Abstract
Centrosome aberrations are commonly observed in human tumors and correlate with tumor aggressiveness and poor prognosis. Extra centrosomes drive mitotic errors that have been implicated in promoting tumorigenesis in mice. However, centrosome aberrations can also disrupt tissue architecture and confer invasive properties that may facilitate the dissemination of metastatic cells. Recent work has shown that centrosome defects facilitate invasion through cell-autonomous and non-cell-autonomous mechanisms, suggesting cancer cells can benefit from centrosome aberrations present in a subset of the tumor cell population. Here we discuss how centrosome defects promote invasive behaviors that may contribute to initial steps in the metastatic cascade.
LoMastro and Holland ETOC
Centrosome aberrations are commonly observed in human tumors and correlate with tumor aggressiveness. Recent work has shown that centrosome defects facilitate invasion through both cell-autonomous and non-cell-autonomous mechanisms. In this Perspective, LoMastro and Holland discuss how centrosome defects promote invasive behaviors that may contribute to the metastatic cascade.
Centrosome abnormalities frequently occur in human tumors
The centrosome is the major microtubule organizing center in animal cells and functions in controlling cell polarity, motility, proliferation, and division (Nigg and Holland, 2018; Conduit, et al., 2015; Fu, et al., 2015; Bornens, 2012; Nigg and Raff, 2009). Centrosomes are generally comprised of a pair of microtubule-based structures called centrioles that are embedded in a pericentriolar matrix (PCM) (Woodruff, et al., 2014). Centrosomes form the poles of the bipolar microtubule spindle during mitosis and organize the microtubule cytoskeleton of many cell types during interphase. In quiescent cells, the older of the two centrioles functions as a basal body that assembles a primary cilium, which plays an important role in cell signaling (Sanchez and Dynlacht, 2016).
Centrosome number is tightly controlled in healthy proliferating cells. In G1 phase, cells contain a single centrosome that duplicates once, producing two centrosomes which form the bipolar spindle in mitosis (Nigg and Holland, 2018; Firat-Karalar and Stearns, 2014). The two centrosomes are equally partitioned during mitosis so that each daughter receives a single copy of the organelle. This duplication and segregation cycle maintains the correct number of centrosomes from one generation to the next. Proper control of centrosome number is critical for the appropriate functioning of the organelle in signaling and cell division (Nigg and Raff, 2009).
While centrosome homeostasis is strictly maintained in healthy cells, centrosome aberrations are commonly observed in human tumors. Centrosome defects in tumors can be broadly classified into numerical or structural alterations. Numerical alterations are an increase in centrosome copy number and may arise from defects in centrosome duplication, or perhaps more often, as a consequence of failed cell divisions. In contrast to centrosome amplification, structural centrosome abnormalities in human tumors are less well characterized, with the most straightforward defects being an increase in centrosome size as result of an expansion of the PCM (Schnerch and Nigg, 2016).
Structural and numerical centrosome aberrations are frequently observed in solid and hematological malignancies (Godinho and Pellman, 2014; Guo, et al., 2007; Kramer, et al., 2005; Pihan, et al., 2003; Sato, et al., 1999; Pihan, et al., 1998). Centrosome aberrations are observed at all stages of tumor development, and the severity of the defects correlates with increased tumor aggressiveness and poor prognosis in some cancer types (Hsu, et al., 2005; Skyldberg, et al., 2001; Sato, et al., 1999). In addition, karyotype analysis of tumors has drawn strong links between centrosome amplification and aneuploidy (Kramer, et al., 2005; Miki, et al., 2004; Krämer, et al., 2003). Structural abnormalities such as altered centrosome size or shape and increased centriole length have also been observed in tumors (Marteil, et al., 2018; Lingle and Salisbury, 1999; Lingle, et al., 1998). Although structural and numerical alterations often co-occur in tumors and induce some common effects, they can also promote mechanistically distinct cell behaviors as described further below (Arnandis, et al., 2018; Ganier, et al., 2018b; Ganier, et al., 2018d; Godinho, et al., 2014).
Studying sea urchin embryos more than 100 years ago, Theodor Boveri proposed that centrosome amplification promotes chromosome segregation errors, which drive tumorigenesis (Boveri, 1914). Today, it is widely accepted that increases in centrosome number disrupt the fidelity of cell division, leading to both numerical and structural alterations in the tumor cell karyotype (Crasta, et al., 2012; Ganem and Pellman, 2012; Janssen, et al., 2011; Ganem, et al., 2009; Silkworth, et al., 2009). A strong connection between centrosome amplification and tumorigenesis was first made in flies, where transplanted tissue containing extra centrosomes formed tumors that were capable of metastasizing (Basto, et al., 2008). Moreover, experimentally inducing centrosome amplification in mouse models by increasing the levels of Polo-like kinase 4 (Plk4), the master regulator of centrosome biogenesis, has been shown to promote tumorigenesis (Levine, et al., 2017; Sercin, et al., 2016; Coelho, et al., 2015). The tumors that form in mice with extra centrosomes show recurrent chromosomal copy number changes, suggesting that mitotic errors are likely to be a major contributor to tumorigenesis in this model (Levine, et al., 2017). However, the effect of extra centrosomes in vivo is context-dependent (Nigg and Holland, 2018). In the mouse brain, centrosome amplification promoted cell death leading to microcephaly (Marthiens, et al., 2013). Moreover, in the skin epidermis, centrosome amplification did not initiate spontaneous tumorigenesis or enhance the development of carcinogen-induced skin tumors (Vitre, et al., 2015), but was able to accelerate tumors that develop in the absence of the tumor suppressor TP53 (Sercin, et al., 2016).
In addition to playing important roles in cell division, centrosomes also organize the interphase microtubule network that controls cell shape, polarity and motility. Structural and numerical centrosome alterations can, therefore, reorganize the microtubule cytoskeleton and disrupt tissue architecture, potentially providing a platform for metastatic cell dissemination (Nigg, et al., 2017; Schnerch and Nigg, 2016). Indeed, recent work shows that centrosome aberrations may facilitate the dissemination of potentially metastatic cells through multiple distinct mechanisms (Arnandis, et al., 2018; Ganier, et al., 2018a; Ganier, et al., 2018c; Godinho, et al., 2014). In this review, we first outline the key steps required for metastasis and then discuss work suggesting that centrosome aberrations can contribute to the initial steps in the metastatic cascade.
Fundamentals of metastasis
The overwhelming majority of cancer mortality is caused by metastasis, a process in which cancer cells disseminate from the primary tumor and seed new colonies at distant sites (Siegel, et al., 2018). The dissemination and metastatic outgrowth of cancer cells is a complex multi-step process. It involves the local invasion of primary tumor cells into surrounding tissue, intravasation of these cells into the circulatory system, and subsequent extravasation back through the vascular walls. In this way, cancer cells travel to the parenchyma of a distant tissue and seed microscopic colonies that proliferate to form metastatic lesions (Lambert, et al., 2017).
Invasion is the first step of metastasis and refers to the ability of cancer cells to escape the site of the primary tumor and enter into surrounding normal tissue. A central process in invasion is the activation of a cell biological program termed the Epithelial to Mesenchymal Transition (EMT). The EMT program causes epithelial cells to adopt mesenchymal cell traits, such as increased motility, loss of cell-cell adhesions and the ability to degrade components of the surrounding extracellular matrix (ECM). Induction of this developmental program is considered a key initiating step in metastasis that allows single cells to exit the primary tumor site and invade surrounding parenchyma (Mittal, 2018; Kalluri and Weinberg, 2009).
The traditional view of metastasis is that activation of the EMT program allows single cells to disseminate from the primary tumor and seed the formation of clonal secondary tumors. However, more recent work has established that invasion by primary tumors often involves the collective migration of cohorts of cells that subsequently seed polyclonal metastatic outgrowths (Cheung and Ewald, 2016; Cheung, et al., 2016; Gundem, et al., 2015; Maddipati and Stanger, 2015; Aceto, et al., 2014). The organization of these invasive tumor cell clusters contradicts the expected behavior of cells that have undergone complete EMT in that they retain cell-cell adhesion junctions (Chung, et al., 2016; Veracini, et al., 2015). However, the EMT program is not a binary switch, as carcinoma cells can adopt a mixture of epithelial and mesenchymal traits (Lambert, et al., 2017). Moreover, in collectively migrating tumor cells, mesenchymal traits are occasionally observed in cells at the invasive front, possibly facilitating ECM degradation and the migration of the tumor cell cluster into the surrounding stroma (Westcott, et al., 2015; Ye, et al., 2015). It therefore likely that the EMT program works together with additional pathways to promote metastatic dissemination.
After invasion into neighboring stromal tissue, cancer cells must then intravasate into the vasculature and travel to a distant site to colonize a secondary tumor. This process depends on multiple signaling pathways, proteases, and interactions with nearby cells to allow invasive cells to adhere to, and pass through, the endothelial membrane (Chiang, et al., 2016). Once in the circulation, tumor cells travel as single cells or clusters until they become lodged in microvessels of distant tissues (Au, et al., 2016; Aceto, et al., 2014). The transit of circulating tumor cells (CTCs) can be aided by interactions with platelets, macrophages, and neutrophils, which coat CTCs to protect them from immune attack and facilitate docking at a distant site (Lambert, et al., 2017).
CTCs are present for short periods in the circulation before becoming lodged in microvessels, where they may breach vessel walls and extravasate into the parenchyma. The mechanisms that control the subsequent proliferation of cancer cells in foreign tissue environments remain unclear. Successful colonization requires adaptive behaviors and the development of a metastatic niche through the expression of supportive growth factors and signaling molecules (Massagué and Obenauf, 2016). Furthermore, in some sites, disseminated tumor cells are able to remain dormant for many years before re-initiating tumor growth and colonizing a metastatic lesion (Braun, et al., 2005).
Centrosome aberrations promote invasive phenotypes
The molecules and pathways required for tumor cell dissemination and subsequent metastatic outgrowth are beginning to come into focus. Nevertheless, we lack a comprehensive understanding of which features of the primary tumor are the major contributors to metastatic disease. As discussed earlier, centrosome aberrations are common in human tumors. New evidence suggests that these alterations can contribute to tumor cell dissemination through at least four different mechanisms, each of which is discussed in more detail below.
Centrosome aberrations induce formation of invadopodia
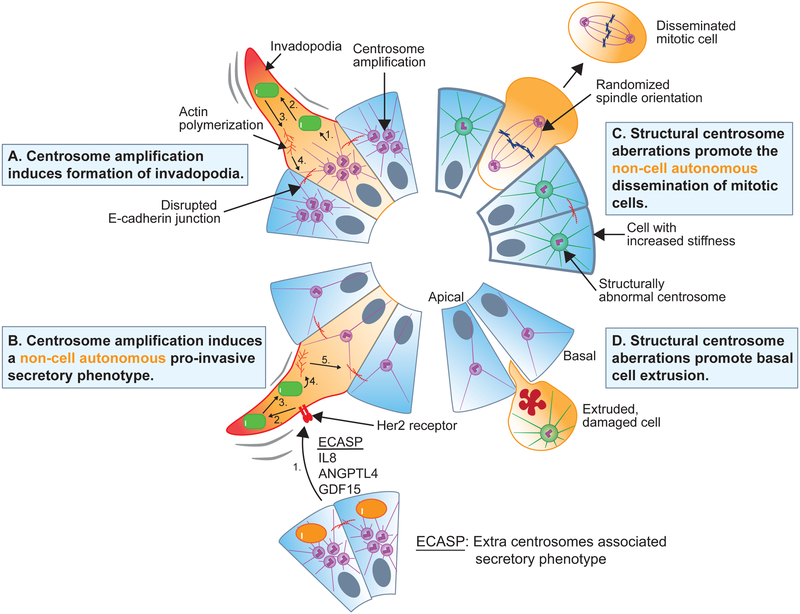
Centrosome amplification triggered by overexpression of Plk4 has been shown to promote to the creation of invasive protrusions (invadopodia) in mammary epithelial acini grown in a three-dimensional culture (3D) system (Figure 1A) (Godinho, et al., 2014). Invasive protrusions were accompanied by the degradation of ECM components, and in some instances resulted in the collective invasion of cells into the surrounding matrix. The increase in centrosomal microtubule nucleation in cells with extra centrosomes promoted activation of the small GTPase Rac1. Rac1 activity, in turn, initiated actin polymerization that disrupted cell-cell adhesion and promoted cell migration. Rac1 signaling is frequently upregulated in tumors, where it has been shown to promote invasion and metastasis (Figure 1A) (Bid, et al., 2013). A similar invasive phenotype can be triggered by overexpression of Ninein-like protein (Nlp), a centrosome protein that interacts with the γ-tubulin ring complex to promote microtubule nucleation (Ganier, et al., 2018d; Casenghi, et al., 2003). Nlp overexpression leads to an increase in centrosome size that resembles structural centrosome alterations in cancer cells (Schnerch and Nigg, 2016). These findings suggest that centrosome aberrations may constitute a general mechanism for promoting Rac1 activation, the disruption of cell-cell junctions, and cell motility during tumorigenesis.
Figure 1. Mechanisms by which centrosome aberrations can promote invasive phenotypes.
A. Centrosome amplification induced by Plk4 overexpression leads to increased microtubule nucleation and dynamics. This increases Rac1 signaling (1), resulting in the activation of the Arp2/3 complex (2), increased actin polymerization (3), and weakening of E-cadherin junctions (4) to promote the formation of invasive protrusions (invadopodia) in cells with extra centrosomes.
B. Pro-invasive factors secreted from cells with extra centrosomes promote the formation of invadopodia in cells with normal centrosome numbers. This extra centrosomes-associated secretory pathway (ECASP) is dependent on elevated reactive oxygen species in cells with amplified centrosomes. The secreted pro-invasive factors activate receptors (1), leading to increased Rac1 signaling (2), activation of the Arp2/3 complex (3), increased actin polymerization (4), and weakening of E-cadherin junctions (5) to promote invadopodia formation in cells with normal centrosome numbers.
C. Structural centrosome aberrations induced by overexpression of Nlp lead to increased microtubule nucleation and stability. This increases interphase cell stiffness and disrupts E-cadherin junctions, promoting the selective extrusion of softer mitotic cells from the epithelium.
D. Structural centrosome aberrations induced by overexpression of Nlp and CEP131 promote basal extrusion of damaged cells.
In addition to a primary role as a microtubule organizing center, the centrosome has been shown to directly nucleate actin filament assembly in an Arp2/3-dependent manner in vitro (Farina, et al., 2016). In lymphocytes, Arp2/3-mediated actin nucleation at the centrosome is required to link this organelle to the nucleus (Obino, et al., 2016). The removal of centrosomal Arp2/3 following lymphocyte activation promotes centrosome detachment from the nucleus and recruitment to the immune synapse. Although centrosome-mediated actin assembly has not yet been implicated in promoting lamellipodium formation or cell movement, this represents another mechanism by which centrosomes could contribute to the cytoskeletal reorganization that precedes invasion.
Centrosome amplification induces a pro-invasive secretory phenotype
In addition to promoting invadopodia formation through increased Rac1 signaling, centrosome amplification has been shown to promote the secretion of pro-invasive factors that induce non-cell autonomous invasion (Figure 1B) (Arnandis, et al., 2018). Conditioned media from cells with extra centrosomes induced the formation of invasive protrusions in 3D organoid cultures of cells with normal centrosome numbers. Cells with extra centrosomes were shown to secrete multiple pro-invasive factors that have been previously linked to cancer, invasion, and migration (such as IL-8, ANGPTL4 and GDF-15). Rac1 signaling was not required for the secretion of pro-invasive factors from cells with extra centrosomes, but it was required for the formation of invadopodia in cells with normal centrosomes responding to the secreted factors (Figure 1B). This suggests that the non-cell autonomous extra centrosomes-associated secretory pathway (ECASP) is distinct from the previously reported pathway that promotes the cell-autonomous formation of invasive protrusions in cells with supernumerary centrosomes (Godinho, et al., 2014). Exactly how extra centrosomes promote the ECASP remains unclear, but the response relies partly on elevated levels of reactive oxygen species in cells with centrosome amplification. While much remains to be understood about the role of the ECASP in tumor development, the discovery of this pathway provides a mechanism explaining how extra centrosomes could induce paracrine invasion in nearby cells with a normal centrosome content (Arnandis, et al., 2018). In the future, it will be interesting to test whether structural centrosome alterations can induce a pro-invasive secretory phenotype similar to that observed in cells with extra centrosomes.
Structural centrosome alterations promote the non-cell autonomous dissemination of mitotic cells
Although significant effort has focused on how centrosome amplification can contribute to tumorigenesis, the impact of structural centrosome aberrations has received much less attention. This is partly because defining centrosome structural defects is subjective, and it is less clear how to model these alterations experimentally. Recently, Nlp overexpression was used to experientially produce structural centrosome alterations that are similar to those observed in cancer. Indeed, elevated expression of Nlp is commonly found in human tumors (Yu, et al., 2009; Qu, et al., 2008), and promotes tumorigenesis in mice (Shao, et al., 2010).
Nlp-induced structural centrosome aberrations promoted the extrusion of individual cells from acini in a 3D culture model (Figure 1C) (Ganier, et al., 2018d). This “budding” phenotype occurred specifically in mitotic cells without structural centrosome aberrations and could be prevented by blocking cells from entering into mitosis. Most of the extruded cells were viable, and some continued to proliferate after escaping from the epithelium, indicating that epithelial budding may promote metastatic dissemination of nearby cells with normal centrosomes. Interestingly, budding was not observed in cells with extra centrosomes, demonstrating that structural and numerical centrosome abnormalities can promote distinct types of invasive behaviors.
The mechanisms by which structural centrosome aberrations promote mitotic cell budding are twofold. Nlp-induced structural centrosome aberrations triggered increased Rac1 signaling and actin polymerization, leading to weakening of E-cadherin mediated cell-cell junctions and randomized mitotic spindle orientation. However, centrosome amplification also increased Rac1 signaling and disrupted E-cadherin junctions, but did not induce a budding phenotype, suggesting that additional alterations are necessary for Nlp-induced dissemination. In contrast to cells with extra centrosomes, Nlp-induced structural centrosome aberrations markedly increased the stiffness of interphase cells in the epithelial sheet through increased microtubule nucleation and stability. This suggests that epithelia with a high density of Nlp-overexpressing cells may selectively squeeze out softer mitotic cells with destabilized E-cadherin junctions (Figure 1C). Importantly, the extruded mitotic cells did not often carry the centrosome alterations themselves, indicating that budding is a non-cell autonomous process that relies on cooperation between cells in an epithelium.
Structural centrosome alterations promote basal-cell extrusion
In addition to mitotic cell budding, structural centrosome aberrations have also been shown to promote the preferential extrusion of damaged cells towards the basal surface of epithelial monolayers (Figure 1D). Epithelia typically dispose of damaged cells by extruding them apically into the luminal cavity (Slattum and Rosenblatt, 2014). However, a switch in the directionality of cell extrusion has been observed in epithelia harboring oncogenic mutations (Ohsawa, et al., 2018; Gu, et al., 2015). If cell death is circumvented, basal extrusion may promote the accumulation of cells outside of the epithelial sheet, providing a first step towards metastatic dissemination. Similar to oncogenic mutations, centrosome structural aberrations induced by overexpression of Nlp have been shown to sensitize damaged epithelial cells to basal extrusion (Figure 1D). In addition, overexpression of the centrosome protein CEP131 creates distinct alterations in centrosome structure that promote the basal extrusion of dying cells with CEP131-induced structural centrosome aberrations in the absence of any external damaging agent (Ganier, et al., 2018b). If extruded cells harbor additional oncogenic alterations that promote survival, it is plausible that a reversal in the directionality of cell extrusion caused by centrosome aberrations could contribute to the dissemination of metastatic cells.
Implications for metastasis
In summary, recent work has uncovered multiple mechanisms by which centrosome alterations may contribute to metastasis by facilitating invasion. This suggests that centrosome aberrations can influence cancer progression beyond simply promoting mitotic defects. Centrosome defects cause invadopodia formation and basal cell extrusion, which could act as first steps in the dissemination of genetically unstable cells with metastatic potential. Intriguingly, centrosome aberrations may also facilitate invasion in a non-cell autonomous manner by inducing a pro-invasive secretory phenotype or promoting mitotic cell budding. These findings could explain why centrosome aberrations are often associated with advanced tumor stage and metastasis (Hsu, et al., 2005; Neben, et al., 2003; Skyldberg, et al., 2001; Sato, et al., 1999). In addition, the non-cell autonomous effects of centrosome alterations suggest that cells with centrosome aberrations could promote invasive behavior in surrounding cells that lack these defects. In this manner, tumor cells can broadly benefit from the centrosome aberrations present in only a subset of cells in the tumor population.
Many of the experiments examining the impact of centrosome aberrations on invasion have focused on 3D culture models of mammary epithelial cells. However, metastasis is a highly inefficient process, with the vast majority of disseminating cells destined to be eliminated or enter into a state of dormancy (Massagué and Obenauf, 2016; Chambers, et al., 2002). It, therefore, remains unclear from current experimental approaches whether centrosome aberrations can give rise to invasive cells that survive long-term and seed metastatic lesions in vivo. Moreover, it is unknown whether the phenotypes observed in cell culture will translate to tissues in vivo considering the responses to centrosome alterations may vary in different tissue types. Therefore, although in vitro studies will continue to be important to define molecular mechanisms, in vivo animal models and intravital tumor imaging will be increasingly required to define to what extent, and by which mechanisms, centrosome aberrations contribute to metastasis.
Perspective
Experimental work in mouse models has demonstrated that structural and numerical centrosome aberrations are sufficient to cause tumorigenesis (Levine, et al., 2017; Sercin, et al., 2016; Coelho, et al., 2015; Shao, et al., 2010). What remains to be clarified are the main mechanisms by which centrosome defects contribute to tumor formation and/or progression. It is now clear that structural and numerical alterations in centrosomes can promote distinct changes in cell physiology and behavior. This emphasizes the need to carefully define the properties of the centrosome aberrations present in various human tumors. However, while alterations in centrosome number are relatively simple to evaluate, determining what constitutes a structural defect in the centrosome is at present, relatively subjective. This issue is compounded by the fact that most studies analyzing primary tumors only use markers for the PCM and not the centriole. Consequently, centrosome defects such as altered centrosome size or shape, increased centriole length, and an expanded PCM cannot be easily distinguished. For example, centrosome amplification and PCM fragmentation arise through different mechanisms, but both lead to the formation of supernumerary PCM foci (Maiato and Logarinho, 2014). There is, therefore, a need to develop better methods to analyze and classify centrosome aberrations in human tumors, to understand both the prevalence and consequences of these defects. Doing so could allow different centrosome alterations to be used as diagnostic or prognostic markers in human tumors.
An additional area of focus is to discern the origin of centrosome aberrations in human tumors. Genes encoding centrosome proteins are rarely found to be mutated in tumors, but the misregulated expression of centrosome components is more common (Gonczy, 2015; Chan, 2011; Nigg and Raff, 2009). Given that centrosome aberrations can promote invasive phenotypes through non-cell-autonomous mechanisms, it is plausible that only a portion of cells in a primary tumor harbor the genetic alterations responsible for causing the centrosome defects (Arnandis, et al., 2018; Ganier, et al., 2018c). In addition, changes in cell cycle progression, DNA damage and failed cell divisions are common deficits in tumors that can indirectly impact centrosome number (Douthwright and Sluder, 2014; Inanc, et al., 2010; Ganem, et al., 2009). Relevant to understanding the origin of centrosome defects in cancer is the question of how faithfully the current experimental models recapitulate the centrosome alterations observed in human tumors. For example, Plk4 has been implicated in centrosome-independent functions, and these may contribute to the phenotypes observed following overexpression of the kinase (Kazazian, et al., 2017; Rosario, et al., 2015). Moreover, it is unclear how closely Nlp overexpression mirrors centrosome structural alterations observed in tumors. Ultimately, improved understanding of the origins of centrosome alterations in cancer will lead to the creation of better experimental models that will more closely phenocopy the defects observed in tumors. The development of these models will allow a more in-depth analysis of the contribution of structural and numerical centrosome alterations to invasion and metastatic disease in vivo.
Acknowledgments
This work was supported by the National Institutes of Health (R01GM114119), a National Institute of Health training grant (T32GM007445), a National Science Foundation Graduate Research Fellowship, and an American Cancer Society Scholar Grant (RSG-16–156-01-CCG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al. (2014). Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnandis T, Monteiro P, Adams SD, Bridgeman VL, Rajeeve V, Gadaleta E, Marzec J, Chelala C, Malanchi I, Cutillas PR, et al. (2018). Oxidative Stress in Cells with Extra Centrosomes Drives Non-Cell-Autonomous Invasion. Developmental Cell 47, 409–424.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au SH, Storey BD, Moore JC, Tang Q, Chen Y-L, Javaid S, Sarioglu AF, Sullivan R, Madden MW, O’Keefe R, et al. (2016). Clusters of circulating tumor cells traverse capillary-sized vessels. Proceedings of the National Academy of Sciences 113, 4947–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basto R, Brunk K, Vinadogrova T, Peel N, Franz A, Khodjakov A, and Raff JW (2008). Centrosome amplification can initiate tumorigenesis in flies. Cell 133, 1032–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bid HK, Roberts RD, Manchanda PK, and Houghton PJ (2013). RAC1: An Emerging Therapeutic Option for Targeting Cancer Angiogenesis and Metastasis. Molecular Cancer Therapeutics 12, 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornens M (2012). The centrosome in cells and organisms. Science 335, 422–6. [DOI] [PubMed] [Google Scholar]
- Boveri T (1914). Zur Frage der Entstenhung Maligner Tumoren. Jena, Gustav Fischer Verlag. [Google Scholar]
- Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, et al. (2005). A Pooled Analysis of Bone Marrow Micrometastasis in Breast Cancer. New England Journal of Medicine 353, 793–802. [DOI] [PubMed] [Google Scholar]
- Casenghi M, Meraldi P, Weinhart U, Duncan PI, Körner R, and Nigg EA (2003). Polo-like Kinase 1 Regulates Nlp, a Centrosome Protein Involved in Microtubule Nucleation. Developmental Cell 5, 113–125. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, and MacDonald IC (2002). Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer 2, 563–572. [DOI] [PubMed] [Google Scholar]
- Chan JY (2011). A clinical overview of centrosome amplification in human cancers. Int J Biol Sci 7, 1122–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KJ, and Ewald AJ (2016). A collective route to metastasis: Seeding by tumor cell clusters. Science (New York, N.Y.) 352, 167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, Gorin MA, Verdone JE, Pienta KJ, Bader JS, et al. (2016). Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci U S A 113, E854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang SPH, Cabrera RM, and Segall JE (2016). Tumor cell intravasation. Am J Physiol Cell Physiol 311, C1–C14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y-C, Wei W-C, Hung C-N, Kuo J-F, Hsu C-P, Chang K-J, and Chao W-T (2016). Rab11 collaborates E-cadherin to promote collective cell migration and indicates a poor prognosis in colorectal carcinoma. European Journal of Clinical Investigation 46, 1002–1011. [DOI] [PubMed] [Google Scholar]
- Coelho PA, Bury L, Shahbazi MN, Liakath-Ali K, Tate PH, Wormald S, Hindley CJ, Huch M, Archer J, Skarnes WC, et al. (2015). Over-expression of Plk4 induces centrosome amplification, loss of primary cilia and associated tissue hyperplasia in the mouse. Open Biol 5, 150209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conduit PT, Wainman A, and Raff JW (2015). Centrosome function and assembly in animal cells. Nat Rev Mol Cell Biol 16, 611–24. [DOI] [PubMed] [Google Scholar]
- Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, and Pellman D (2012). DNA breaks and chromosome pulverization from errors in mitosis. Nature 482, 53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douthwright S, and Sluder G (2014). Link between DNA damage and centriole disengagement/reduplication in untransformed human cells. Journal of cellular physiology 229, 1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina F, Gaillard J, Guerin C, Coute Y, Sillibourne J, Blanchoin L, and Thery M (2016). The centrosome is an actin-organizing centre. Nat Cell Biol 18, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firat-Karalar EN, and Stearns T (2014). The centriole duplication cycle. Philos Trans R Soc Lond B Biol Sci 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Hagan IM, and Glover DM (2015). The centrosome and its duplication cycle. Cold Spring Harb Perspect Biol 7, a015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem NJ, Godinho SA, and Pellman D (2009). A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem NJ, and Pellman D (2012). Linking abnormal mitosis to the acquisition of DNA damage. J Cell Biol 199, 871–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganier O, Schnerch D, and Nigg EA (2018a). Structural centrosome aberrations sensitize polarized epithelia to basal cell extrusion. Open Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganier O, Schnerch D, and Nigg EA (2018b). Structural centrosome aberrations sensitize polarized epithelia to basal cell extrusion. Open Biology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganier O, Schnerch D, Oertle P, Lim RY, Plodinec M, and Nigg EA (2018c). Structural centrosome aberrations promote non-cell-autonomous invasiveness. Embo J 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganier O, Schnerch D, Oertle P, Lim RY, Plodinec M, and Nigg EA (2018d). Structural centrosome aberrations promote non-cell-autonomous invasiveness. The EMBO Journal, e98576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho SA, and Pellman D (2014). Causes and consequences of centrosome abnormalities in cancer. Philosophical Transactions of the Royal Society B: Biological Sciences 369, 20130467–20130467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho SA, Picone R, Burute M, Dagher R, Su Y, Leung CT, Polyak K, Brugge JS, Thery M, and Pellman D (2014). Oncogene-like induction of cellular invasion from centrosome amplification. Nature 510, 167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P (2015). Centrosomes and cancer: revisiting a long-standing relationship. Nat Rev Cancer 15, 639–52. [DOI] [PubMed] [Google Scholar]
- Gu Y, Shea J, Slattum G, Firpo MA, Alexander M, Mulvihill SJ, Golubovskaya VM, and Rosenblatt J (2015). Defective apical extrusion signaling contributes to aggressive tumor hallmarks. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, Brewer DS, Kallio HML, Högnäs G, Annala M, et al. (2015). The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H. q., Gao M, Ma J, Xiao T, Zhao L. l., Gao Y, and Pan Q. j. (2007). Analysis of the cellular centrosome in fine-needle aspirations of the breast. Breast Cancer Research 9, R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L-C, Kapali M, DeLoia JA, and Gallion HH (2005). Centrosome abnormalities in ovarian cancer. International Journal of Cancer 113, 746–751. [DOI] [PubMed] [Google Scholar]
- Inanc B, Dodson H, and Morrison CG (2010). A centrosome-autonomous signal that involves centriole disengagement permits centrosome duplication in G2 phase after DNA damage. Mol Biol Cell 21, 3866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen A, van der Burg M, Szuhai K, Kops GJ, and Medema RH (2011). Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science 333, 1895–8. [DOI] [PubMed] [Google Scholar]
- Kalluri R, and Weinberg RA (2009). The basics of epithelial-mesenchymal transition. Journal of Clinical Investigation 119, 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian K, Go C, Wu H, Brashavitskaya O, Xu R, Dennis JW, Gingras AC, and Swallow CJ (2017). Plk4 Promotes Cancer Invasion and Metastasis through Arp2/3 Complex Regulation of the Actin Cytoskeleton. Cancer Res 77, 434–447. [DOI] [PubMed] [Google Scholar]
- Kramer A, Neben K, and Ho AD (2005). Centrosome aberrations in hematological malignancies. Cell Biol Int 29, 375–83. [DOI] [PubMed] [Google Scholar]
- Krämer A, Schweizer S, Neben K, Giesecke C, Kalla J, Katzenberger T, Benner A, Müller-Hermelink HK, Ho AD, and Ott G (2003). Centrosome aberrations as a possible mechanism for chromosomal instability in non-Hodgkin’s lymphoma. In Leukemia, pp. 2207–2213. [DOI] [PubMed] [Google Scholar]
- Lambert AW, Pattabiraman DR, and Weinberg RA (2017). Emerging Biological Principles of Metastasis. Cell 168, 670–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MS, Bakker B, Boeckx B, Moyett J, Lu J, Vitre B, Spierings DC, Lansdorp PM, Cleveland DW, Lambrechts D, et al. (2017). Centrosome Amplification Is Sufficient to Promote Spontaneous Tumorigenesis in Mammals. Dev Cell 40, 313–322.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle WL, Lutz WH, Ingle JN, Maihle NJ, and Salisbury JL (1998). Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc Natl Acad Sci U S A 95, 2950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle WL, and Salisbury JL (1999). Altered Centrosome Structure Is Associated with Abnormal Mitoses in Human Breast Tumors. Am J Pathol 155, 1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddipati R, and Stanger BZ (2015). Pancreatic Cancer Metastases Harbor Evidence of Polyclonality. Cancer Discov 5, 1086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H, and Logarinho E (2014). Mitotic spindle multipolarity without centrosome amplification. Nat Cell Biol 16, 386–94. [DOI] [PubMed] [Google Scholar]
- Marteil G, Guerrero A, Vieira AF, de Almeida BP, Machado P, Mendonca S, Mesquita M, Villarreal B, Fonseca I, Francia ME, et al. (2018). Over-elongation of centrioles in cancer promotes centriole amplification and chromosome missegregation. Nat Commun 9, 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthiens V, Rujano MA, Pennetier C, Tessier S, Paul-Gilloteaux P, and Basto R (2013). Centrosome amplification causes microcephaly. Nat Cell Biol 15, 731–40. [DOI] [PubMed] [Google Scholar]
- Massagué J, and Obenauf AC (2016). Metastatic colonization by circulating tumour cells. Nature 529, 298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki R, Okuda M, Oikawa T, Watanabe M, Ma Z, Matsumoto K, Iwata H, and Inokuma H (2004). Centrosome amplification and chromosomal instability in feline lymphoma cell lines. The Journal of veterinary medical science / the Japanese Society of Veterinary Science 66, 797–805. [DOI] [PubMed] [Google Scholar]
- Mittal V (2018). Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol 13, 395–412. [DOI] [PubMed] [Google Scholar]
- Neben K, Giesecke C, Schweizer S, Ho AD, and Krä A (2003). Centrosome aberrations in acute myeloid leukemia are correlated with cytogenetic risk profile. 101, 289–291. [DOI] [PubMed] [Google Scholar]
- Nigg EA, and Holland AJ (2018). Once and only once: mechanisms of centriole duplication and their deregulation in disease. Nat Rev Mol Cell Biol 19, 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA, and Raff JW (2009). Centrioles, centrosomes, and cilia in health and disease. Cell 139, 663–78. [DOI] [PubMed] [Google Scholar]
- Nigg EA, Schnerch D, and Ganier O (2017). Impact of Centrosome Aberrations on Chromosome Segregation and Tissue Architecture in Cancer. Cold Spring Harb Symp Quant Biol 82, 137–144. [DOI] [PubMed] [Google Scholar]
- Obino D, Farina F, Malbec O, Saez PJ, Maurin M, Gaillard J, Dingli F, Loew D, Gautreau A, Yuseff MI, et al. (2016). Actin nucleation at the centrosome controls lymphocyte polarity. Nat Commun 7, 10969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa S, Vaughen J, and Igaki T (2018). Cell Extrusion: A Stress-Responsive Force for Good or Evil in Epithelial Homeostasis. Developmental cell 44, 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihan GA, Purohit A, Wallace J, Knecht H, Woda B, Quesenberry P, and Doxsey SJ (1998). Centrosome defects and genetic instability in malignant tumors. Cancer Res 58, 3974–85. [PubMed] [Google Scholar]
- Pihan GA, Purohit A, Wallace J, Knecht H, Woda B, Quesenberry P, Doxsey SJ, Skyldberg B, Fujioka K, Hellström A. c., et al. (2003). Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res 58, 1–7. [PubMed] [Google Scholar]
- Qu D, Qu H, Fu M, Zhao X, Liu R, Sui L, and Zhan Q (2008). Increased expression of Nlp, a potential oncogene in ovarian cancer, and its implication in carcinogenesis. Gynecologic oncology 110, 230–6. [DOI] [PubMed] [Google Scholar]
- Rosario CO, Kazazian K, Zih FSW, Brashavitskaya O, Haffani Y, Xu RSZ, George A, Dennis JW, and Swallow CJ (2015). A novel role for Plk4 in regulating cell spreading and motility. Oncogene 34, 3441–3451. [DOI] [PubMed] [Google Scholar]
- Sanchez I, and Dynlacht BD (2016). Cilium assembly and disassembly. Nat Cell Biol 18, 711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Mizumoto K, Nakamura M, Nakamura K, Kusumoto M, Niiyama H, Ogawa T, and Tanaka M (1999). Centrosome abnormalities in pancreatic ductal carcinoma. Clin Cancer Res 5, 963–70. [PubMed] [Google Scholar]
- Schnerch D, and Nigg EA (2016). Structural centrosome aberrations favor proliferation by abrogating microtubule-dependent tissue integrity of breast epithelial mammospheres. Oncogene 35, 2711–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sercin O, Larsimont JC, Karambelas AE, Marthiens V, Moers V, Boeckx B, Le Mercier M, Lambrechts D, Basto R, and Blanpain C (2016). Transient PLK4 overexpression accelerates tumorigenesis in p53-deficient epidermis. Nat Cell Biol 18, 100–10. [DOI] [PubMed] [Google Scholar]
- Shao S, Liu R, Wang Y, Song Y, Zuo L, Xue L, Lu N, Hou N, Wang M, Yang X, et al. (2010). Centrosomal Nlp is an oncogenic protein that is gene-amplified in human tumors and causes spontaneous tumorigenesis in transgenic mice. The Journal of clinical investigation 120, 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, and Jemal A (2018). Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 68, 7–30. [DOI] [PubMed] [Google Scholar]
- Silkworth WT, Nardi IK, Scholl LM, and Cimini D (2009). Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS One 4, e6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyldberg B, Fujioka K, Hellström A-C, Sylvén L, Moberger B, and Auer G (2001). Human Papillomavirus Infection, Centrosome Aberration and Genetic Stability in Cervical Lesions. Modern Pathology 14, 279–284. [DOI] [PubMed] [Google Scholar]
- Slattum GM, and Rosenblatt J (2014). Tumour cell invasion: an emerging role for basal epithelial cell extrusion. Nature reviews. Cancer 14, 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veracini L, Grall D, Schaub S, Divonne S.B.-d.l.F., Etienne-Grimaldi M-C, Milano G, Bozec A, Babin E, Sudaka A, Thariat J, et al. (2015). Elevated Src family kinase activity stabilizes E-cadherin-based junctions and collective movement of head and neck squamous cell carcinomas. Oncotarget 6, 7570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitre B, Holland AJ, Kulukian A, Shoshani O, Hirai M, Wang Y, Maldonado M, Cho T, Boubaker J, Swing DA, et al. (2015). Chronic centrosome amplification without tumorigenesis. Proc Natl Acad Sci U S A 112, E6321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott JM, Prechtl AM, Maine EA, Dang TT, Esparza MA, Sun H, Zhou Y, Xie Y, and Pearson GW (2015). An epigenetically distinct breast cancer cell subpopulation promotes collective invasion. J Clin Invest 125, 1927–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff JB, Wueseke O, and Hyman AA (2014). Pericentriolar material structure and dynamics. Philos Trans R Soc Lond B Biol Sci 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, and Weinberg RA (2015). Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525, 256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Song Y, Zhang Q, and Zhan Q (2009). Ninein-like protein is overexpressed in head and neck squamous cell carcinoma and contributes to cancer growth and resistance to apoptosis. Oncology reports 22, 789–98. [DOI] [PubMed] [Google Scholar]