Abstract
Objective:
To review methodology informing evidence-based guideline development and integration of guidelines into clinical care through shared decision making (SDM) and highlight challenges to SDM in disorders of consciousness.
Methods:
We describe guideline development strategies and implications for use, approaches to SDM generally and with surrogate decision-makers, and considerations when implementing the prolonged disorders of consciousness guideline into clinical care.
Results:
Clinical practice guidelines aim to improve high-quality patient care and outcomes by assessing the best medical evidence and incorporating this into care recommendations. This is accomplished through transparent methodology and compliance with published standards. Guidelines support SDM with patients and surrogate decision makers. Effective SDM can be challenging in conditions such as prolonged disorders of consciousness where surrogates are required, but assessment of patient values and incorporation of these values into SDM is ethically critical.
Conclusions:
Recently published disorders of consciousness guideline recommendations provide strategies for clinicians to enhance quality care for individuals with prolonged disorders of consciousness. They also provide details helping clinicians partner with individuals with disorders of consciousness and their surrogates. Further research is needed into many aspects of caring for individuals with disorders of consciousness and optimal strategies for partnering with surrogates in decision making.
Keywords: Guidelines as Topic [MeSH], Guideline Adherence [MeSH], Clinical Decision-Making [MeSH], Shared Decision Making, Vegetative State [MeSH term under “Persistent Vegetative State”], Unresponsive Wakefulness Syndrome, Minimally Conscious State [MeSH term under “Persistent Vegetative State”], Consciousness Disorders [MeSH], Medical Ethics [MeSH], Neuroethics
In 2018, the Amercian Congress of Rehabilitation Medicine (ACRM), American Academy of Neurology (AAN), and National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) co-published a systematic review1 and associated evidence-based guideline2 on the diagnosis, prognosis, natural history, and treatment of individuals with a disorder of consciousness lasting at least 28 days. While guidelines are years in development, publication is only the first step in the intended goal of improving patient outcomes. Research suggests that compliance with guidelines is variable and often poor,3–5 limiting impact. Barriers to guideline implementation include:
attitudes towards guidelines, including lack of confidence in development (credibility),6,8 beliefs regarding utility/applicability of the guideline in general,7,8 clinician judgement regarding whether following the guideline will lead to desired outcomes,6,7 and confidence in one’s ability to follow guideline recommendations,6,7 and
clinician behaviors, including assessing applicability to individual patients,6,8 and reconciling patient preferences with guideline recommendations.6,7
Clinican behaviors can be affected by the availability of tools to enhance guideline use and environmental factors such as time, resources, and organizational constraints.6–8 In the context of a guideline on individuals with disorders of consciousness, one particular challenge is translation of the recommendations into a context of care where decisions are often made with surrogates and not patients because of their decisional incapacity. This paper addresses the analytics of the evidence base review and the construction of the practice guideline as well as their applicability to shared decision making (SDM) with surrogates. These topics have ethical salience for guideline adoption and clinical practice when caring for individuals with prolonged disorders of consciousness.
WHAT ARE GUIDELINES?
In the past, “guidelines” were often expert consensus statements on management of a medical condition. This changed with the availability of online databases such as MEDLINE, which allows high-volume searching of scientific publications. Concomitantly, the broader sociology of medicine moved from consensus- to evidence-based approaches.9 The Institute of Medicine (IOM, renamed the National Academy of Medicine) reserves the term “clinical practice guideline” to describe “recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options.”10
Numerous standards exist for the development of high quality clinical practice guidelines, including ones from the IOM10,11 and the Guidelines International Network.12 The Appraisal of Guidelines Research & Evaluation Enterprise II (AGREE-II) instrument is the most commonly used tool for assessing guideline quality and reporting.13 These documents identify that trustworthy clincal practice guidelines utilize a transaprent and explict development methodology to limit bias, identify scope and objectives, include relevant stakeholders throughout development, address conflicts of interest, create or use a systematic review, engage a specific process for recommendation development, craft clear recommendations, undergo external stakeholder review at draft stages, and identify a plan for update.10–14 Before using clinical practice guidelines, clinicians need to asess both guideline quality and relevance to a particular clinical scenario.14
Clinicians should also be aware of the framework used for specific guidelines. The AAN guideline development methodology used by the recently published disorders of consciousness guideline takes an individual patient approach to development.15 This involves weighing patient-specific factors in recommendation development such as individual risks and benefits, variation in patient preferences, and patient costs.15 Other developers, particularly those associated with governments, health systems, or payors, take societal, population, or payor views. Such frameworks (e.g. those used by the National Institute for Health and Care Excellence in the United Kingdom16) account for resource utilization and cost-utility at a population level, potentially resulting in conflicts between indivduals who may benefit from a drug based on clinical trials and those able to access it through medical providers.17
Guideline recommendations aim to optimize patient care,10 but they are not rules for practice. Individual decisions require SDM between patients (or surrogates, where relevant) and clinicians. This process comprises considering the best medical evidence alongside a patient’s values and preferences to partner to make the best decision for that patient in that circumstance.18 Guidelines are a powerful tool to promote SDM. Conclusion statements in the systematic reviews accompanying guidelines present the evidence and an assessment of confidence in that evidence. This provides clinicians with the best medical evidence to review with patients and surrogates. Guidelines also highlight when high-quality evidence is lacking.
Recommendations provide patients/surrogates and clinicians with strategies to optimize patient care and an assessment of how likely such strategies are to improve care (resulting in the “level of obgliation,” typically A, B, or C). In the AAN system, Level A recommendations are the strongest recommendations, but they are uncommon. It is expected that following these recommendations will improve health-related outcomes in almost all circumstances and that almost all patients in the relevant circumstance will desire that the recommendation be followed. Level A recommendations are associated with “must” language, but the “almost all” language acknowledges that there may be rare patients/surrogates who choose paths different than the recommended course. Level B recommendations use “should” language. It is expected that most patients will want to adhere to Level B recommendations. Following Level B recommendations is expected to improve health-related outcomes in most circumstances. Level C recommendations are the weakest allowable recommendations and use “may” language. Following Level C recommendations might improve health-related outcomes in some circumstances.15 The implications of each recommendation level allow for incorporation of patient values and preferences through SDM.
SHARED DECISION MAKING
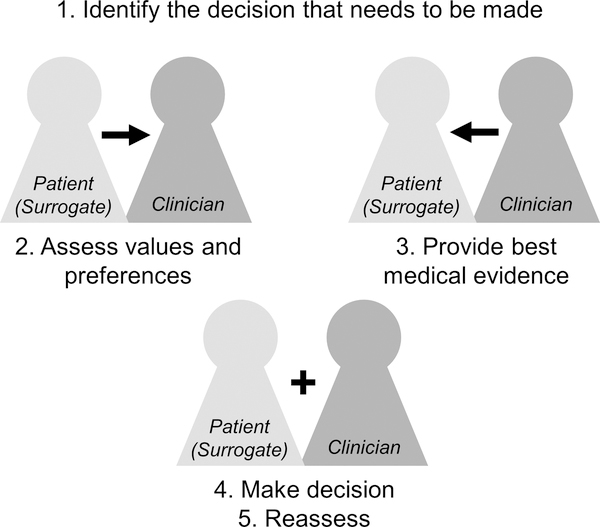
As noted above, SDM is typically a collaboration between physicians, patients, and sometimes others (e.g. families) where involved parties weigh patients’ values and preferences alongside the best available evidence to partner to make the best decisions for that patient in their current circumstance. SDM involves a bidirectional exchange (Figure), where patients (or surrogates) inform clinicians of their values and preferences, clinicians present the best medical evidence, and they partner to make the final decision. SDM is described as “the pinnacle of patient-centered care”19 and is rooted in concepts of self-determination and relational autonomy.20,21
Figure. The Five Steps of Shared Decision-Making.
After identification of a decision that needs to be made, shared decision making involves a bidirectional exchange of information, where patients or surrogates provide patient values and preferences and clinicians provide the best medical evidence (e.g. from systematic reviews and guidelines). Participants than partner to weigh evidence alongside values and preferences to make the best decision for that patient at that time. Shared decision making also involves reassessment over time as results of the decision are appreciated and as circumstances change.
Beyond ethical imperatives, SDM can improve patient knowledge/understanding, satisfaction, and trust.22 Decision aids used in SDM increase knowledge, decrease patients’ indecision and decisional conflict, and result in more decisions for less-aggressive care.23 SDM models identify potential benefits for patients, clinicians, and organizations/systems.24
SDM is a model distinct from paternalistic approaches but also emphasizes that information alone is insufficient for quality decision-making (Table 1).21,25 In “informed decision making,” the role of the clinicians is limited to information transfer, but this assumes that clinicians accurately communicate information, that patients are able to fully understand it, and that information alone is enough to make decisions. Clinician participation in decision-making, however, helps clarify misperceptions regarding costs and benefits, mitigate challenges with health literacy and risk interpretation, and balance patient intuitive and emotional processing.26 SDM recognizes that each partner in health care encounters has information critical for making optimal decisions – clinicians possess medical knowledge regarding effectiveness and risks of different approaches whereas patients know the outcomes that will most impact their well-being and the risks that they are willing to take – and that the process of information exchange has additional value.
Table 1.
| Decision-Making Model | Description | Information Flow | Decision-Maker | Assumptions |
|---|---|---|---|---|
| Parentalistic | Clinician assesses situation and makes decision | Clinician to patient; limited | Clinician | Clinicians are best suited to make medical decisions; patients cannot be trusted to choose well for themselves |
| Clinician as best agent | Clinician makes decision while considering patient values and preferences | Bidirectional | Clinician | Clinicians can know patient’s preferences well enough to make the same decision that patient would make if he or she had medical knowledge |
| SDM | Discussion of patient values and medical evidence to make a shared decision | Bidirectional | Patient and clinician (partnership) | Patients and clinicians are able to partner to integrate information from both parties to make decisions |
| Informed decision making | Clinician provides medical information to patient and leaves patient to make decision without further input | Clinician to patient | Patient | Patient fully understands technical information regarding choices; patient can apply information to life context |
”Patient” in this context could also mean surrogate in relevant clinical scenarios; SDM: shared decision making
Shared decision making with surrogates
In the context of disorders of consciousness, working with decision-making surrogates is critical, but understanding the role of surrogates in SDM is in its nascency. Step 3 of SDM (Figure 1) is based on systematic reviews, guidelines, and other medical evidence and is not dependent on the decision-maker. However, the understanding and expression of patient values and preferences (step 2) and making and reassessing decisions (steps 4, 5) are both influenced by the person filling the decision-making role. In the context of disorders of consciousness and other conditions where patients are unable to speak for themselves, surrogates are responsible for expressing patient values and partnering with clinicians and medical teams in decision-making. Surrogates may be family members or holders of health care agency. The practice guideline for individuals with prolonged disorders of consciousness highlights the important role of patient preferences and surrogate involvement in defining care and stresses the importance ofeducating surrogate decision makers regarding patient prognosis and long-term planning tasks.2 As noted in an accompanying publication, however, “this is a complex task and the admonition to engage in conversations with families will not necessarily translate into effective strategies to work with surrogate decision-makers.”27
Conducting SDM with surrogates has particular challenges. These include surrogate discomfort with the decision-making role, emotional stressors on the surrogate relating to the patient’s health state, changing family roles and decisional responsibilities, uncertainty of patient wishes, the import of many decisions (e.g. relating to life-sustaining care), physician comfort with discussing prognosis, physician communication skills, and logistical requirements for arranging meetings, particularly if multidisciplinary input is required.28,29
Several recent publications address the concept of SDM in the intensive care unit setting where surrogates are often required. The American College of Critical Care Medicine and American Thoracic Society Ethics Committees endorse use of SDM in critical care settings and recommend using SDM when defining goals of care and making major treatment decisions.30 They acknowledge that both clinician-directed and surrogate-directed models are ethically supported (when patients are unable to participate) and suggest that clinicians tailor the process to surrogate preferences.30 Surrogates differ in views regarding whether clinicians or surrogates should control value-sensitive life support decisions,31 but research suggests that clinicians rarely ask surrogates about the role they desire to play.32
Advance care planning (ACP) documentation can be useful in highlighting patients’ pre-expressed wishes when assessing patient values and preferences in intensive care units, but limitations include the absence of ACP, lack of discussions between patients and surrogates regarding ACP contents and patient wishes, and interpretation of ACP documentation in specific clinical scenarios.28 Interviews of surrogates of individuals in vegetative state/unresponsive wakefulness syndrome (VS/UWS) revealed that surrogates interpreted life-sustaining treatments differently than commonly expressed in ACP. Surrogates based decisions not only on their interpretation of the patient’s ACP but also on expectations of improvement and a perceived moral obligation not to harm the patient.33
APPLYING THE DISORDERS OF CONSCIOUSNESS GUIDELINE IN CLINICAL PRACTICE
The ACRM/AAN/NIDILRR guideline on caring for individuals with prolonged disorders of consciousness was developed using the AAN’s guideline methodology,15 which is based on the IOM standards for trustworthy guideline development.10 The guideline specifically advises on care for individuals already experiencing a prolonged course of ≥28 days duration. Only one recommendation targets individuals earlier in their course, stating that clinicians must avoid statements suggesting a universally poor prognosis for individuals with a disorder of consciousness in the first 28 days postinjury based on evidence suggesting the possibility of meaningful recovery even in individuals with more prolonged courses.1,2
Not all guideline recommendations deal specifically with decisions requiring surrogate discussions. Many of the diagnostic and prognostic recommendations guide optimal approaches to assessment including using standardized measures, serial evaluations, and arousal-enhancing techniques, treating comorbidities that could confound diagnosis, and selecting scales and tools (e.g. electroencephalography, imaging) to assist prognostication.2 Some diagnostic approaches – such as use of functional imaging – may benefit from SDM prior to ordering, whether used in research or off-label clinical contexts. It is argued that researchers should disclose the results of functional neuroimaging studies used to detect covert awareness,34 but the implications of such findings remain incompletely understood.1 Surrogates need to be aware of such uncertainty prior to agreeing to testing.
Many of the recommendations have implications for SDM, either by highlighting issues that clinicians must or should discuss with surrogates (counseling recommendations) or recommending specific approaches to care that require a surrogate decision. Three of the eight prognostic recommendations are counseling recommendations, addressing expected outcomes based on prognostic factors and advising families to engage in long-term planning when prognosis suggests severe long-term disability.2 Such discussions are not necessarily tied to a single decision, but provide needed background for accurate decision-making.
Consistent with SDM principals, the guideline states that “clinicians must identify patient and family preferences early and throughout provision of care to help guide the decision-making process for persons with prolonged DoC (Level A).”2 While research supports a role for prescribing amantadine for individuals with traumatic disorders of consciousness,1,2 treatments for disorders of consciousness remain limited and none are approved by the Food and Drug Administration for this purpose. Guideline recommendations highlight important information for families to know, e.g. the uncertainy regarding pain and suffering experienced by individuals with disorders of consciousness and lack of evidence for therapies other than amantadine. They also describe approaches clinicians should take (e.g. treating signs of pain and suffering).2
To incorporate guideline recommendations into SDM, clinicians can use the evidence presented in the systematic review1 to counsel surrogates regarding the best medical evidence. A summary of key information from the systematic review and guideline is available for families and caregivers (https://www.aan.com/Guidelines/Home/GetGuidelineContent/930) and this can be helpful in identifying key vocabulary and messages from the systematic review and guideline publications. SDM is likely best accomplished through plain-language meetings with key team members in a quiet room free of distractions and with opportunities for families to speak and weigh decisions.28,35
REASSESSMENT
Reassessment is a critical final step of SDM (Figure). For decisions that result in ongoing management strategies (e.g. use of amantadine or artificial nutrition and hydration), decisions made through SDM must be reassessed based on response to the intervention and changing circumstances over time. Decisions must also be assessed as individuals with disorders of consciousness experience recovery. The recently published systematic review found that over 75% of individuals in posttraumatic VS/UWS recovered consciousness (i.e., recovered to at least a minimally conscious state); recovery of individuals with nontraumatic VS/UWS was substantially less.1 Other research showed that approximately 20% of surviving patients with a post-traumatic disorder of consciousness admitted to inpatient rehabilitation improved to where they could live at home without supervision and/or were judged to have empolyment potential.36 Individuals with residual cognitive deficits relating to their disorder of consciousness will need ongoing decisional support from their surrogates, but engaging them in SDM to the extent that they are able to participate is ethically critical as their status improves.27 This is particularly important as individuals with severe disability rate their own quality of life substantially higher than others37 and can have mental health ratings consistent with population norms even in the context of limited physical abilities resulting from traumatic brain injury.38
CONCLUSIONS
Clinical practice guidelines aim to improve high-quality patient care and outcomes by assessing the best medical evidence and transparently incorporating it into recommendations for care. Guidelines are instrumental in supporting SDM with patients and surrogate decision makers. Effective SDM can be challenging in conditions such as prolonged disorders of consciousnes where surrogates are required, but assessment of patient values and incorporation of these values into SDM remains ethically imperative. Recently published disorders of consciousness guideline recommendations provide strategies for clinicians to enhance quality care for individuals with prolonged disorders of consciousness and also provide details that will help clinicians partner with patients with disorders of consciousness and their surrogates. Further research is needed into many aspects of caring for individuals with disorders of consciousness and optimal strategies for partnering with their surrogates in decision making.
Acknowledgments
Source of Funding & Disclaimer:
MJA is supported by an ARHQ K08 career development award (K08HS24159) through which this manuscript was developed.* AHRQ played no role in study design, the collection, analysis, or interpretation of data, or writing the manuscript. This work was not performed through Dr. Armstrong’s work for the American Academy of Neurology. The content is solely the responsibility of Dr. Armstrong and does not necessarily represent the official views of AHRQ, the American Academy of Neurology, or others involved in the disorders of consciousness guideline development.
Conflicts of Interest:
MJA: M.J. Armstrong is supported by an ARHQ K08 career development award (K08HS24159) on the topic of patient engagement in guidelines. She also receives research support from a 1Florida ADRC (AG047266) pilot grant and as the local PI of a Lewy Body Dementia Association Research Center of Excellence. She receives royalties from the publication of the book Parkinson’s Disease: Improving Patient Care. She receives compensation from the American Academy of Neurology for work as an evidence-based medicine methodology consultant and has participated as faculty in the annual meeting of the American Academy of Neurology and for Medscape CME.
Footnotes
AHRQ shares the public access policy of the NIH.
References
- 1.Giacino JT, Katz DI, Schiff ND, et al. Comprehensive systematic review update summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018;91(10):461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018;91(10):450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645. [DOI] [PubMed] [Google Scholar]
- 4.Sheldon TA, Cullum N, Dawson D, et al. What’s the evidence that NICE guidance has been implemented? Results from a national evaluation using time series analysis, audit of patients’ notes, and interviews. BMJ. 2004;239(7473):999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Runciman WB, Hunt TD, Hannaford NA, et al. CareTrack: assessing the appropriateness of health care delivery in Australia. Med J Aust. 2012;197(2):100–105. [DOI] [PubMed] [Google Scholar]
- 6.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvment. JAMA. 1999;282(15):1458–1465. [DOI] [PubMed] [Google Scholar]
- 7.Cochrane LJ, Olson CA, Murray S, Dupuis M, Tooman T, Hayes S. Gaps between knowing and doing: understanding and assessing the barriers to optimal health care. J Contin Educ Health Prof. 2007;27(2):94–102. [DOI] [PubMed] [Google Scholar]
- 8.Kastner M, Bhattacharyya O, Hayden L, et al. Guideline update is influenced by six implementability domains for creating and communicating guidelines: a realist view. J Clin Epidemiol. 2015;68(5):498–509. [DOI] [PubMed] [Google Scholar]
- 9.Solomon M Making Medical Knowledge. New York: Oxford University Press; 2015. [Google Scholar]
- 10.Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E, eds. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press; 2011. http://www.nationalacademies.org/hmd/Reports/2011/Clinical-Practice-Guidelines-We-Can-Trust.aspx. Accessed August 18, 2017. [PubMed] [Google Scholar]
- 11.Institute of Medicine. Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC: The National Academies Press; 2011. 10.17226/13059. Accessed June 23, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P; Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156(7):525–531. [DOI] [PubMed] [Google Scholar]
- 13.Brouwers M, Kho ME, Browman GP, et al. AGREE II: Advancing guideline development, reporting and evaluation in healthcare. CMAJ. 2010;182(18):E839–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong MJ, Gronseth GS. Approach to assessing and using clinical practice guidelines. Neurol Clin Pract. 2018;8(1):58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gronseth GS, Cox J, Gloss D, et al. Clinical Practice Guideline Process Manual, 2017 ed. Minneapolis, MN: The American Academy of Neurology; 2017. [Google Scholar]
- 16.Developing NICE guidelines: the manual. nice.org.uk/process/pmg20. Published October 2014; updated April 2017. Accessed August 21, 2018.
- 17.Hawkes N NICE approval of new hepatitis drug could result in £700m bill for NHS. BMJ. 2015;351:h5554. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong MJ, Shulman LM, Vandigo J, Mullins CD. Patient engagement and shared decision making: what do they look like in neurology practice? Neurol Clin Pract. 2016;6(2):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–781. [DOI] [PubMed] [Google Scholar]
- 20.Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681–692. [DOI] [PubMed] [Google Scholar]
- 22.Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35(1):114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. [DOI] [PubMed] [Google Scholar]
- 24.Elwyn G, Frosch DL, Kobrin S. Implementing shared decision-making: consider all the consequences. Implement Sci. 2016;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seaburg L, Hess EP, Coylewright M, Ting HH, McLeod CJ, Montori VM. Shared decision making in atrial fibrillation: where we are and where we should be going. Circulation. 2014;129(6):704–710. [DOI] [PubMed] [Google Scholar]
- 26.Ubel PA. Beyond costs and benefits: understanding how patients make health care decisions. Oncologist. 2010;15 Suppl 1:5–10. [DOI] [PubMed] [Google Scholar]
- 27.Fins JJ, Bernat JL. Ethical, palliative, and policy considerations in disorders of consciousness. Neurology. 2018;91(10):471–475. [DOI] [PubMed] [Google Scholar]
- 28.Cai X, Robinson J, Muehlschlegel S, et al. Patient preferences and surrogate decision making in neuroscience intensive care units. Neurocrit Care. 2015;23(1):131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White DB. Rethinking interventions to improve surrogate decision making in intensive care units. Am J Crit Care. 2011;20(3):252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kon AA, Davidson JE, Morrison W, et al. Shared decision making in ICUs: an American College of Critical Care Medicine and American Thoracic Society policy statement. Crit Care Med. 2016;44(1):188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson SK, Bautista CA, Hong SY, Weissfeld L, White DB. An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care units. Am J Respir Crit Care Med. 2011;183(7):915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White DB, Malvar G, Karr J, Lo B, Curtis JR. Expanding the paradigm of the physician’s role in surrogate decision-making: an empirically derived framework. Crit Care Med. 2010;38(3):743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuehlmeyer K, Borasio GD, Jox RJ. How family caregivers’ medical and moral assumptions influence decision making for patients in the vegetative state: a qualitative interview study. J Med Ethics. 2012;38(6):332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham M, Weijer C, Peterson A, et al. Acknowledging awareness: informing families of individual research results for patients in the vegetative state. J Med Ethics. 2015;41(7):534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94(3):291–309. [DOI] [PubMed] [Google Scholar]
- 36.Nakase-Richardson R, Whyte J, Giacino JT, et al. Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI Model Systems Programs. J Neurotrauma. 2012;29(1):59–65. [DOI] [PubMed] [Google Scholar]
- 37.Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med. 1999;48(8):977–988. [DOI] [PubMed] [Google Scholar]
- 38.Honeybul S, Janzen C, Kruger K, Ho K. Decompressive craniectomy and the disability paradox [abstract]. Aust Crit Care. 2016;29:119–120. [Google Scholar]