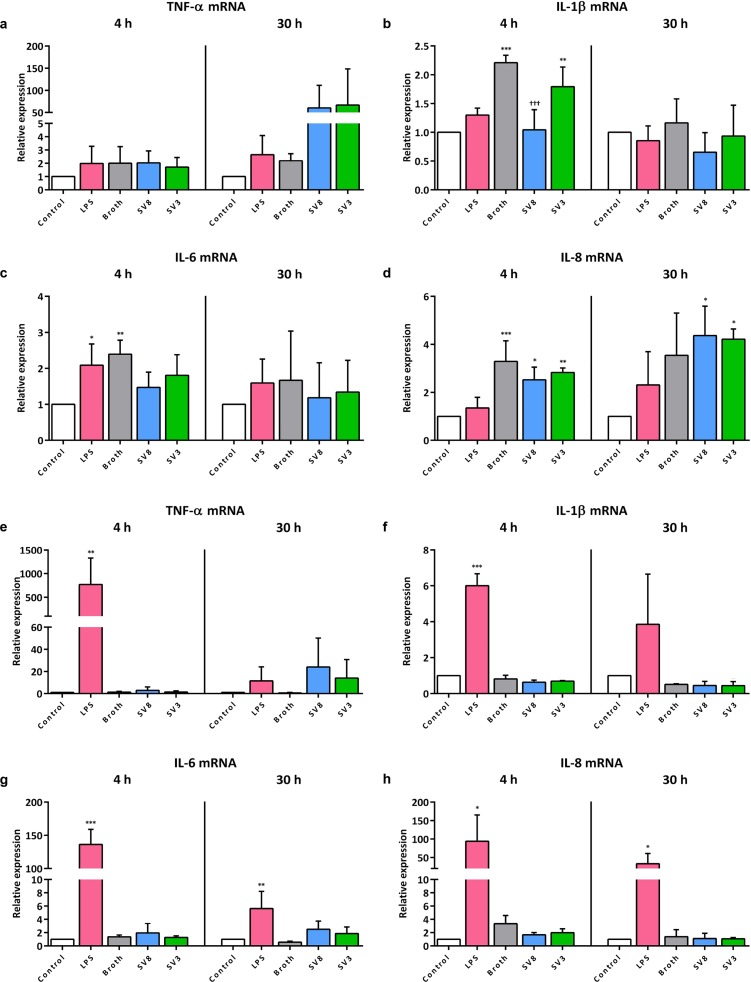
In A549 cells, no significant TNF-α, IL-1β, IL-6, and IL-8 mRNA responses were measurable upon Ureaplasma exposure if compared to broth and control, since broth itself had a certain cytokine-inducing effect (Fig 4A–4D). Exposure of A549 cells to LPS for 4 h induced a significant increase of IL-6 mRNA (2.1 ± 0.6-fold, p = 0.0430, vs. control), whereas LPS did not influence mRNA levels of TNF-α, IL-1β, and IL-8, regardless of the duration of stimulation (Fig 4A–4D).
Fig 4. Ureaplasma-driven mRNA expression of pro-inflammatory cytokines in A549 cells and HPMEC.
In A549 cells, mRNA levels of TNF-α (a), IL-1β (b), IL-6 (c), and IL-8 (d) were assessed via qRT-PCR after 4 and 30 h stimulation. Similarly, mRNA expression in HPMEC was determined for TNF-α (e), IL-1β (f), IL-6 (g), and IL-8 (h). Data are presented as means ± SD from n ≥ 3 independent experiments. LPS stimulated cells were compared vs. control, cells exposed to Ureaplasma isolates vs. control and vs. broth. * p < 0.05, ** p < 0.01, and *** p < 0.001 compared to untreated controls; ††† p < 0.001 compared to cells treated with broth. SV8: Ureaplasma urealyticum serovar 8, SV3: Ureaplasma parvum serovar 3.
In HPMEC, Ureaplasma spp. similarly did not have any significant effects on mRNA levels of all given cytokines (Fig 4E–4H). LPS was able to significantly induce mRNA expression of TNF-α (4 h: 770 ± 557-fold, p = 0.0097, vs. control), IL-1β (4 h: 6.0 ± 0.7-fold, p < 0.001), IL-6 (4 h: 136 ± 22.8-fold, p < 0.001, 30 h: 5.6 ± 2.6-fold, p = 0.0048), and IL-8 (4 h: 94.0 ± 71.1-fold, p = 0.0140, 30 h: 33.0 ± 27.8-fold, p = 0.0307) (Fig 4E–4H).
Caspase and cytokine responses upon co-stimulation of pulmonary epithelial and endothelial cells
Co-stimulation of A549 cells or HPMEC with LPS and Ureaplasma isolates did not significantly aggravate effects observed after mono-stimulation with one or the other stimulus regarding caspase 3, 4, 5, 8, and 9 mRNA expression, cleaved caspase 3, caspase 8, and cleaved caspase 9 protein levels, or TNF-α, IL-1β, IL-6, and IL-8 mRNA levels (S2 Fig–S4 Fig).
Discussion
This is the first in vitro study assessing influences of Ureaplasma spp. on pulmonary epi- and endothelial cells. Results indicate Ureaplasma-driven suppression of apoptosis in pulmonary epithelial cells and a simultaneous pro-apoptotic capacity of Ureaplasma spp. in pulmonary endothelial cells. Influencing caspase levels and activity may be one of the mechanisms Ureaplasma spp. employ to relevantly interfere with immunological processes.
We could demonstrate that exposure of A549 cells to Ureaplasma spp. resulted in a distinct reduction of caspase mRNA and lacking protein responses (Fig 2), whereas in HPMEC, Ureaplasma isolates did not influence mRNA expression, but promoted caspase activation and protein increase (Fig 3). These divergent results regarding mRNA and protein levels can be explained by the meticulous multistep process in caspase protein production and activation (Fig 5A) [27, 43]. The Ureaplasma-induced caspase mRNA suppression we could demonstrate in A549 cells may result in impaired protein production. Consequently, we observed no relevant pathogen-induced caspase protein increase in A549 cells. Caspase mRNA synthesis in HPMEC appeared mostly unaffected by Ureaplasma-stimulation. These cells, consequently, were able to exercise an unconfined caspase response regarding protein abundance and enzyme activity.
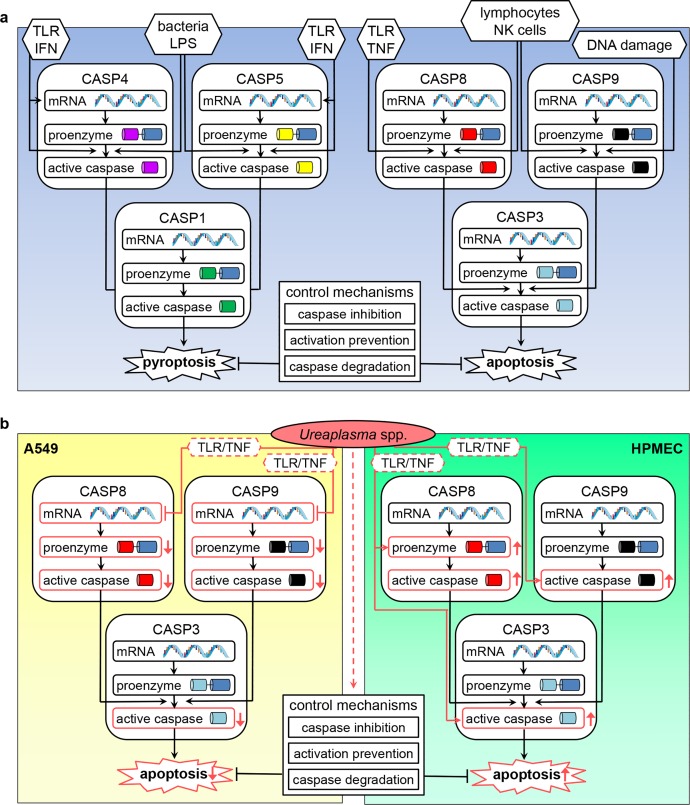
Fig 5. Cascade of caspase activation and potential pathways influenced by Ureaplasma spp.
Simplified scheme (a) depicting caspase activation processes. Multiple trigger factors can initiate apoptosis and pyroptosis. Under engagement of several additional proteins not mentioned here, initiator caspases 4 and 5 for pyroptosis or 8 and 9 for apoptosis are produced and activated. These subsequently activate effector caspases 1 or 3. Caspases are activated by cleavage or dimerization, often followed by a maturational process. Control mechanisms confine programmed cell death [26, 27, 43–45]. The potential influence of Ureaplasma spp. on apoptosis according to our results is illustrated in (b). Pathways affected by Ureaplasma isolates are marked in red. In A549 cells, Ureaplasma-triggered reduction of caspase 8 and 9 mRNA may result in an absent increase in protein production and active caspases. Ultimately, effector caspase 3 remains unactivated and apoptosis is impaired. In HPMEC, Ureaplasma spp. seem to enhance caspase 8 protein and caspase 9 activity. Both caspases may subsequently activate effector caspase 3 and induce apoptosis. It remains to be determined if the caspase 3 activation observed is directly due to Ureaplasma or a consequence of initiator caspase activation. Hypothesized underlying mechanisms Ureaplasma isolates may engage are indicated with a dashed line. Ⱶ inhibit / down-regulate; ← activate / up-regulate. CASP: caspase; IFN: interferon; LPS: lipopolysaccharide; NK: natural killer; TLR: toll-like receptor; TNF: tumor necrosis factor. Illustrations: https://smart.servier.com/.
To fully appreciate the implications of Ureaplasma-driven caspase modulation, the specific functions of individual caspases have to be considered. Caspases 4 and 5 are inflammatory caspases which, via caspase 1, trigger pyroptosis of macrophages, epithelial cells, and lymphocytes as an effective defense mechanism against intracellular pathogens [26] (Fig 5A). Caspases 3, 8, and 9, on the contrary, are primarily apoptotic caspases, with caspase 3 being a key effector, and caspase 8 and 9 initiating apoptosis [26] (Fig 5A).
Ureaplasma-associated down-regulation of caspase 8 and 9 mRNA in A549 cells and consecutively absent protein responses may therefore suppress apoptosis (Fig 5B). Apoptosis as an immune defense mechanism is particularly relevant in elimination of intracellular pathogens [45]. Given the ability of Ureaplasma spp. to invade host cells [46, 47], down-regulation of caspases may function as an escape mechanism Ureaplasma spp. use to prevent eradication. In line with this hypothesis, we did not observe an impact of Ureaplasma isolates on cell viability in A549 cells (Fig 1). Impaired apoptosis and subsequently reduced pathogen eradication may ultimately facilitate chronic Ureaplasma colonization. Ureaplasma spp. are known to cause long-lasting pulmonary infections [48], and BPD-development in preterm infants, to name one example, has been associated with long-term respiratory tract Ureaplasma colonization in particular [49]. Our findings may therefore be of considerable clinical relevance.
Whereas several intracellular pathogens appear to be able to suppress apoptosis, the capacity for down-regulation or inactivation of caspases has been recognized for only a few of them [50, 51]. Our data are the first to indicate Ureaplasma-associated down-regulation of apoptotic caspases, which may be relevant not only in pulmonary cells.
Ureaplasma-driven enhanced caspase 8 protein levels and enzyme activity of caspases 3 and 9 in HPMEC (Figs 3 and 5B), in contrast, provide first in vitro evidence for a pro-apoptotic effect of Ureaplasma spp. in pulmonary endothelial cells. We could confirm Ureaplasma-driven cell death in HPMEC by flow cytometric viability assessment (Fig 1B).
Apoptosis has a dual role with both beneficial as well as harmful effects. It contributes to immune defense and repair processes on the one hand, but facilitates tissue damage on the other [45]. A meticulous balance between growth and apoptosis is essential for normal lung development in utero as well as after birth [52]. Ureaplasma-driven increase or inhibition of apoptosis may disturb this physiological balance and may begin to impair structural lung development even prenatally, with severe implications as, for example, seen in BPD pathogenesis. Indeed, early structural lung tissue impairment such as pulmonary fibrosis could be demonstrated in Ureaplasma-infected preterm infants [53], and increased apoptotic activity was demonstrated within the lungs of preterm infants having developed BPD [54].
Underlying mechanisms Ureaplasma spp. use to modulate caspases remain to be determined. Ureaplasma spp. are known to engage toll-like receptor (TLR) signaling and induce TNF-α [33, 55]. TLR as well as TNF-α are relevant initiators of apoptosis and inflammatory cell death [45] (Fig 5A). Another contributing factor may be intracellular Ureaplasma invasion with subsequent ammonia production and pH shift. Ureaplasma spp. can cause fatal hyperammonemia in lung transplant patients [56], but data regarding the influence of ammonia or pH on apoptosis and caspases are inconclusive [57–59]. Last but not least, Ureaplasma spp. might interfere with inhibition or degradation of caspases (Fig 5).
Ureaplasma isolates did not relevantly modulate inflammatory responses in this study. We found only non-significantly reduced inflammatory caspase levels in A549 cells (Fig 2), and, opposed to LPS, Ureaplasma spp. did not evoke distinct cytokine reactions in both pulmonary cell lines. This is in line with data from animal studies describing only mild pulmonary inflammatory reactions upon Ureaplasma exposure [48].
With this study, we describe distinct differences in the inflammatory responses of pulmonary epithelial and endothelial cells (Figs 1–4). Alveolar epithelial cells fulfill certain immunological functions and interact with, but do not belong to professional immunological cells [18, 60]. This possibly reduces their ability to effectively target infections. Other studies also reported insignificant cytokine induction in A549 cells [61, 62], and pathogen persistence is predominantly described in epithelial cells [63, 64]. Contrarily, opposed to earlier perceptions, pulmonary endothelial cells are increasingly regarded relevant in immune recruitment and production of pro-inflammatory proteins [65, 66]. This is reflected by our observation of Ureaplasma- or LPS-induced caspase and cytokine enhancements in HPMEC. Of note, we could recently demonstrate Ureaplasma-driven apoptosis in HBMEC [28], possibly indicating a higher vulnerability of endothelial cells in general for Ureaplasma-induced cell death. In any case, as many studies suggest a cross-talk between epithelial and endothelial pulmonary cells [67], affection of the one is likely to bear consequences for the other.
In this study, U. urealyticum serovar 8 appeared to evoke stronger reactions than U. parvum serovar 3 (Figs 1B, 2F, 3F and 3G). It is uncertain in how far this relates to in vivo conditions, since literature often does not distinguish between serovars. A relevant quantity of clinically isolated Ureaplasma was furthermore revealed to be hybrids of different serovars [3, 68]. U. urealyticum were nonetheless reported to cause male urethritis and endometritis in pregnancy more frequently than U. parvum, and relevant genetic differences among serovars were described [69, 70]. Compared to U. parvum, more frequent horizontal gene transfer and a higher phospholipase activity, both virulence determinants, were reported in U. urealyticum [70, 71]. However, several studies did not find relevant differences between serovars and many authors conclude that Ureaplasma strains do no generally differ in their pathogenicity, but that host characteristics may determine individual inflammatory responses [1, 3, 70].
Our results contradict the one previous study describing apoptosis in Ureaplasma-exposed A549 cells [36]. These authors, however, used heat-inactivated Ureaplasma isolates. Although this is common practice, heat exposure may not only kill Ureaplasma cells, but is also likely to destroy their immunogenic surface proteins, impairing host immune responses [72]. In our experience, heat-inactivated Ureaplasma spp. tend to lose their pathogenicity. The use of viable Ureaplasma isolates is therefore advantageous and a strength of this study. It does, however, require a complex culture medium. Although we implemented growth of Ureaplasma isolates in yeast-free medium and furthermore ruled out endotoxin contamination, broth itself bore a certain immunogenic effect (Figs 1–4). A reason for this might be cell affection by the altered composition of A549 / HPMEC growth medium after addition of Ureaplasma broth. Particularly urea has been shown to be potentially cytotoxic [73] and may therefore have induced at least parts of the broth effects observed in this study. Another potential limitation of this study is the use of well-established, but adult cell lines. Although Ureaplasma infections may affect immunocompromised adults, preterm and term neonates are those with the highest susceptibility [9, 17]. Future studies should therefore be conducted with cell lines of neonatal origin. Furthermore, in vitro settings can never fully represent in vivo conditions, where complex interactions between numerous mediators and cell types have to be taken into consideration. In this study, we concentrated on a purposive selection of caspases and cytokines, and we can correlate mRNA levels and enzyme activity or protein levels for only a few of them. Nonetheless, we provide first evidence for Ureaplasma-driven, cell-type specific interference with caspases and cell death. These findings once more suggest a profound clinical relevance of Ureaplasma spp. and should now encourage further research.
Conclusions
Pathogen-triggered inflammation usually evokes fierce immune reactions, condoning tissue damage, but ultimately resulting in pathogen eradication. The clinical significance of respiratory tract Ureaplasma colonization, however, may be based less on fulminant and temporary inflammatory reactions, but rather on chronic, subclinical infections, in which even mild inflammatory effects cause long-term sequelae. A key pathomechanism Ureaplasma spp. seem to employ is an interference with the caspase system. On the one hand, Ureaplasma-driven increases of caspase protein expression and activity in pulmonary endothelial cells may cause apoptosis and thus relevantly contribute to structural lung impairment. On the other hand, Ureaplasma spp. down-regulate caspase mRNA levels in pulmonary epithelial cells, thereby potentially suppressing programmed cell death as an important immune defense mechanism. Combined, these processes may facilitate chronic infections, long-term lung injury, and possibly inflammatory lung diseases such as BPD. This study provides additional evidence for the growing perception that Ureaplasma spp. are no innocent bystanders, but most likely much more relevant than contemplated to date.
Supporting information
This diagram illustrates the gating strategy used to determine the caspase positive, viable cells depicted in Figs 2F–2H and 3F–3H as well as S2 Fig and S3 Fig. Unstimulated, stained control cells were gated via forward and side scatter, doublets were excluded, and events in the caspase positive, viability dye negative quadrant were depicted in the respective figure. CASP: caspase.
(TIF)
Following 4 and 30 h of co-stimulation of A549 cells, caspase mRNA levels were assessed via qRT-PCR (a-e), and relative expression was calculated using the ΔΔCT method. Flow cytometry was used to determine caspase protein or activity after 24 h stimulation (f-h), the respective gating strategy is illustrated in S1 Fig. Data are shown as means ± SD and were obtained from n ≥ 3 independent experiments. # p < 0.05, ## p < 0.01, and ### p < 0.001 compared to cells treated with LPS; † p < 0.05, †† p < 0.01, and ††† p < 0.001 compared to cells treated with broth+LPS. SV8: Ureaplasma urealyticum serovar 8, SV3: Ureaplasma parvum serovar 3.
(TIF)
After 4 and 30 h of co-stimulation of A549 cells, caspase mRNA levels were assessed via qRT-PCR (a-e), and relative expression was calculated using the ΔΔCT method. Flow cytometry was used to determine caspase protein or activity after 24 h stimulation (f-h), the respective gating strategy is illustrated in S1 Fig. Data are shown as means ± SD and were obtained from n ≥ 3 independent experiments. # p < 0.05 and ### p < 0.001 compared to cells treated with LPS; ††† p < 0.001 compared to cells treated with broth+LPS. SV8: Ureaplasma urealyticum serovar 8, SV3: Ureaplasma parvum serovar 3.
(TIF)
Cytokine mRNA levels were assessed via qRT-PCR in A549 cells (a-d) and HPMEC (e-h) following 4 and 30 h of co-stimulation. Data are presented as means ± SD from n ≥ 3 independent experiments. # p < 0.05 and ## p < 0.01 compared to cells treated with LPS; † p < 0.05 compared to cells treated with broth+LPS. SV8: Ureaplasma urealyticum serovar 8, SV3: Ureaplasma parvum serovar 3.
(TIF)
Acknowledgments
We thank Brigitte Wollny and Silvia Seidenspinner for their excellent technical assistance. We would furthermore like to thank Birgit Henrich, Institute of Medical Microbiology and Hospital Hygiene, University Clinic Duesseldorf, Germany, for determination of Ureaplasma copy numbers.
KG’s work was supported by the Interdisciplinary Center for Clinical Research (IZKF) at the University of Wuerzburg, Germany.
Abbreviations
- ATCC
American Tissue Culture Collection
- BPD
bronchopulmonary dysplasia
- CCU
color-changing units
- FCS
fetal calf serum
- HBMEC
human brain microvascular endothelial cells
- HPMEC
human pulmonary microvascular endothelial cells
- ICAM-1
intercellular adhesion molecule 1
- IL
interleukin
- LPS
lipopolysaccharide
- qRT-PCR
real time quantitative reverse transcriptase polymerase chain reaction
- SD
standard deviation
- spp.
species
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
- U.
Ureaplasma
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18(4):757–89. Epub 2005/10/15. 10.1128/CMR.18.4.757-789.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198(1):43 e1–5. Epub 2008/01/02. 10.1016/j.ajog.2007.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sweeney EL, Dando SJ, Kallapur SG, Knox CL. The Human Ureaplasma Species as Causative Agents of Chorioamnionitis. Clin Microbiol Rev. 2017;30(1):349–79. Epub 2016/12/16. 10.1128/CMR.00091-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney EL, Kallapur SG, Gisslen T, Lambers DS, Chougnet CA, Stephenson SA, et al. Placental Infection With Ureaplasma species Is Associated With Histologic Chorioamnionitis and Adverse Outcomes in Moderately Preterm and Late-Preterm Infants. J Infect Dis. 2016;213(8):1340–7. Epub 2015/12/17. 10.1093/infdis/jiv587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panero A, Pacifico L, Rossi N, Roggini M, Chiesa C. Ureaplasma urealyticum as a cause of pneumonia in preterm infants: analysis of the white cell response. Arch Dis Child Fetal Neonatal Ed. 1995;73(1):F37–40. Epub 1995/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viscardi RM. Ureaplasma species: role in neonatal morbidities and outcomes. Arch Dis Child Fetal Neonatal Ed. 2014;99(1):F87–92. 10.1136/archdischild-2012-303351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaser K, Speer CP. Neonatal CNS infection and inflammation caused by Ureaplasma species: rare or relevant? Expert Rev Anti Infect Ther. 2015;13(2):233–48. 10.1586/14787210.2015.999670 . [DOI] [PubMed] [Google Scholar]
- 8.Glaser K, Wohlleben M, Speer CP. An 8-month history of meningitis in an extremely low birth weight infant?—Long-lasting Infection with Ureaplasma parvum. Z Geburtshilfe Neonatol. 2015;219(1):52–6. 10.1055/s-0034-1395537 . [DOI] [PubMed] [Google Scholar]
- 9.Fernandez R, Ratliff A, Crabb D, Waites KB, Bharat A. Ureaplasma Transmitted From Donor Lungs Is Pathogenic After Lung Transplantation. Ann Thorac Surg. 2017;103(2):670–1. Epub 2017/01/23. 10.1016/j.athoracsur.2016.09.026 . [DOI] [PubMed] [Google Scholar]
- 10.Sung TJ, Xiao L, Duffy L, Waites KB, Chesko KL, Viscardi RM. Frequency of ureaplasma serovars in respiratory secretions of preterm infants at risk for bronchopulmonary dysplasia. Pediatr Infect Dis J. 2011;30(5):379–83. Epub 2010/11/26. 10.1097/INF.0b013e318202ac3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groneck P, Schmale J, Soditt V, Stutzer H, Gotze-Speer B, Speer CP. Bronchoalveolar inflammation following airway infection in preterm infants with chronic lung disease. Pediatr Pulmonol. 2001;31(5):331–8. Epub 2001/05/08. . [DOI] [PubMed] [Google Scholar]
- 12.Kasper DC, Mechtler TP, Bohm J, Petricevic L, Gleiss A, Spergser J, et al. In utero exposure to Ureaplasma spp. is associated with increased rate of bronchopulmonary dysplasia and intraventricular hemorrhage in preterm infants. J Perinat Med. 2011;39(3):331–6. Epub 2011/04/30. 10.1515/JPM.2011.022 . [DOI] [PubMed] [Google Scholar]
- 13.Speer CP. Chorioamnionitis, postnatal factors and proinflammatory response in the pathogenetic sequence of bronchopulmonary dysplasia. Neonatology. 2009;95(4):353–61. 10.1159/000209301 . [DOI] [PubMed] [Google Scholar]
- 14.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res. 1999;46(6):641–3. Epub 1999/12/10. . [DOI] [PubMed] [Google Scholar]
- 15.Collins JJ, Kallapur SG, Knox CL, Nitsos I, Polglase GR, Pillow JJ, et al. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol. 2010;299(6):L852–60. Epub 2010/10/12. 10.1152/ajplung.00183.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moss TJ, Knox CL, Kallapur SG, Nitsos I, Theodoropoulos C, Newnham JP, et al. Experimental amniotic fluid infection in sheep: effects of Ureaplasma parvum serovars 3 and 6 on preterm or term fetal sheep. Am J Obstet Gynecol. 2008;198(1):122 e1-8. Epub 2008/01/02. 10.1016/j.ajog.2007.06.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silwedel C, Speer CP, Glaser K. Ureaplasma-associated prenatal, perinatal, and neonatal morbidities. Expert Rev Clin Immunol. 2017;13(11):1073–87. Epub 2017/09/19. 10.1080/1744666X.2017.1381559 . [DOI] [PubMed] [Google Scholar]
- 18.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2014;16(1):27–35. 10.1038/ni.3045 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willems CH, Zimmermann LJ, Sanders PJ, Wagendorp M, Kloosterboer N, Cohen Tervaert JW, et al. Alveolocapillary model system to study alveolar re-epithelialization. Exp Cell Res. 2013;319(1):64–74. Epub 2012/10/02. 10.1016/j.yexcr.2012.09.010 . [DOI] [PubMed] [Google Scholar]
- 20.Kuehn A, Kletting S, de Souza Carvalho-Wodarz C, Repnik U, Griffiths G, Fischer U, et al. Human alveolar epithelial cells expressing tight junctions to model the air-blood barrier. ALTEX. 2016;33(3):251–60. Epub 2016/03/18. 10.14573/altex.1511131 . [DOI] [PubMed] [Google Scholar]
- 21.Bartels H. Air-Blood Barrier in the Human-Lung—Freeze-Fracture Study. Cell Tissue Res. 1979;198(2):269–85. ISI:A1979HE22900008. [DOI] [PubMed] [Google Scholar]
- 22.Mul FPJ, Zuurbier AEM, Janssen H, Calafat J, van Wetering S, Hiemstra PS, et al. Sequential migration of neutrophils across monolayers of endothelial and epithelial cells. Journal of Leukocyte Biology. 2000;68(4):529–37. 10.1189/jlb.68.4.529 [DOI] [PubMed] [Google Scholar]
- 23.Chau A, Markley JC, Juang J, Tsen LC. Cytokines in the perinatal period—Part I. Int J Obstet Anesth. 2016;26:39–47. Epub 2016/03/14. 10.1016/j.ijoa.2015.12.005 . [DOI] [PubMed] [Google Scholar]
- 24.Shaalan A, Carpenter G, Proctor G. Caspases are key regulators of inflammatory and innate immune responses mediated by TLR3 in vivo. Mol Immunol. 2018;94:190–9. Epub 2018/01/15. 10.1016/j.molimm.2017.12.018 . [DOI] [PubMed] [Google Scholar]
- 25.Cullen SP, Henry CM, Kearney CJ, Logue SE, Feoktistova M, Tynan GA, et al. Fas/CD95-induced chemokines can serve as "find-me" signals for apoptotic cells. Mol Cell. 2013;49(6):1034–48. Epub 2013/02/26. 10.1016/j.molcel.2013.01.025 . [DOI] [PubMed] [Google Scholar]
- 26.Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16(1):7–21. Epub 2015/12/15. 10.1038/nri.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326 (Pt 1):1–16. Epub 1997/08/15. 10.1042/bj3260001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silwedel C, Haarmann A, Fehrholz M, Claus H, Speer CP, Glaser K. More than just inflammation: Ureaplasma species induce apoptosis in human brain microvascular endothelial cells. J Neuroinflammation. 2019;16(1):38 Epub 2019/02/16. 10.1186/s12974-019-1413-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fehrholz M, Speer CP, Kunzmann S. Caffeine and rolipram affect Smad signalling and TGF-beta1 stimulated CTGF and transgelin expression in lung epithelial cells. PloS one. 2014;9(5):e97357 Epub 2014/05/16. 10.1371/journal.pone.0097357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unger RE, Krump-Konvalinkova V, Peters K, Kirkpatrick CJ. In vitro expression of the endothelial phenotype: comparative study of primary isolated cells and cell lines, including the novel cell line HPMEC-ST1.6R. Microvasc Res. 2002;64(3):384–97. . [DOI] [PubMed] [Google Scholar]
- 31.Krump-Konvalinkova V, Bittinger F, Unger RE, Peters K, Lehr HA, Kirkpatrick CJ. Generation of human pulmonary microvascular endothelial cell lines. Lab Invest. 2001;81(12):1717–27. Epub 2001/12/14. . [DOI] [PubMed] [Google Scholar]
- 32.Shepard MC, Lunceford CD. Serological typing of Ureaplasma urealyticum isolates from urethritis patients by an agar growth inhibition method. J Clin Microbiol. 1978;8(5):566–74. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glaser K, Silwedel C, Fehrholz M, Waaga-Gasser AM, Henrich B, Claus H, et al. Ureaplasma Species Differentially Modulate Pro- and Anti-Inflammatory Cytokine Responses in Newborn and Adult Human Monocytes Pushing the State Toward Pro-Inflammation. Front Cell Infect Microbiol. 2017;7(484):480 10.3389/fcimb.2017.00484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mobius N, Brenneisen W, Schaeffer A, Henrich B. Protocol for the rapid detection of the urogenital tract mollicutes and Chlamydia with concomitant LGV-(sub)typing. Methods Mol Biol. 2012;903:235–53. Epub 2012/07/12. 10.1007/978-1-61779-937-2_15 . [DOI] [PubMed] [Google Scholar]
- 35.Silwedel C, Speer CP, Haarmann A, Fehrholz M, Claus H, Buttmann M, et al. Novel insights into neuroinflammation: bacterial lipopolysaccharide, tumor necrosis factor alpha, and Ureaplasma species differentially modulate atypical chemokine receptor 3 responses in human brain microvascular endothelial cells. J Neuroinflammation. 2018;15(1):156 Epub 2018/05/25. 10.1186/s12974-018-1170-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li YH, Chen M, Brauner A, Zheng C, Skov Jensen J, Tullus K. Ureaplasma urealyticum induces apoptosis in human lung epithelial cells and macrophages. Biol Neonate. 2002;82(3):166–73. Epub 2002/10/10. 10.1159/000063616 . [DOI] [PubMed] [Google Scholar]
- 37.Viscardi RM, Atamas SP, Luzina IG, Hasday JD, He JR, Sime PJ, et al. Antenatal Ureaplasma urealyticum respiratory tract infection stimulates proinflammatory, profibrotic responses in the preterm baboon lung. Pediatr Res. 2006;60(2):141–6. 10.1203/01.pdr.0000228322.73777.05 . [DOI] [PubMed] [Google Scholar]
- 38.Kallapur SG, Kramer BW, Knox CL, Berry CA, Collins JJ, Kemp MW, et al. Chronic fetal exposure to Ureaplasma parvum suppresses innate immune responses in sheep. J Immunol. 2011;187(5):2688–95. 10.4049/jimmunol.1100779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Carson-Walter E, Walter KA. Chemokine receptor CXCR7 is a functional receptor for CXCL12 in brain endothelial cells. PloS one. 2014;9(8):e103938 Epub 2014/08/02. 10.1371/journal.pone.0103938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fehrholz M, Bersani I, Kramer BW, Speer CP, Kunzmann S. Synergistic effect of caffeine and glucocorticoids on expression of surfactant protein B (SP-B) mRNA. PLoS One. 2012;7(12):e51575 10.1371/journal.pone.0051575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall OJ. PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics. 2004;20(15):2471–2. Epub 2004/04/10. 10.1093/bioinformatics/bth254 . [DOI] [PubMed] [Google Scholar]
- 42.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 43.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284(33):21777–81. Epub 2009/05/29. 10.1074/jbc.R800084200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61–75. Epub 2017/05/04. 10.1111/imr.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17(3):151–64. Epub 2017/02/01. 10.1038/nri.2016.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marques LM, Ueno PM, Buzinhani M, Cortez BA, Neto RL, Yamaguti M, et al. Invasion of Ureaplasma diversum in Hep-2 cells. BMC Microbiol. 2010;10:83 10.1186/1471-2180-10-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buzinhani M, Yamaguti M, Oliveira RC, Cortez BA, Marques LM, Machado-Santelli GM, et al. Invasion of Ureaplasma diversum in bovine spermatozoids. BMC Res Notes. 2011;4:455 Epub 2011/10/29. 10.1186/1756-0500-4-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viscardi RM, Kaplan J, Lovchik JC, He JR, Hester L, Rao S, et al. Characterization of a murine model of Ureaplasma urealyticum pneumonia. Infect Immun. 2002;70(10):5721–9. 10.1128/IAI.70.10.5721-5729.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castro-Alcaraz S, Greenberg EM, Bateman DA, Regan JA. Patterns of colonization with Ureaplasma urealyticum during neonatal intensive care unit hospitalizations of very low birth weight infants and the development of chronic lung disease. Pediatrics. 2002;110(4):e45 Epub 2002/10/03. . [DOI] [PubMed] [Google Scholar]
- 50.Schwartz JT, Barker JH, Kaufman J, Fayram DC, McCracken JM, Allen L-AH. Francisella tularensis Inhibits the Intrinsic and Extrinsic Pathways To Delay Constitutive Apoptosis and Prolong Human Neutrophil Lifespan. The Journal of Immunology. 2012;188(7):3351–63. 10.4049/jimmunol.1102863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedrich A, Pechstein J, Berens C, Luhrmann A. Modulation of host cell apoptotic pathways by intracellular pathogens. Curr Opin Microbiol. 2017;35:88–99. Epub 2017/03/21. 10.1016/j.mib.2017.03.001 . [DOI] [PubMed] [Google Scholar]
- 52.Del Riccio V, van Tuyl M, Post M. Apoptosis in lung development and neonatal lung injury. Pediatr Res. 2004;55(2):183–9. Epub 2003/11/25. 10.1203/01.PDR.0000103930.93849.B2 . [DOI] [PubMed] [Google Scholar]
- 53.Viscardi RM, Manimtim WM, Sun CC, Duffy L, Cassell GH. Lung pathology in premature infants with Ureaplasma urealyticum infection. Pediatr Dev Pathol. 2002;5(2):141–50. 10.1007/s10024-001-0134-y . [DOI] [PubMed] [Google Scholar]
- 54.Hargitai B, Szabo V, Hajdu J, Harmath A, Pataki M, Farid P, et al. Apoptosis in various organs of preterm infants: histopathologic study of lung, kidney, liver, and brain of ventilated infants. Pediatr Res. 2001;50(1):110–4. Epub 2001/06/23. 10.1203/00006450-200107000-00020 . [DOI] [PubMed] [Google Scholar]
- 55.Shimizu T, Kida Y, Kuwano K. Ureaplasma parvum lipoproteins, including MB antigen, activate NF-{kappa}B through TLR1, TLR2 and TLR6. Microbiology. 2008;154(Pt 5):1318–25. Epub 2008/05/03. 10.1099/mic.0.2007/016212-0 . [DOI] [PubMed] [Google Scholar]
- 56.Bharat A, Cunningham SA, Scott Budinger GR, Kreisel D, DeWet CJ, Gelman AE, et al. Disseminated Ureaplasma infection as a cause of fatal hyperammonemia in humans. Sci Transl Med. 2015;7(284):284re3 Epub 2015/04/24. 10.1126/scitranslmed.aaa8419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang F, Chen S, Jiang Y, Zhao Y, Sun L, Zheng B, et al. Effects of ammonia on apoptosis and oxidative stress in bovine mammary epithelial cells. Mutagenesis. 2018;33(4):291–9. Epub 2018/09/06. 10.1093/mutage/gey023 . [DOI] [PubMed] [Google Scholar]
- 58.Kosenko E, Kaminsky Y, Solomadin I, Marov N, Venediktova N, Felipo V, et al. Acute ammonia neurotoxicity in vivo involves increase in cytoplasmic protein P53 without alterations in other markers of apoptosis. J Neurosci Res. 2007;85(11):2491–9. Epub 2007/06/07. 10.1002/jnr.21385 . [DOI] [PubMed] [Google Scholar]
- 59.Sergeeva TF, Shirmanova MV, Zlobovskaya OA, Gavrina AI, Dudenkova VV, Lukina MM, et al. Relationship between intracellular pH, metabolic co-factors and caspase-3 activation in cancer cells during apoptosis. Biochim Biophys Acta Mol Cell Res. 2017;1864(3):604–11. Epub 2017/01/09. 10.1016/j.bbamcr.2016.12.022 . [DOI] [PubMed] [Google Scholar]
- 60.Hiemstra PS, McCray PB Jr., Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J. 2015;45(4):1150–62. Epub 2015/02/24. 10.1183/09031936.00141514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kato T, Kobayashi K, Suzukawa M, Saito M, Okuda K, Koyama K, et al. Epithelial-mesenchymal transition of human lung adenocarcinoma A549 cells up-regulates cytokine production upon LPS stimulation. Allergol Int. 2017;66S:S56–S8. Epub 2017/07/18. 10.1016/j.alit.2017.06.014 . [DOI] [PubMed] [Google Scholar]
- 62.Patil RH, Naveen Kumar M, Kiran Kumar KM, Nagesh R, Kavya K, Babu RL, et al. Dexamethasone inhibits inflammatory response via down regulation of AP-1 transcription factor in human lung epithelial cells. Gene. 2018;645:85–94. Epub 2017/12/19. 10.1016/j.gene.2017.12.024 . [DOI] [PubMed] [Google Scholar]
- 63.Lamberti Y, Gorgojo J, Massillo C, Rodriguez ME. Bordetella pertussis entry into respiratory epithelial cells and intracellular survival. Pathog Dis. 2013;69(3):194–204. Epub 2013/07/31. 10.1111/2049-632X.12072 . [DOI] [PubMed] [Google Scholar]
- 64.Garzoni C, Francois P, Huyghe A, Couzinet S, Tapparel C, Charbonnier Y, et al. A global view of Staphylococcus aureus whole genome expression upon internalization in human epithelial cells. BMC Genomics. 2007;8:171 Epub 2007/06/16. 10.1186/1471-2164-8-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells–conditional innate immune cells. J Hematol Oncol. 2013;6:61 10.1186/1756-8722-6-61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teijaro John R, Walsh Kevin B, Cahalan S, Fremgen Daniel M, Roberts E, Scott F, et al. Endothelial Cells Are Central Orchestrators of Cytokine Amplification during Influenza Virus Infection. Cell. 2011;146(6):980–91. 10.1016/j.cell.2011.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang L, Taneja R, Wang W, Yao LJ, Veldhuizen RA, Gill SE, et al. Human alveolar epithelial cells attenuate pulmonary microvascular endothelial cell permeability under septic conditions. PloS one. 2013;8(2):e55311 Epub 2013/02/09. 10.1371/journal.pone.0055311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao L, Paralanov V, Glass JI, Duffy LB, Robertson JA, Cassell GH, et al. Extensive horizontal gene transfer in ureaplasmas from humans questions the utility of serotyping for diagnostic purposes. J Clin Microbiol. 2011;49(8):2818–26. Epub 2011/06/24. 10.1128/JCM.00637-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zimmerman CU, Rosengarten R, Spergser J. Interaction of the putative tyrosine recombinases RipX (UU145), XerC (UU222), and CodV (UU529) of Ureaplasma parvum serovar 3 with specific DNA. FEMS Microbiol Lett. 2013;340(1):55–64. Epub 2013/01/12. 10.1111/1574-6968.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paralanov V, Lu J, Duffy LB, Crabb DM, Shrivastava S, Methe BA, et al. Comparative genome analysis of 19 Ureaplasma urealyticum and Ureaplasma parvum strains. BMC Microbiol. 2012;12:88 Epub 2012/06/01. 10.1186/1471-2180-12-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Silva NS, Quinn PA. Endogenous activity of phospholipases A and C in Ureaplasma urealyticum. J Clin Microbiol. 1986;23(2):354–9. Epub 1986/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peltier MR, Freeman AJ, Mu HH, Cole BC. Characterization of the macrophage-stimulating activity from Ureaplasma urealyticum. Am J Reprod Immunol. 2007;57(3):186–92. Epub 2007/02/14. 10.1111/j.1600-0897.2006.00460.x . [DOI] [PubMed] [Google Scholar]
- 73.Glinos AD, Bardi GN, Dermitzaki KC, Perez SA, Talieri MJ. Cytokinetic and cytotoxic effects of urea on HeLa cells in suspension cultures. J Natl Cancer Inst. 1983;71(6):1211–9. Epub 1983/12/01. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This diagram illustrates the gating strategy used to determine the caspase positive, viable cells depicted in Figs 2F–2H and 3F–3H as well as S2 Fig and S3 Fig. Unstimulated, stained control cells were gated via forward and side scatter, doublets were excluded, and events in the caspase positive, viability dye negative quadrant were depicted in the respective figure. CASP: caspase.
(TIF)
Following 4 and 30 h of co-stimulation of A549 cells, caspase mRNA levels were assessed via qRT-PCR (a-e), and relative expression was calculated using the ΔΔCT method. Flow cytometry was used to determine caspase protein or activity after 24 h stimulation (f-h), the respective gating strategy is illustrated in S1 Fig. Data are shown as means ± SD and were obtained from n ≥ 3 independent experiments. # p < 0.05, ## p < 0.01, and ### p < 0.001 compared to cells treated with LPS; † p < 0.05, †† p < 0.01, and ††† p < 0.001 compared to cells treated with broth+LPS. SV8: Ureaplasma urealyticum serovar 8, SV3: Ureaplasma parvum serovar 3.
(TIF)
After 4 and 30 h of co-stimulation of A549 cells, caspase mRNA levels were assessed via qRT-PCR (a-e), and relative expression was calculated using the ΔΔCT method. Flow cytometry was used to determine caspase protein or activity after 24 h stimulation (f-h), the respective gating strategy is illustrated in S1 Fig. Data are shown as means ± SD and were obtained from n ≥ 3 independent experiments. # p < 0.05 and ### p < 0.001 compared to cells treated with LPS; ††† p < 0.001 compared to cells treated with broth+LPS. SV8: Ureaplasma urealyticum serovar 8, SV3: Ureaplasma parvum serovar 3.
(TIF)
Cytokine mRNA levels were assessed via qRT-PCR in A549 cells (a-d) and HPMEC (e-h) following 4 and 30 h of co-stimulation. Data are presented as means ± SD from n ≥ 3 independent experiments. # p < 0.05 and ## p < 0.01 compared to cells treated with LPS; † p < 0.05 compared to cells treated with broth+LPS. SV8: Ureaplasma urealyticum serovar 8, SV3: Ureaplasma parvum serovar 3.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.