Abstract
While the human immunodeficiency virus (HIV) epidemic was initially characterized by a high prevalence of severe and wide-spread neurological pathologies, the development of better treatments to suppress viremia over years and even decades have mitigated many of the severe neurological pathologies previously observed. Despite effective treatment, mild neurocognitive impairment and premature cognitive aging are observed in HIV-infected individuals, suggesting a changing but ongoing role of HIV infection in the central nervous system (CNS). Although current therapies are effective in suppressing viremia, they are not curative and patients must remain on life-long treatment or risk recrudescence of virus. Important for the development and evaluation of a cure for HIV will be animal models that recapitulate critical aspects of infection in vivo. In the following, we seek to summarize some of the recent developments in humanized mouse models and their usefulness in modeling HIV infection of the CNS and HIV cure strategies.
Introduction
Infection with human immunodeficiency virus (HIV), the underlying cause of acquired immune deficiency syndrome (AIDS), results in system-wide manifestations of disease. The central nervous system (CNS) is one of the affected systems, and in the absence of treatment, infection can result in severe brain pathology and cognitive/behavioral abnormalities. These neurological deficiencies are grouped under the umbrella term of HIV-1 associated neurocognitive disorders (HAND). Effective treatment of HIV with combination antiretroviral therapy (cART) has led to a decrease in the frequency of severe HAND (HIV-associated dementia or HAD) from 10–15% to 2% (Heaton, Clifford et al. 2010). However, asymptomatic forms of HAND exist, and HIV infection is associated with premature cognitive aging, despite effective cART (Cohen, Seider et al. 2015). The asymptomatic form of HAND, termed asymptomatic neurocognitive impairment (ANI), is included for research purposes but not clinical diagnosis, and is detected via direct neuropsychological assessment (Heaton, Clifford et al. 2010).
The recent and dogma-redefining discovery that the brain has a lymphatic system, consisting of lymphatic vessels adjacent to the dura that empty into the periphery (Aspelund, Antila et al. 2015, Louveau, Smirnov et al. 2015), suggest that better systems to study the interactions between the CNS and peripheral immune system will be key to modeling HIV infection in the brain. A better understanding of the mechanisms involved in viral trafficking and cART penetration into the brain and whether or not the brain represents a tissue reservoir of HIV that cannot be cleared with cART (or where HIV persists despite prolonged cART) are all important for developing more effective treatments to target HIV infection in the CNS.
Humanized mice models
Mice that are reconstituted with human cells offer a unique small animal model for studying HIV infection, as all strains of mice are refractory to HIV infection. Humanized mice are created by implanting human tissues and/or injecting human cells into various strains of immunodeficient mice (Figure 1); these animals have been extensively used successfully to study HIV pathogenesis, transmission and persistence in vivo.
Figure 1: Schematic of humanized mouse models for evaluating NeuroHIV infection and/or cure strategies.
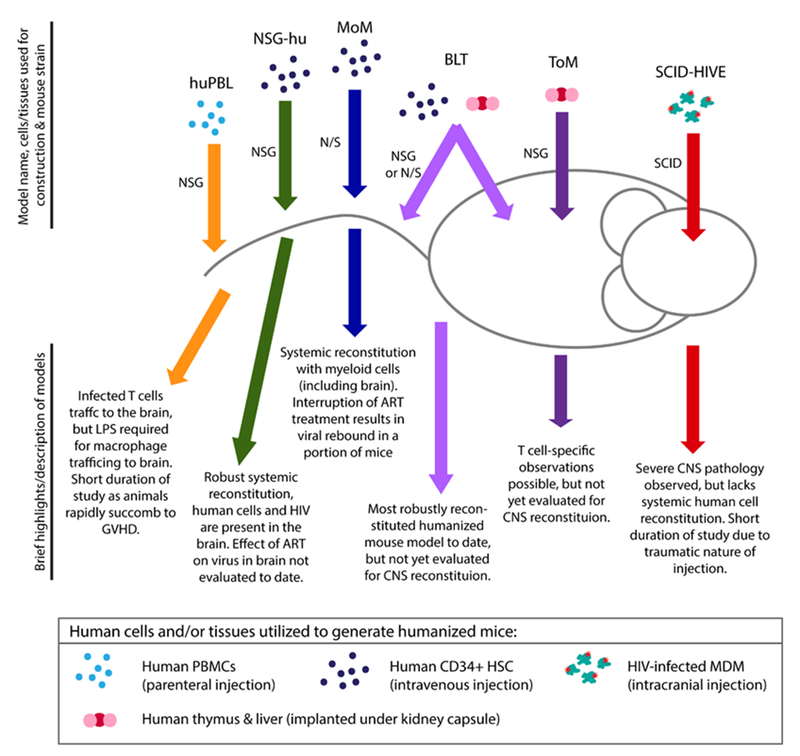
The name of each model is followed by the human cells/tissues and immunodeficient mouse strains used in the generation of each type of humanized mouse. Brief description of advantages and disadvantages of each model are listed.
SCID-HIVE model
To determine whether or not HIV-infected cells directly contribute to HIV-associated encephalitis (HIVE), HIV-infected human myeloid cells have been directly injected into the brains of severe combined immune deficient (SCID) mice, which lack murine B and T cells (Tyor, Power et al. 1993, Persidsky, Limoges et al. 1996, Persidsky, Buttini et al. 1997, Persidsky and Gendelman 1997, Persidsky, Ghorpade et al. 1999). This direct injection model results in pathologic changes in about a week; pathology includes encephalitis, astrogliosis (activated astrocytes), and increased levels of mononuclear phagocyte trafficking.
Recently, this model has been used investigate strategies to mitigate HIV-induced inflammation in the CNS. CEP-1347, an inhibitor of mixed multilineage kinases, has shown promise in animal models of Huntington’s disease (Apostol, Simmons et al. 2008, Conforti, Ramos et al. 2008). CEP-1347 has also been shown to reduce microglia inflammation in vitro (Lund, Porzgen et al. 2005). In SCID-HIVE mice, CEP-1347 was able to reduce the levels of microglia activation (microgliosis), as measured by decreases in Iba-1 expression (Eggert, Dash et al. 2010). An IFN-alpha inhibitor, B18R, was shown to effectively cross the blood-brain barrier (BBB) and was able to resolve some of the pathologies present in the SCID-HIVE model (Fritz-French, Shawahna et al. 2014). Decreased presence of mouse mononuclear phagocytes, decreased expression of IFN stimulated genes (ISGs), and decreased levels of mouse microtubule-associated protein-2 (MAP-2) were noted in B18R treated mice, compared with untreated SCID-HIVE mice. However, the levels of astrogliosis were unaffected in mice treated with B18R, which remained significantly higher than seen in mice injected with uninfected cells. A Jak/STAT inhibitor, ruxolitnib, was also evaluated for its ability to reduce HIV-associated inflammation in the SCID-HIVE model (Haile, Gavegnano et al. 2016). Dosing with ruxolitnib (20 or 50 mg/kg twice per day) was able to significantly reduce astrogliosis in treated mice, as measured by a reduction in glial fibrillary acidic protein (GFAP) expression.
A number of the pathologies associated with the SCID-HIVE model are mitigated or reduced in the presence of cART (Cook, Dasgupta et al. 2005, Cook-Easterwood, Middaugh et al. 2007, Koneru, Olive et al. 2014), including astrogliosis, mononuclear cell infiltration and restored neuronal integrity (reduced MAP-2 levels). This suggests that HIV replication may directly contribute to the extent of brain pathology in this model. They also demonstrate that ART consisting of atazanavir, tenofovir and emtricitabine was able to effectively penetrate into the brain within one hour of injection (Koneru, Olive et al. 2014). A significant decrease, approximately 4-fold, in the numbers of p24+ cells in treated animals compared with saline-treated mice was observed. The authors conclude that this regimen was insufficient to eradicate virus from the brain, as p24+ cells were present in the brains of cART treated mice; however, animals were treated for a period of 10 days, a relatively short time. Nevertheless, this study provides unique information regarding drug penetrance into the brain and demonstrates partial control of infection in the brains of SCID-HIVE mice on cART.
PBMC-transplanted NSG mouse model or NSG-huPBL or Hu-PBMC-NSG
The NSG-huPBL model is generated by intraperitoneal injection of healthy human donor PBMCs into NSG mice, and systemic distribution of human cells is observed in these mice (Kim, Choi et al. 2016, Wu, Liu et al. 2016). After intraperitoneal exposure to virus, cell-free HIV-RNA and p24 antigen can be detected in the peripheral blood of animals, and the ratio of CD4+ to CD8+ T cells declines rapidly. HIV infected human T cells (p24+/CD3+ dual positive) were present in the meninges and cortex of infected animals (Wu, Liu et al. 2016). Cell-free HIV-RNA and p24 was detectable in brain homogenates of infected mice. Microgliosis, astrogliosis and neuronal dropout were noted in the brains of mice infected with HIV-1JR-FL, although this was not noted in mice infected with HIV-1BJZ57, compared with uninfected controls. The level of microgliosis observed was correlated with the amount of p24 present in the brain (Wu, Liu et al. 2016). While macrophages are absent in the brains of huPBL mice, administration of lipopolysaccharide (LPS) to these mice induces macrophage trafficking into the CNS (Miura, Misawa et al. 2003).
Treatment with cART consisting of azidotymidine, indinavir and atazanavir suppressed plasma viremia in HIV-infected NSG-huPBL mice (Kim, Choi et al. 2016). Interruption of treatment resulted in rebound of virus in the plasma in a group of animals. Furthermore, treatment with a neutralizing antibody, 068P, prevented the decline in the CD4:CD8 ratio, suggesting that HIV infection could be inhibited with this antibody (Kim, Choi et al. 2016). However, direct evidence of viral control (p24 production or HIV-RNA levels) was not shown.
CD34-transplanted NSG mouse model or hu-NSG
The hu-NSG model is generated by intravenously or intrahepatically injecting human CD34+ hematopoietic stem cells, derived from cord blood, bone marrow, or fetal liver, into pre-conditioned NSG mice (Gorantla, Makarov et al. 2010, Choi, Chun et al. 2011, Dash, Gorantla et al. 2011, Dash, Gendelman et al. 2012). In this model, human cells are present systemically as well as in the CNS, including the cortex, meninges and brain stem (Gorantla, Makarov et al. 2010). As these animals are reconstituted systemically, they are susceptible to HIV transmission via parenteral routes and support long-term HIV infection (Denton and Garcia 2011, Krisko, Martinez-Torres et al. 2013). In hu-NSG mice infected intraperitoneally with a macrophage-tropic HIV isolate (HIV-1ADA), multi-spliced and unspliced HIV-RNA was detected by semi-nested qPCR in the brains of a portion of animals (Arainga, Su et al. 2016). However, total and integrated DNA levels in the brains of infected mice were below the limit of detection.
A recent manuscript described a macrophage (“mature macrophage” HLA-DR+/CD14++/CD16+, typically referred to as intermediate phenotype) reservoir for HIV after treatment with long-acting cART (Arainga, Edagwa et al. 2017). In this paper, hu-NSG mice were infected with HIV-1ADA and were either untreated, treated with a 2-drug ART regimen (cabotegravir and rilpivirine) or a 4-drug ART regimen (cabotegravir, rilpivirine, lamivudine and abacavir). Animals were treated for 4 weeks prior to necropsy. Various T cell populations, monocytes/macrophages and CD34 HSCs were sorted from the blood, spleen and bone marrow. The frequency of viral RNA (multi-spliced and unspliced) and viral DNA (total and integrated) in the sorted monocytes/macrophages (CD14+/CD16+) was greatest in the spleens of animals treated with the 4-drug ART regimen (Arainga, Edagwa et al. 2017). This finding seems unusual as one would expect to find the most virus in the untreated animals or animals treated with only two drugs. This could be the result of poor cART penetration, or contamination with T cells in the sorted monocyte/macrophage fraction.
Myeloid only mouse (MoM) model
The recently described myeloid only mouse model, or MoM, is generated by injecting human hematopoietic stem cells into pre-conditioned NOD/SCID mice (Honeycutt, Wahl et al. 2016, Honeycutt, Thayer et al. 2017). These animals are reconstituted with human myeloid and B cells and permit for the systemic analysis of HIV infection in tissue macrophages. A major benefit of this model is that the presence of virus in macrophages cannot be attributed to phagocytosis of infected T cells (Baxter, Russell et al. 2014, Calantone, Wu et al. 2014). Using flow cytometry, both classical (CD14+/CD16-) and intermediate (CD14+/CD16+) macrophages were present in the brains of MoM. HIV-infected cells were clearly detected in the brains of infected MoM (Honeycutt, Wahl et al. 2016). HIV infection was associated with higher numbers of human hematopoietic cells and macrophages present in the brain compared with uninfected controls. Notably, human macrophages were present in the cerebellum, caudate putamen, cerebral cortex, ventral striatum and brain stem (Honeycutt, Wahl et al. 2016). HIV-RNA was detected by PCR analysis in 57% of samples analyzed, demonstrating that human T cells are not necessary to traffic virus to or maintain infection in the brain. Further analysis demonstrated the presence of HIV-infected cells throughout the brains of MoM. Regions where HIV p24+ cells were observed included the cerebellum, cerebral cortex, corpus callosum, midbrain, brain stem and caudate putamen (Honeycutt, Wahl et al. 2016).
While most studies of HIV persistence have focused on T cells, the MoM model offers a unique model to study persistence exclusively in tissue macrophages. This is critically important when developing strategies for targeting HIV infection in the CNS, as the predominant targets for infection of the brain are myeloid-derived cells such as macrophages and microglia. ART treatment rapidly reduced the plasma viral load and levels of cell-associated virus in the tissues of treated MoM, compared to untreated animals (Honeycutt, Thayer et al. 2017). The half-life of infected cells in this model was calculated to be approximately a day, suggesting that the number of productively infected cells would decay to approximately 1% after a week of cART treatment (Honeycutt, Thayer et al. 2017). After cART interruption, re-establishment of systemic infection was observed in a third of MoM, and this viral rebound was associated with higher pre-ART viral burden. This study suggests a role for macrophages in HIV persistence during treatment and suggests the possibility that persistent HIV infection of brain myeloid-derived cells could re-start infection systemically when therapy is removed
Humanized Bone marrow/Liver/Thymus (BLT) mouse model
Humanized BLT mice are created by implanting human thymus and liver tissues under the kidney capsule of NSG mice; these animals are also injected with autologous hematopoietic stem cells. The result is a robust small animal model that is reconstituted with a variety of human immune cells including T cells, myeloid cells, B cells, and NK cells. These animals are susceptible to both parenteral and mucosal routes of transmission (Sun, Denton et al. 2007, Denton, Estes et al. 2008, Denton, Krisko et al. 2010). There is no peer-reviewed publication detailing the characterization of the brain of this model system. However, some information regarding the brains of BLT mice has been reported. Specifically, human hematopoietic cells are present in the brains of BLT mice, including T cells, B cells, and myeloid cells; HIV is detectable in the brains of systemically infected animals (Brew, Robertson et al. 2015).
Implementation of cART consisting of tenofovir, raltegravir and emtricitabine in HIV-infected BLT mice suppresses viremia in the plasma and tissues (Denton, Olesen et al. 2012, Denton, Long et al. 2014). Latently infected resting CD4+ memory T cells are present in the BLT mouse model (Denton, Olesen et al. 2012, Marsden, Kovochich et al. 2012). Latently infected resting CD4+ T cells are present at a frequency of about 8 infectious units per million cells in this model (Denton, Olesen et al. 2012). Rapid viral rebound is observed in this model after ART-interruption. The BLT mouse model has proved especially useful in the evaluation of latency reversing agents (LRAs). A combination of three LRAs (vorinostat, I-BET151 and CTLA-4) in combination with three antibodies (3BNC117, 10–174 and PG16) were able to prevent viral rebound in over 50% of HIV-infected BLT mice. Additionally, two groups have demonstrated that targeting the interferon response during suppressive cART treatment restored T cell immune function, reduced size of the viral reservoir, and delayed viral rebound after ART-interruption (Cheng, Ma et al. 2017, Zhen, Rezek et al. 2017). These results indicate the potential usefulness of BLT mice for future study of HIV infection in the CNS.
Humanized T cell only mouse (ToM) model
Implantation of human thymus and liver tissues under the kidney capsule of NSG mice leads to systemic reconstitution with human T cells, exclusively (Honeycutt, Wahl et al. 2013). These T cell only mice, or ToM, have been used to study HIV infection in vivo without contribution from human antigen presenting cells (Honeycutt, Wahl et al. 2013, Honeycutt, Wahl et al. 2016). T cell only mice support sustained HIV replication, and institution of cART in these animals leads to undetectable plasma viremia over time. More importantly, analysis of resting memory CD4+ T cells from these mice demonstrate that latency is established at similar levels to that present in patients that initiated therapy during the chronic phase of infection (Honeycutt, Wahl et al. 2013). Like for BLT mice, there is no information published as of this date regarding the repopulation of the brains of ToM with human T cells. However, if T cells are present in the brains of these animals it will allow for the investigation of the role of T cells in HIV infection and persistence in the CNS.
Strengths and limitations of current humanized mouse models
One major benefit of using humanized mouse models for HIV in general, and for studies focusing on the CNS in particular, is the fact that these animals are highly customizable. Systemic or local human reconstitution can be achieved with a variety of cell types, which can shape the types of questions that can be addressed. In humans, studies are limited to imaging (non-invasive), cerebral spinal fluid sampling (invasive, and not clear how representative of the brain), and post-mortem analysis. The use of humanized mice allows for controlled experiments, which can have defined endpoints with whole brain analysis. Humanized mice are reconstituted with the natural targets of HIV infection, human T cells and human myeloid cells, and allow scientists to mimic natural routes of infection with full-length, replication-competent viruses. By using cells and/or tissues from a variety of donors in experiments, humanized mice can also represent some of the genetic variations present in the general population.
The recent observation that macrophages in culture remain in a G0-terminally differentiated state, as opposed to the G1-like state found in tissue macrophages further highlights the need for models where human macrophages can be studied in vivo (Mlcochova, Sutherland et al. 2017). The G1-like state present in tissue macrophages is thought to allow HIV-1 to bypass the replication restriction generally imposed by SAMHD1, and likely explains how tissue macrophages are able to sustain HIV replication over time in vivo (Mlcochova, Sutherland et al. 2017). In this respect, humanized mice reconstituted with myeloid cells, including huNSG, huNOG, MoM and BLT mice, offer a unique advantage over culture systems.
Each humanized mouse model also has its limitations. For the SCID-HIVE model, the major limitation is the traumatic nature of humanization (direct injection into the brain), which leads to brain pathology even when uninfected cells are used, although the pathology is more pronounced in HIV-infected versus uninfected cells. Studies in SCID-HIVE mice have generally been limited to four weeks or less as a robust graft-versus-host-disease (GVHD) occurs, which prevents long-term analysis of HIV persistence in these animals. In the myeloid-only mouse model, there is a time restraint for study, as NOD/SCID mice succumb to formation of thymic lymphomas which are ultimately lethal (generally 9–14 months of age) (Prochazka, Gaskins et al. 1992). However, infection studies upwards of 22 weeks have been shown in this model (Honeycutt, Thayer et al. 2017). In models reconstituted with human PBMCs, GVHD occurs rapidly and limits the lifespan of the animals (Ali, Flutter et al. 2012).
In summary, in all humanized mouse models, the human immune cells exist in a chimeric environment and must interact with the endogenous mouse cells. However, there is a remarkable correspondence between the human condition and each model is able to recapitulate specific key aspects of HIV infection, including routes of transmission, response to cART, and establishment of viral persistence (Table 1).
Table 1:
Comparison of humanized mouse models for studies of NeuroHIV and HIV cure studies.
| Parameter | SCID HIVE | hu-NSG | NSG-hu-PBL | MoM | BLT | ToM |
|---|---|---|---|---|---|---|
| Human cells present in CNS | Y | Y | Y | Y | Y* | nd |
| CNS pathology evaluated | Y | Y | Y | Y | nd | nd |
| Virus present in CNS | Y | Y | Y | Y | Y* | nd |
| ART evaluated in CNS | Y | nd | nd | nd | Y* | nd |
| Human cells present systemically | nd | Y | Y | Y | Y | Y |
| Susceptible to parenteral HIV transmission | nd | Y | Y | Y | Y | Y |
| Susceptible to mucosal HIV transmission | nd | nd | nd | nd | Y | nd |
| Model for HIV persistence/latency | nd | Y | nd | Y | Y | Y |
Y=yes, N=no, nd=not done, Y*=some information available, but lacking primary publication.
Interesting topics for future study
Immune activation in the brains of humanized mice
In response to chronic infection with HIV, a state of persistent immune activation occurs systemically. In the CNS, this chronic immune activation can lead to detrimental pathologies. While the robust immune response is extremely beneficial during early/acute infection, long-term chronic inflammation leads to detrimental effects, including immune exhaustion (Deeks, Odorizzi et al. 2017). By tempering the levels of immune activation via antagonism of the interferon response in humanized BLT mice infected with HIV, improved functioning of CD8+ T cells and a possible decrease in the viral reservoir has been reported (Cheng, Ma et al. 2017, Zhen, Rezek et al. 2017). Unfortunately, the CNS was not evaluated in these studies. Several studies in SCID-HIVE mice have begun to address this phenomenon in the CNS, including treatment with CEP-1347, B18R, and ruxolitnib (described above). However, it is not clear whether the pathology observed in the SCID-HIVE model is representative of the majority of patients in the post-ART era.
CD4+ and CD8+ T cell infiltration in the brains of HIV-infected humanized mice
Encephalitis in patients on cART was associated with infiltration with CD8+ lymphocytes, suggesting these cells may have a role in HAND (Gray, Lescure et al. 2013). By using systemically reconstituted models, such as huNSG, BLT mice and T cell-only mice (ToM), it may be possible to elucidate the underlying mechanisms driving T cell infiltration into the brain. However, this will be dependent on the integrity of the blood-brain barrier in these models.
Summary.
Humanized mouse models reflect key aspects of HIV infection in patients and provide useful research platforms for studying infection in vivo. More widespread inclusion of the CNS into current studies of humanized mouse models, especially with regard to persistence and eradication studies, will advance the field considerably. This will be especially important in studies using stem cell transplanted models, which allow for prolonged observation (>20 weeks) and could be used to elucidate the effects of long-term cART on the brain. Overall, humanized mice have thus far proved to be valuable in describing the pathogenesis of HIV infection and represent a potentially useful tool for both NeuroHIV and HIV cure research.
Acknowledgements
This work has been supported in part by the Virology Training Grant (T32 AI-007419) (JBH) and grants from the National Institute of Mental Health (NIMH) (MH-108179), the National Institute of Allergy and Infectious Diseases (NIAID) (AI-111899), the UNC CFAR (P30 AI050410) and CARE [a Martin Delaney Collaboratory of the NIAID, National Institute of Neurological Disorders and Stroke (NINDS), National Institute On Drug Abuse (NIDA) and the NIMH of the National Institutes of Health, grant number 1UM1AI126619–01] (JVG).
Footnotes
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. JBH no conflict of interest. JVG no conflict of interest.
References:
- Ali N, Flutter B, Sanchez Rodriguez R, Sharif-Paghaleh E, Barber LD, Lombardi G and Nestle FO (2012). “Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rgammanull mice display a T-effector memory phenotype.” PLoS One 7(8): e44219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol BL, Simmons DA, Zuccato C, Illes K, Pallos J, Casale M, Conforti P, Ramos C, Roarke M, Kathuria S, Cattaneo E, Marsh JL and Thompson LM (2008). “CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice.” Mol Cell Neurosci 39(1): 8–20. [DOI] [PubMed] [Google Scholar]
- Arainga M, Edagwa B, Mosley RL, Poluektova LY, Gorantla S and Gendelman HE (2017). “A mature macrophage is a principal HIV-1 cellular reservoir in humanized mice after treatment with long acting antiretroviral therapy.” Retrovirology 14(1): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arainga M, Su H, Poluektova LY, Gorantla S and Gendelman HE (2016). “HIV-1 cellular and tissue replication patterns in infected humanized mice.” Sci Rep 6: 23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H and Alitalo K (2015). “A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules.” J Exp Med 212(7): 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AE, Russell RA, Duncan CJ, Moore MD, Willberg CB, Pablos JL, Finzi A, Kaufmann DE, Ochsenbauer C, Kappes JC, Groot F and Sattentau QJ (2014). “Macrophage infection via selective capture of HIV-1-infected CD4+ T cells.” Cell Host Microbe 16(6): 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew BJ, Robertson K, Wright EJ, Churchill M, Crowe SM, Cysique LA, Deeks S, Garcia JV, Gelman B, Gray LR, Johnson T, Joseph J, Margolis DM, Mankowski JL and Spencer B (2015). “HIV eradication symposium: will the brain be left behind?” J Neurovirol 21(3): 322–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calantone N, Wu F, Klase Z, Deleage C, Perkins M, Matsuda K, Thompson EA, Ortiz AM, Vinton CL, Ourmanov I, Lore K, Douek DC, Estes JD, Hirsch VM and Brenchley JM (2014). “Tissue myeloid cells in SIV-infected primates acquire viral DNA through phagocytosis of infected T cells.” Immunity 41(3): 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Ma J, Li J, Li D, Li G, Li F, Zhang Q, Yu H, Yasui F, Ye C, Tsao LC, Hu Z, Su L and Zhang L (2017). “Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs.” J Clin Invest 127(1): 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B, Chun E, Kim M, Kim SY, Kim ST, Yoon K, Lee KY and Kim SJ (2011). “Human T cell development in the liver of humanized NOD/SCID/IL-2Rgamma(null)(NSG) mice generated by intrahepatic injection of CD34(+) human (h) cord blood (CB) cells.” Clin Immunol 139(3): 321–335. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Seider TR and Navia B (2015). “HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease?” Alzheimers Res Ther 7(1): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti P, Ramos C, Apostol BL, Simmons DA, Nguyen HP, Riess O, Thompson LM, Zuccato C and Cattaneo E (2008). “Blood level of brain-derived neurotrophic factor mRNA is progressively reduced in rodent models of Huntington’s disease: restoration by the neuroprotective compound CEP-1347.” Mol Cell Neurosci 39(1): 1–7. [DOI] [PubMed] [Google Scholar]
- Cook-Easterwood J, Middaugh LD, Griffin WC 3rd, Khan I and Tyor WR (2007). “Highly active antiretroviral therapy of cognitive dysfunction and neuronal abnormalities in SCID mice with HIV encephalitis.” Exp Neurol 205(2): 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JE, Dasgupta S, Middaugh LD, Terry EC, Gorry PR, Wesselingh SL and Tyor WR (2005). “Highly active antiretroviral therapy and human immunodeficiency virus encephalitis.” Ann Neurol 57(6): 795–803. [DOI] [PubMed] [Google Scholar]
- Dash PK, Gendelman HE, Roy U, Balkundi S, Alnouti Y, Mosley RL, Gelbard HA, McMillan J, Gorantla S and Poluektova LY (2012). “Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice.” AIDS 26(17): 2135–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Gorantla S, Gendelman HE, Knibbe J, Casale GP, Makarov E, Epstein AA, Gelbard HA, Boska MD and Poluektova LY (2011). “Loss of neuronal integrity during progressive HIV-1 infection of humanized mice.” J Neurosci 31(9): 3148–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Odorizzi PM and Sekaly RP (2017). “The interferon paradox: can inhibiting an antiviral mechanism advance an HIV cure?” J Clin Invest 127(1): 103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK, Powell DA, Payne D, Haase AT and Garcia JV (2008). “Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice.” PLoS Med 5(1): e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton PW and Garcia JV (2011). “Humanized mouse models of HIV infection.” AIDS Rev 13(3): 135–148. [PMC free article] [PubMed] [Google Scholar]
- Denton PW, Krisko JF, Powell DA, Mathias M, Kwak YT, Martinez-Torres F, Zou W, Payne DA, Estes JD and Garcia JV (2010). “Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice.” PLoS One 5(1): e8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton PW, Long JM, Wietgrefe SW, Sykes C, Spagnuolo RA, Snyder OD, Perkey K, Archin NM, Choudhary SK, Yang K, Hudgens MG, Pastan I, Haase AT, Kashuba AD, Berger EA, Margolis DM and Garcia JV (2014). “Targeted cytotoxic therapy kills persisting HIV infected cells during ART.” PLoS Pathog 10(1): e1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, Swanson MD, Chateau M, Nochi T, Krisko JF, Spagnuolo RA, Margolis DM and Garcia JV (2012). “Generation of HIV latency in humanized BLT mice.” J Virol 86(1): 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert D, Dash PK, Gorantla S, Dou H, Schifitto G, Maggirwar SB, Dewhurst S, Poluektova L, Gelbard HA and Gendelman HE (2010). “Neuroprotective activities of CEP-1347 in models of neuroAIDS.” J Immunol 184(2): 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz-French C, Shawahna R, Ward JE, Maroun LE and Tyor WR (2014). “The recombinant vaccinia virus gene product, B18R, neutralizes interferon alpha and alleviates histopathological complications in an HIV encephalitis mouse model.” J Interferon Cytokine Res 34(7): 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla S, Makarov E, Finke-Dwyer J, Castanedo A, Holguin A, Gebhart CL, Gendelman HE and Poluektova L (2010). “Links between progressive HIV-1 infection of humanized mice and viral neuropathogenesis.” Am J Pathol 177(6): 2938–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray F, Lescure FX, Adle-Biassette H, Polivka M, Gallien S, Pialoux G and Moulignier A (2013). “Encephalitis with infiltration by CD8+ lymphocytes in HIV patients receiving combination antiretroviral treatment.” Brain Pathol 23(5): 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile WB, Gavegnano C, Tao S, Jiang Y, Schinazi RF and Tyor WR (2016). “The Janus kinase inhibitor ruxolitinib reduces HIV replication in human macrophages and ameliorates HIV encephalitis in a murine model.” Neurobiol Dis 92(Pt B): 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I and Group C (2010). “HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study.” Neurology 75(23): 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt JB, Thayer WO, Baker CE, Ribeiro RM, Lada SM, Cao Y, Cleary RA, Hudgens MG, Richman DD and Garcia JV (2017). “HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy.” Nat Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt JB, Wahl A, Archin N, Choudhary S, Margolis D and Garcia JV (2013). “HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model.” Retrovirology 10: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt JB, Wahl A, Baker C, Spagnuolo RA, Foster J, Zakharova O, Wietgrefe S, Caro-Vegas C, Madden V, Sharpe G, Haase AT, Eron JJ and Garcia JV (2016). “Macrophages sustain HIV replication in vivo independently of T cells.” J Clin Invest 126(4): 1353–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KC, Choi BS, Kim KC, Park KH, Lee HJ, Cho YK, Kim SI, Kim SS, Oh YK and Kim YB (2016). “A Simple Mouse Model for the Study of Human Immunodeficiency Virus.” AIDS Res Hum Retroviruses 32(2): 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koneru R, Olive MF and Tyor WR (2014). “Combined antiretroviral therapy reduces brain viral load and pathological features of HIV encephalitis in a mouse model.” J Neurovirol 20(1): 9–17. [DOI] [PubMed] [Google Scholar]
- Krisko JF, Martinez-Torres F, Foster JL and Garcia JV (2013). “HIV restriction by APOBEC3 in humanized mice.” PLoS Pathog 9(3): e1003242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH and Kipnis J (2015). “Structural and functional features of central nervous system lymphatic vessels.” Nature 523(7560): 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Porzgen P, Mortensen AL, Hasseldam H, Bozyczko-Coyne D, Morath S, Hartung T, Bianchi M, Ghezzi P, Bsibsi M, Dijkstra S and Leist M (2005). “Inhibition of microglial inflammation by the MLK inhibitor CEP-1347.” J Neurochem 92(6): 1439–1451. [DOI] [PubMed] [Google Scholar]
- Marsden MD, Kovochich M, Suree N, Shimizu S, Mehta R, Cortado R, Bristol G, An DS and Zack JA (2012). “HIV latency in the humanized BLT mouse.” J Virol 86(1): 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Misawa N, Kawano Y, Okada H, Inagaki Y, Yamamoto N, Ito M, Yagita H, Okumura K, Mizusawa H and Koyanagi Y (2003). “Tumor necrosis factor-related apoptosis-inducing ligand induces neuronal death in a murine model of HIV central nervous system infection.” Proc Natl Acad Sci U S A 100(5): 2777–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlcochova P, Sutherland KA, Watters SA, Bertoli C, de Bruin RA, Rehwinkel J, Neil SJ, Lenzi GM, Kim B, Khwaja A, Gage MC, Georgiou C, Chittka A, Yona S, Noursadeghi M, Towers GJ and Gupta RK (2017). “A G1-like state allows HIV-1 to bypass SAMHD1 restriction in macrophages.” EMBO J 36(5): 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Buttini M, Limoges J, Bock P and Gendelman HE (1997). “An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis.” J Neurovirol 3(6): 401–416. [DOI] [PubMed] [Google Scholar]
- Persidsky Y and Gendelman HE (1997). “Development of laboratory and animal model systems for HIV-1 encephalitis and its associated dementia.” J Leukoc Biol 62(1): 100–106. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Ghorpade A, Rasmussen J, Limoges J, Liu XJ, Stins M, Fiala M, Way D, Kim KS, Witte MH, Weinand M, Carhart L and Gendelman HE (1999). “Microglial and astrocyte chemokines regulate monocyte migration through the blood-brain barrier in human immunodeficiency virus-1 encephalitis.” Am J Pathol 155(5): 1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet HS, Epstein L, Gelbard H, Flanagan E, Reinhard J, Pirruccello SJ and Gendelman HE (1996). “Human immunodeficiency virus encephalitis in SCID mice.” Am J Pathol 149(3): 1027–1053. [PMC free article] [PubMed] [Google Scholar]
- Prochazka M, Gaskins HR, Shultz LD and Leiter EH (1992). “The nonobese diabetic scid mouse: model for spontaneous thymomagenesis associated with immunodeficiency.” Proc Natl Acad Sci U S A 89(8): 3290–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Denton PW, Estes JD, Othieno FA, Wei BL, Wege AK, Melkus MW, Padgett-Thomas A, Zupancic M, Haase AT and Garcia JV (2007). “Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1.” J Exp Med 204(4): 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Power C, Gendelman HE and Markham RB (1993). “A model of human immunodeficiency virus encephalitis in scid mice.” Proc Natl Acad Sci U S A 90(18): 8658–8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Liu L, Cheung KW, Wang H, Lu X, Cheung AK, Liu W, Huang X, Li Y, Chen ZW, Chen SM, Zhang T, Wu H and Chen Z (2016). “Brain Invasion by CD4(+) T Cells Infected with a Transmitted/Founder HIV-1BJZS7 During Acute Stage in Humanized Mice.” J Neuroimmune Pharmacol 11(3): 572–583. [DOI] [PubMed] [Google Scholar]
- Zhen A, Rezek V, Youn C, Lam B, Chang N, Rick J, Carrillo M, Martin H, Kasparian S, Syed P, Rice N, Brooks DG and Kitchen SG (2017). “Targeting type I interferon-mediated activation restores immune function in chronic HIV infection.” J Clin Invest 127(1): 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]


