Abstract
Coxiella burnetii (C. burnetii), a zoonotic bacterium, has recently been identified in several marine mammal species on the Pacific Coast of North America but little is known about the epidemiology, transmission, and pathogenesis in these species. To address this knowledge gap, we tested sera archived from northern fur seals (NFS, Callorhinus ursinus; n=236) and Steller sea lions (SSL, Eumatopias jubatus; n=72) sampled in Alaska for presence of C. burnetii antibodies, and vaginal swabs from NFS (n=40) for C. burnetii by qPCR. The seroprevalence of NFS samples from 1994 was 49%, while the prevalence in samples from 2009 and 2011 was 69%, and this difference was significant. The seroprevalence of SSL samples from 2007–2011 was 59%. All NFS vaginal swabs were negative for C. burnetii, despite an 80% seroprevalence of the matched sera. The significant increase in seroprevalence in NFS from 1994 to 2011 suggests that the pathogen may be increasingly common, or that there is marked temporal variation, within the vulnerable NFS population. The high seroprevalence in SSL suggests that this pathogen may also be significant in the endangered SSL population. These results confirm that C. burnetii is more prevalent within these populations than previously known; more research is needed to determine how this bacterium may affect individual, population, and reproductive health of marine mammals.
Keywords: Coxiella burnetii, marine mammals, northern fur seal, seroprevalence, Steller sea lion
Coxiella burnetii (C. burnetii), causative agent of human “Q fever,” is an obligate intracellular Gram-negative bacterium that is shed in reproductive tissues of infected females during parturition and transmitted via aerosolization and inhalation (Maurin and Raoult, 1999). Although infection is often subclinical in many species, it can present clinically as infertility and abortion in ruminants (McQuiston et al., 2002). The bulk of knowledge of coxiellosis pertains to terrestrial species, but case reports and studies of C. burnetii infection in marine mammals increasingly suggest that the pathogen may be common and clinically significant in marine mammals. C. burnetii placentitis was documented in an ailing, and later euthanized, pregnant Pacific harbor seal (Phoca vitulina richardsi) found on a beach in Northern California in 1998 (Lapointe et al., 1999). C. burnetii was also found in the placenta of a dead pregnant Steller sea lion (SSL, Eumatopias jubatus) on the coast of Washington in 2008 (Kersh et al., 2010). A 2010 cross-sectional survey of Pacific harbor seals, harbor porpoises (Phocoena phocoena), and SSL collected in the Pacific Northwest suggested that C. burnetii infection is commonplace among multiple species of marine mammals in this region (Kersh et al., 2012).
The significance of this reproductive pathogen in more northern regions is unclear. The northern fur seal (NFS, Callorhinus ursinus) is listed as a vulnerable species by the International Union for Conservation of Nature (IUCN), and in 2010, 75% of 146 sampled placentas from Saint Paul Island, Alaska, were positive for the pathogen by quantitative polymerase chain reaction (qPCR) (Duncan et al., 2012a). The western stock of SSL, occupying much of the same range as the NFS, is listed as an endangered species (IUCN), but nothing is known about the prevalence of C. burnetii infection within this population. To better characterize the epidemiology of C. burnetii in a subset of Alaskan pinnipeds, we conducted a serosurvey using archived samples from NFS and SSL sampled at varying time points between 1994 and 2011. To examine shedding in these species, we tested a subset of vaginal swabs from these animals for presence of the IS1111 and com1 genomic regions of C. burnetii by qPCR.
Serum samples were collected by venipuncture from live NFS and SSL during routine animal capture activities focused on animal behavior and physiology. Subadult male NFS were sampled immediately after being killed for subsistence harvest by native Alaskans. Vaginal swabs (sterile polyester, Puritan medical, Guilford, Maine) were collected from adult female NFS only during the same operations. All samples were frozen at −80° Celsius until thawed for analysis. Indirect immunofluorescensce assay (IFA) was conducted on serum samples at the Centers for Disease Control and Prevention Q Fever Laboratory in Atlanta, Georgia, as previously reported (Kersh et al., 2012). Serial dilutions of sera from 1:128 to 1:16384 were plated on slides coated with acetone-fixed C. burnetii strains Nine Mile Phase 1 and Phase 2. Binding was visualized using a fluorescein isothiocyanate (FITC) conjugated goat anti-dog secondary antibody (KPL, Gaithersburg, MD). To eliminate reporting of false positives from cross-reactivity of antibodies, a positive result cutoff was set at 1:128 similar to previous marine mammal studies (Kersh et al., 2012).
Genomic DNA was extracted from adult NFS vaginal swabs from 2009 and 2011 using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, California) according to manufacturer’s directions. Quantitative PCR was used to test the extracted DNA for C. burnetii com1 and IS1111 gene targets as previously described (Kersh et al., 2010). Quantitative PCR was conducted utilizing the Applied Biosystems TaqMan® Universal PCR Master Mix Kit, No AmpErase® UNG (Applied Biosystems, Foster City, California) and an Applied Biosystems 7500 96-well plate thermocycler. Descriptive and comparative statistics were conducted using commercially available software (SPSS 20, IBM, Inc.). The frequency of positive titers was compared between years, sexes, and age classes using the chi-square test.
Serology was conducted on a total of 236 NFS samples collected from Saint Paul Island, Alaska (Figure 1). Samples included 72 sub-adult males from 1994, 30 adult female samples from 2009, 80 sub-adult male samples from 2011, 24 adult female samples from 2011, and 30 three-month-old female pup samples from 2011. The seroprevalence of NFS per year and stratified by sex and age is presented by year (Table 1). Overall, 63% of samples had a titer of 1:128 or greater. The frequency of positive titers varied significantly between years (p<0.001), with an apparent increase from 49% in 1994 to 69% in 2009 and 2011. Overall, 65% and 61% of seropositive animals were females and males, respectively; this difference was not significant (p=0.514). Only 2009 and 2011 samples were included in the age class analysis to eliminate the effect of an apparent temporal increase; 69% of these samples had a titer and the frequency of seropositive animals varied significantly between age groups (p=0.046), with titers present in 50% of pups, 73% of sub-adults, and 74% of adults.
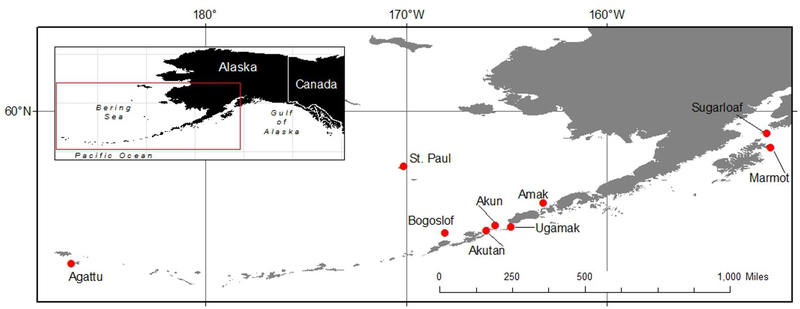
Figure 1.
Northern fur seal (Callorhinus ursinus) and Steller sea lion (Eumatopias jubatus) serum and vaginal swab samples were collected at the plotted sites in Alaska from 1994 to 2011.
Table 1.
1994 to 2011 Temporal Seroprevalence by Sex and Age in Saint Paul Island, Alaska, Northern Fur Seal (Callorhinus ursinus) Population
| Year | Sex | Age | Positive Titer | Total | % Seropositive |
|---|---|---|---|---|---|
| 1994 | Male | Sub-Adult | 35 | 72 | 49% |
| 2009 | Female | Adult | 27 | 30 | 90% |
| 2011 | Female | Adult | 13 | 24 | 54% |
| Male | Sub-Adult | 58 | 80 | 73% | |
| Female | Pup | 15 | 30 | 50% | |
| Total 2011 | 86 | 134 | 64% | ||
| All Years | 148 | 236 | 63% | ||
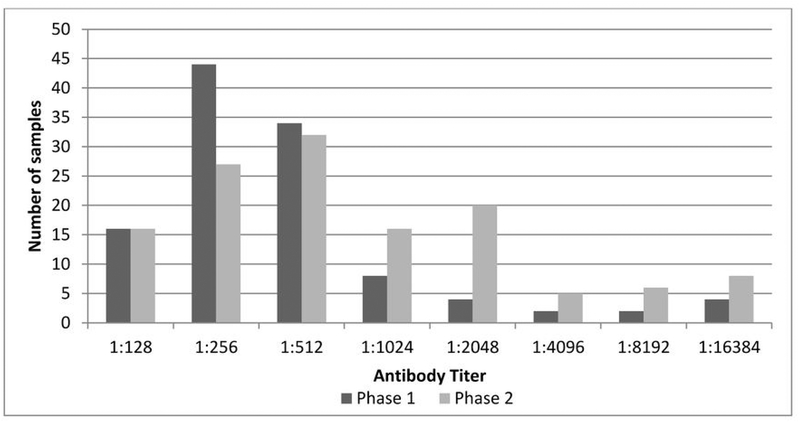
The frequency and range of titers to phase 1 and phase 2 antibodies are presented in figure 2. There was a wide range of titers, from 1:128 to 1:16384, for both phase 1 and phase 2, with 64 animals having a titer of 1:256 for phase 1 and 51 animals having a titer of 1:512 to phase 2. Positive (greater than or equal to 1:128) phase 2 titers were more common (n=145) than positive phase 1 titers (n=129), but the majority of NFS (85%) had both phase 1 and phase 2 titers.
Figure 2.
Frequency Distribution of Anti-C. burnetii Phase 1 and Phase 2 Antibody Titers in Saint Paul Island, Alaska, Northern Fur Seal (Callorhinus ursinus) Serum Samples from 1994–2011
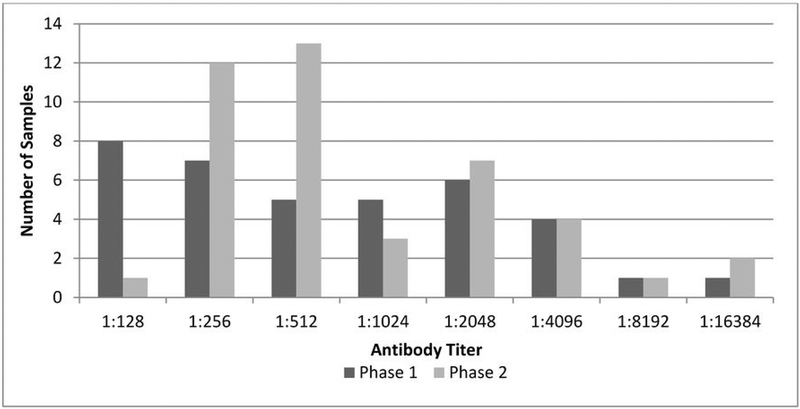
Serology was conducted on a total of 72 SSL samples, including seven samples from 2007 (three female and four male), 18 from 2008 (nine female and nine male), 13 from 2010 (11 female and two male), and 34 from 2011 (19 female and 15 male). All samples were from pups under two months of age at the time of sampling. SSL sera were collected from eight islands in the Gulf of Alaska and Aleutian archipelago (Figure 1), including Agattu (n=10), Akun (n=7), Bogoslof (n=9), Akutan (n=8), Marmot (n=5), Amak (n=10), Sugarloaf (n=9), and Ugamak (n=14). Overall, 59% of samples were positive (Table 2), and there was an increasing trend in seroprevalence over time, however there was no statistical difference in the frequency of positive animals by year (p=0.16). There was no difference in the prevalence of titers in females (60%) compared to males (59%, p=0.721). As with NFS, there was a wide range of titers (Figure 3) with positive phase 1 titers more common (n=44) than positive phase 2 titers (n=39). Most samples were positive for both phase 1 and phase 2 (86%).
Table 2.
2007 to 2011 Temporal Seroprevalence in Western Stock Alaska Steller Sea Lion (Eumatopias jubatus) Pup Samples
| Year | Age | Positive Titer | Total | % Seropositive |
|---|---|---|---|---|
| 2007 | Pup | 2 | 7 | 29% |
| 2008 | Pup | 10 | 18 | 56% |
| 2010 | Pup | 8 | 13 | 62% |
| 2011 | Pup | 24 | 36 | 67% |
| Total | Pup | 44 | 74 | 59% |
Figure 3.
Frequency Distribution of Anti-C. burnetii Phase 1 and Phase 2 Antibody Titers in Western Stock Alaska Steller Sea Lion (Eumatopias jubatus) Serum Samples from 2007–2011
Quantitative PCR was conducted on 40 vaginal swabs from adult female NFS collected in October 2009 (n=29) and October 2011 (n=11). Thirty-two (80%) of these animals were seropositive; seven were seropositive for only phase 2, while 25 were seropositive for both phase 1 and phase 2, and 35% had titers greater than 1:1000. All swabs were negative for C. burnetii by qPCR.
Our results suggest that C. burnetii is a common pathogen in western stock SSL and NFS in Alaska. Previously, C. burnetii in SSL was only identified by polymerase chain reaction in one out of two SSL placental samples from the Pacific Northwest (Kersh et al., 2012). Although sample sizes from some years were small, in the present study there appeared to be an increase in the seroprevalence from 2007 to 2011. Placentitis was evident in C. burnetii infected SSL in a previous case report from 2008 (Kersh et al., 2010) as well as the Pacific Northwest (Kersh et al., 2012), suggesting that infection with this pathogen could have a significant impact on reproductive health, and therefore, population health.
Results of this study show that C. burnetii was present in NFS as early as 1994. When the high prevalence of infection was identified in 2010 (Duncan et al., 2012a), it was unknown if the pathogen was new to the population or simply newly identified. Results of the present study suggest that the pathogen has been present in the population since the early 1990’s, however the prevalence is significantly higher in the 2000’s. The 20% increase in seroprevalence from 1994 to 2009 and 2011 is suggestive of an increasing trend of exposure within the NFS population, but it is also possible that there is cyclical variation in population seroprevalence that could influence the observed difference over time. The high seroprevalence is consistent with the high placental prevalence identified in this NFS population by qPCR previously (Duncan et al., 2012a; Duncan et al., 2012b).
In humans, phase 2 titers are present during acute infection and can remain elevated for years, whereas phase 1 titers are typically only elevated during chronic infection (Peacock et al., 1983). If this antibody-antigen relationship holds true for marine mammals, the frequency of titers to phase 1 and phase 2 C. burnetii antigens suggests that NFS and SSL may experience both acute and chronic infections. Given the aggregation of animals at the time of parturition, it is hypothesized that animals could be re-exposed annually during pupping season.
Vaginal C. burnetii was not detected using the archived NFS vaginal swab samples. Given that 80% of the animals tested were seropositive and the high placental prevalence in this population (Duncan et al., 2012a), this negative finding is interesting. In iatrogenically infected domestic goats, C. burnetii was excreted in vaginal discharge up to 14 days after abortion (Bouvery, 2003). However, given that the NFS swabs were collected in October, three to four months after parturition and when the majority of adult females are pregnant (Gentry, 1998), it is likely the organism had been cleared from the vaginal vault. To better characterize shedding, sample collection would ideally take place closer to time of parturition.
Overall, this study provides increasing epidemiological information regarding C. burnetii in Alaskan marine mammals. Reproductive health is a critical component of population stability in any animal cohort. C. burnetii infection has been shown to significantly alter cellular apoptosis in NFS placentas from Saint Paul Island (Myers et al., 2012) but the impact of this cellular alteration is difficult to extrapolate to the population level. As both of these species have shown recent population declines, we recommend that further investigation into the role of reproductive pathogens, including C. burnetii, be continued for both species.
Acknowledgements
C.M. was supported by an appointment to the Merial Veterinary Scholars Program and funded by the United States Department of Agriculture (USDA), National Institute of Food and Agriculture (NIFA), Animal Health and Disease Program. A.V.K. was supported by an appointment to the Emerging Infectious Diseases (EID) Fellowship program administered by the Association of Public Health Laboratories (APHL) and funded by the Centers for Disease Control and Prevention (CDC). Serum samples from NFS subadult males were collected with cooperation from the Aluet Community of Saint Paul Island Tribal Government.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC or the Department of Health and Human Services. All tissue samples were collected under authority of U.S. Marine Mammal Permit No. 782–1708 issued to the National Marine Mammal Lab, Seattle, Washington. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the National Marine Fisheries Service, NOAA. Reference to trade names does not imply endorsement by the National Marine Fisheries Service, NOAA.
References
- Bouvery NA, Souriau A, Lechopier P, Rodolakis A 2003. Experimental coxiella burnetii infection pregant goats: Excretion routes. Veterinary research(34):423–433. [DOI] [PubMed] [Google Scholar]
- Duncan C, Kersh GJ, Spraker T, Patyk KA, Fitzpatrick KA, Massung RF, Gelatt T. 2012a. Coxiella burnetii in northern fur seal (callorhinus ursinus) placentas from st. Paul island, alaska. Vector Borne Zoonotic Dis 12(3):192–5. [DOI] [PubMed] [Google Scholar]
- Duncan C, Savage K, Williams M, Dickerson B, Kondas A, Fitzpatrick K, Guerrero J, Spraker T, Kersh G. 2012b. Multiple strains of coxiella burnetii are present in the environment of st. Paul island, alaska. Transbound Emerg Dis In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry RL. 1998. Behavior and ecology of the northern fur seal. Princeton, N.J.: Princeton University Press. [Google Scholar]
- Kersh GJ, Lambourn DM, Raverty SA, Fitzpatrick KA, Self JS, Akmajian AM, Jeffries SJ, Huggins J, Drew CP, Zaki SR, Massung RF. 2012. Coxiella burnetii infection of marine mammals in the pacific northwest, 1997–2010. Journal of wildlife diseases 48(1):201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersh GJ, Lambourn DM, Self JS, Akmajian AM, Stanton JB, Baszler TV, Raverty SA, Massung RF. 2010. Coxiella burnetii infection of a steller sea lion (eumetopias jubatus) found in washington state. J Clin Microbiol 48(9):3428–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe JM, Gulland FM, Haines DM, Barr BC, Duignan PJ. 1999. Placentitis due to coxiella burnetii in a pacific harbor seal (phoca vitulina richardsi). J Vet Diagn Invest 11(6):541–3. [DOI] [PubMed] [Google Scholar]
- Maurin M, Raoult D. 1999. Q fever. Clinical microbiology reviews 12(4):518–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston JH, Childs JE, Thompson HA. 2002. Q fever. J Am Vet Med Assoc 221(6):796–9. [DOI] [PubMed] [Google Scholar]
- Myers E, Ehrhart EJ, Charles B, Spraker T, Gelatt T, Duncan C. 2012. Apoptosis in normal and coxiella burnetii-infected placentas from alaskan northern fur seals (callorhinus ursinus). Vet Pathol. Epub ahead of print, Nov. 2. [DOI] [PubMed] [Google Scholar]
- Peacock MG, Philip RN, Williams JC, Faulkner RS. 1983. Serological evaluation of o fever in humans: Enhanced phase i titers of immunoglobulins g and a are diagnostic for q fever endocarditis. Infection and immunity 41(3):1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]