Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating neuromuscular disease characterized by motor neuron loss and prominent skeletal muscle wasting. Despite more than one hundred years of research efforts, the pathogenic mechanisms underlying neuromuscular degeneration in ALS remain elusive. While the death of motor neuron is a defining hallmark of ALS, accumulated evidences suggested that in addition to being a victim of motor neuron axonal withdrawal, the intrinsic skeletal muscle degeneration may also actively contribute to ALS disease pathogenesis and progression. Examination of spinal cord and muscle autopsy/biopsy samples of ALS patients revealed similar mitochondrial abnormalities in morphology, quantity and disposition, which are accompanied by defective mitochondrial respiratory chain complex and elevated oxidative stress. Detailing the molecular/cellular mechanisms and the role of mitochondrial dysfunction in ALS relies on ALS animal model studies. This review article discusses the dysregulated mitochondrial Ca2+ and reactive oxygen species (ROS) signaling revealed in live skeletal muscle derived from the ALS mouse models, and a potential role of the vicious cycle formed between the dysregulated mitochondrial Ca2+ signaling and excessive ROS production in promoting muscle wasting during ALS progression.
Keywords: ALS, skeletal muscle, mitochondria ROS, mitochondrial Ca2+ uptake, mitochondrial dynamics, autophagy
Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive neuromuscular disease characterized by motor neuron death and devastating skeletal muscle wasting. The overall worldwide ALS incidence was reported at 1.75 (1.55–1.96)/100,000 person-years of follow-up [1]. A population-based study in the United Kingdom also showed that lifetime risk of ALS was 1 in 472 for women and 1 in 350 for men [2]. Currently there is no effective treatment for ALS [3]. The expected life span for the majority of ALS patients is usually 3–5 years after disease onset [4]. Despite intensive research effort (to the date of Aug 20, 2018, over 23,500 ALS research articles have been published since 1897), the pathogenic mechanism underlying progressive neuromuscular degeneration in ALS remains obscure [5]. Majority of ALS cases are sporadic (SALS), while about 5~10% cases exhibit an autosomal dominant inheritance, termed familial ALS (FALS) [6]. Although multiple factors could contribute to SALS, both SALS and FALS share similar pathological and clinical phenotypes of neuromuscular degeneration [4, 7], indicating a common mechanism underlying neuromuscular degeneration during ALS disease progression. While the death of motor neurons in the motor cortex of the brain and spinal cord is a defining pathological feature [8], accumulating evidences suggest that defects in other cell types or organs also have critical impact in ALS disease progression [9–21].
As one of the largest tissues in human body, skeletal muscle is responsible for voluntary movements of the entire body and is also essential for maintaining the homeostasis of the whole-body metabolism [22]. ALS patients experience progressive and severe muscle degeneration. Such devastating muscle wasting plays a significant role in the disease progression and life quality of ALS patients [23]. Muscle fibers and the motor neuron communicate at the neuromuscular junction (NMJ). While muscle function is largely controlled by motor neuron, retrograde signals are conducted from muscle back to motor neuron [24, 25]. Although skeletal muscle is a victim of motor neuron axonal withdrawal in the course of ALS progression, studies from us [11, 14, 15, 26] and other research groups [10, 12] support skeletal muscle as a primary recipient of ALS-causing factors. Investigating muscle degeneration at different disease stages shall enhance our understanding of ALS pathophysiology and facilitate the development of therapeutic approaches to sustain muscle function for alleviating the disease progression and improving the life quality of ALS patients.
For a cell, mitochondria are a major source of ATP and reactive oxygen species (ROS, as a byproduct of oxidative phosphorylation) [27]. They are not only essential for energy supply but also determine the survival or death of cells. Because of high demands of energy, neurons are highly dependent on mitochondria for physiological functions. Mitochondrial dysfunction is a major player in neuronal degeneration in ALS. There are multiple recent excellent reviews covering this topic [28–38]. In neurons, mitochondria occupy about 3–8% of the cell volume, and ~50% of the energy is utilized by the Na+/K+ ATPase in maintaining membrane potential and recovery from action potential [39, 40]. Similar to the nervous system, skeletal muscle is a highly energy-demanding organ with mitochondria occupying about 10–15% of the muscle fiber volume [41]. About 10–25% of ATP in muscle is used for sarcoplasmic reticulum Ca2+-ATPase, 65–80% for actomyosin ATPase, and 5–10% for Na+/K+ ATPase [42]. Molecular and functional studies on mitochondrial defects in skeletal muscle are relatively behind those in neurons, possibly because of the traditional, motor neuron centered view of ALS onset. In recent years, more research efforts shifted the attention to the role of muscle in ALS disease onset and progression, and particularly the contribution of mitochondrial dysfunction in muscle degeneration during ALS progression. Muscle uses Ca2+ as a messenger to control cellular events ranging from muscle contraction to cell death. While mitochondrial function in muscle is fine-turned by the intracellular Ca2+ signaling [43], mitochondria also have the ability to shape intracellular Ca2+ signaling profiles in skeletal muscle [14, 44, 45]. There is always a crosstalk between mitochondrial Ca2+ and ROS signaling [46]. It is now agreed that low level mitochondrial ROS serves as an important signaling molecule regulating various physiological process [47], while long lasting and excessive production of ROS put cell under oxidative stress, which is detrimental to cell function [48]. This current review discusses the abnormal mitochondrial Ca2+ and ROS signaling revealed in live skeletal muscle derived from ALS mouse models, and a potential role of the vicious loops formed between the dysregulated mitochondrial Ca2+ signaling and excessive ROS production in promoting muscle wasting during ALS progression.
1. Abnormal mitochondrial morphology and oxidative stress revealed in skeletal muscle samples derived from ALS patients
The ultrastructural studies of spinal cord and muscle autopsy/biopsy samples derived from both SALS and FALS patients revealed remarkable defects in mitochondrial morphology. Early study on the lumber spinal cord of ALS patients by Sasaki and Iwata observed aggregated dark mitochondria in the anterior horn neurons [49]. In the soma of these neurons, mitochondria could also be swollen, accompanied by markedly increased cristae. The multilayer cristae even stacked to form filamentous structures in some cases [49]. The swollen and vacuolated mitochondria with aggregate of tubules, broken cristae, and inclusion bodies were also evident in motor neurons and intra-muscle nerves in other studies [50–52]. Interestingly, the detailed ultrastructural study on muscle samples of ALS patients happened years earlier than studies on motor neurons. In 1966, Afifi et al gave the first description of abnormal mitochondrial disposition and morphology in atrophic muscles of ALS patients [53]. Specifically, in the subsarcolemmal regions, mitochondrial aggregates caused outpouching of the sarcolemmal membrane. In the sarcomere regions, pairs of large, elongated mitochondria parallel to the Z line were observed at the boundaries of degenerating sarcomeres. Ectopically large mitochondria with disorganized and/or discontinuous cristae were spotted at interfilamentous space [53]. Decreased number of mitochondria was also notable in degenerated muscle fibers from ALS patients by Fidziańska et al [54]. The swollen mitochondria characterized by dilatation and disruption of the cristae were further evident in ALS patients’ muscle by Napoli et al (2011) [55] and Chung et al (2002) [56].
Biochemical studies revealed impaired mitochondrial oxidative metabolism in muscle samples derived from ALS patients. Wiedemann et al (1998) incorporated enzyme activity and respiration measurement to demonstrate that NADH dehydrogenase (respiratory chain complex I) had significantly reduced activity. The reduced activity of NADH dehydrogenase was accompanied by decreased maximal respiration capacity, indicating impairment of mitochondrial function in skeletal muscle of SALS patients [57]. Later studies by Vielhaber et al (1999) and Safa Al-Sarraj et al (2014) also reported that cytochrome c oxidase (COX, or respiratory chain complex IV) had reduced activity in ALS patients’ muscle, and the distribution of mitochondria with respiratory chain defects was very heterogeneous [58, 59]. Retrospective histochemical and biochemical studies on the muscle biopsy derived from both FALS and SALS patients have shown that the deficiency of mitochondrial respiratory activity (COX) is correlated with the progression of ALS [60, 61]. Other studies further confirmed the reduced activity of mitochondrial respiratory chain complexes in muscle samples derived from SALS patients [62].
The impairment of mitochondrial oxidative metabolism in skeletal muscle of ALS patients was found to be accompanied by mitochondrial DNA lesions [63–65]. Mitochondrial DNA is vulnerable to ROS mediated oxidative damages, and defects in mitochondrial respiratory chain may boost ROS production [66, 67]. Similar defects in mitochondrial DNA and respiratory chain function were also identified in the spinal cord of ALS patients [68]. Using microarray technology and gene regulatory network analysis, Bernardini et al (2013) systematically identified mitochondrial network genes whose expressions were significantly altered in muscles of ALS patients. Remarkably, the gene network observed in ALS muscle includes genes whose functions connect the muscle structure definition to mitochondrial oxidative phosphorylation and ATP synthesis [69]. Another study used multigene qRT-PCR to demonstrate that in addition to the respiratory chain components (COXIV), the expression levels of genes regulating mitochondrial biogenesis and dynamics were also downregulated in skeletal muscles of ALS patients [70]. Results of those biochemical and multigene expression analysis suggest a potential role of mitochondrial oxidative stress in the occurrence of multi-faceted mitochondrial defects during ALS progression.
Although most ALS are sporadic cases without identified genetic causes, the spinal cord and muscle autopsy/biopsy samples from both sporadic and familial ALS patients all show analogous defects in morphology and biochemical properties of mitochondria. This indicates abnormal mitochondria as a common player in neuromuscular degeneration despite the etiology. As those studies could be only conducted with autopsy/biopsy samples derived from ALS patients, it is not known whether mitochondrial damage is a cause or a consequence of ALS. Answers to these questions rely on ALS animal model studies.
2. Disease-stage-dependent changes in ROS-related mitochondrial dysfunction in skeletal muscle of ALS mouse models
The rodent models expressing human ALS mutations recapitulate many features of the human disease [71–74], and were widely used for investigating pathogenic mechanisms of ALS and for testing preclinical therapies for ALS [72, 75–78]. Similar to the ultrastructural characteristics observed in the spinal cord of ALS patients, the predominant mitochondrial abnormalities were also seen in the spinal cord ventral horn of the transgenic mice with over expression of various ALS-associated human superoxide dismutase 1 (SOD1) mutations [79–81]. The electron microscopy study also revealed that morphological changes of mitochondria in skeletal muscle of ALS SOD1 mutant mice were similar to those observed in human patients [15, 81, 82]. The altered mitochondrial morphology could be associated with mitochondrial respiratory function defects, which are a major source of ROS production [83–85]. Indeed, the molecular and biochemical studies revealed oxidative stress as an essential feature of ALS muscle pathology [18]. The study of Halter et al (2010) showed that accumulation of ROS in skeletal muscle happens early at asymptomatic stages in mutant SOD1 mice [86]. The biochemical studies also link the oxidative stress to abnormal mitochondrial oxidative phosphorylation in skeletal muscle of ALS mouse models. The maximal oxygen consumption rate and ADP affinity significantly decreased in oxidative muscle of SOD1G93A mice, implying defects in mitochondrial oxidative phosphorylation [87]. In addition, elevated NADH content and PGC-1α expression were detected in skeletal muscle from MLC/hSOD1G93A mice, implying a mitochondrial metabolism to a more oxidative phenotype [10]. PGC-1α is known to be induced by oxidative stress and helps modulate intracellular ROS levels [88] through what is known as retrograde mitochondria-nucleus signaling [89]. The proteomics study by Capitanio et al (2012) suggests that ROS accumulation in ALS SOD1G93A skeletal muscle could result from an enhanced mitochondrial oxidative metabolism [90]. As observed in skeletal muscle of ALS patients, multiple signaling pathways could be involved in mitochondria-related oxidative stress in skeletal muscle of ALS mouse models (reviewed in Loeffler et al 2016) [18]. The similar morphological and biochemical changes of mitochondria between muscle and neuron, and between ALS patients and animal models justify the usage of ALS animal model to further understand molecular and cellular mechanisms linking mitochondrial dysfunction to neuromuscular degeneration during ALS progression.
Using chemical ROS indicators (ROS Brite™ 570 and MitoSOX™ Red), our group (Xiao et al, 2018) detected enhanced intracellular and mitochondrial ROS levels in live skeletal muscle fibers derived from SOD1G93A mice before and after ALS disease onset [91]. Due to their non-ratiometric and non-reversible nature, those chemical ROS indicators do not provide quantitative evaluation of the ROS production, do not follow dynamic changes of mitochondrial ROS production in live muscle cells, and do not reveal the correlation between dysregulated mitochondrial ROS production and ALS disease progression. To examine the disease-stage-dependent changes of mitochondrial dysfunction in ALS skeletal muscle, we generated double transgenic mouse model (G93A/cpYFP) that carries human ALS mutation SOD1G93A and mt-cpYFP transgenes [91], in which mt-cpYFP detects dynamic changes of ROS-related mitoflash events at individual mitochondrion level [84, 85, 92–94]. Through examining live skeletal muscle fibers derived from these double transgenic mice, disease-stage-dependent alterations in mitoflash events were revealed. Before ALS symptom onset, the frequency of mitoflash events is doubled, while other kinetic parameters of the signal remain the same as the control. After disease onset, the duration of mitoflash events is significantly prolonged [91]. It is known that mitoflash activity is dynamically coupled with mitochondrial transition pore (mPTP) opening in both cardiac and skeletal muscle, because the transient increase in mt-cpYFP fluorescence was always coupled with the depolarization of mitochondrial inner membrane potential at the same location [84, 95, 96]. Thus, the elongated duration of mitoflash events suggests potential changes in functional status of mPTP in skeletal muscle during ALS progresses. It has been shown that CypD promotes mPTP opening [97–99] and there is a strong connection between CypD-related mPTP opening and mitoflash signaling [84, 93, 96, 100]. Remarkably, the elongated mitoflash activity is associated with increased expression levels of CypD in muscle mitochondria in the later stage of ALS, linking CypD-related mPTP opening to elevated ROS production in ALS muscle [91]. We speculate that increased CypD level in mitochondria of SOD1G93A muscle may contribute to the elongated and irreversible opening of mPTP at the later stage of ALS. Remarkably, through overexpression of ALS mutation SOD1G93A in skeletal muscle of normal mice, we found that ALS mutation directly led to an enhanced mitochondrial ROS production and mPTP-related mitoflash activity in the absence of motor neuron withdrawal [91], demonstrating muscle mitochondria as a primary target of ALS mutation.
Although in recent years mt-cpYFP has been characterized and used as a biomarker of mitochondrial ROS generation [93, 94, 101], debates exist about its sensitivity to ROS and pH [102–104]. cpYFP may report both ROS- and pH-related mitochondrial signaling [95, 105], and is a robust indicator of the dynamic status of mitochondrial function [85, 92, 94, 106, 107]. In the case of studying ALS, the cpYFP mitoflash signal can be used as a quantitative biomarker for evaluating ROS-related mitochondrial dysfunction during ALS disease progression, and the double transgenic G93A/cpYFP mice could provide a unique animal model for testing whether potential therapeutic means could reverse or slow down the mitochondrial dysfunction by examining mitoflash activities.
3. Abnormal mitochondrial dynamics and autophagy activity revealed in live skeletal muscle fibers of ALS mice
Mitochondria are dynamic organelles that are constantly remolded by fusion and fission processes, which define mitochondrial morphology, distribution and function [31, 108, 109]. Abnormal mitochondrial dynamics are implicated in various neurodegenerative disorders [110, 111]. While oxidative stress could lead to mitochondrial fragmentation, mitochondrial dynamics also modulate ROS generation [112–115]. Overexpression of ALS mutant SOD1 in cultured motor neurons led to abnormal mitochondrial dynamics and cell toxicity [116, 117]. Through overexpression of a mitochondria-targeted photoactivatable fluorescent protein (mt-PAGFP) in skeletal muscle of SOD1G93A mice, our group (Luo et al, 2013) examined the migration rate of the PAGFP inside mitochondria network in live muscle fibers, and revealed less-connected mitochondrial networks with reduced rate of fission/fusion in SOD1G93A muscle. Those abnormalities occur in asymptomatic ALS stage, indicating abnormal mitochondrial network and dynamics as early pathological events in ALS muscle [11]. Mounting evidence indicates a vicious cycle between abnormal mitochondrial dynamics and excessive ROS generation [118, 119]. Interestingly, in ALS G93A muscle, the increased ROS generation is associated with reduced mitochondrial dynamics and network, indicating that this vicious feedback cycle could play a role in mitochondrial dysfunction in muscle during ALS progression. Remarkably, overexpression of ALS-causing mutation SOD1G93A in skeletal muscle of normal mice led to similar abnormality in mitochondrial network and dynamics [11], and promoted mitochondrial ROS production [91] in the absence of motor neuron axonal withdrawal, again indicating muscle mitochondria could be directly affected by ALS mutations local to skeletal muscle.
Autophagy, an intracellular process targeting misfolded proteins and damaged organelles for lysosomal degradation, plays crucial roles in mitochondrial quality control [120]. Oxidative stress regulates autophagy via multiple singling pathways in skeletal muscle (see excellent review by Rodney et al 2016) [121]. Dysregulation of autophagy is implicated in various neurodegenerative diseases [122]. Upregulated autophagy activity has been reported in the spinal cord of ALS patients and animal models [123–126]. However, contradictory results were also reported. There was no detectable increase in the expression level of autophagosome marker LC3-II in the spinal cord of ALS G93A mice [127]. The effects of compounds that activate autophagy in ALS mouse models are also contradictory. Some studies show that autophagy activation helped limit ALS progression [128, 129]. Treatment of Rapamycin (an autophagy activator via mTOR inhibition) on ALS animal models had conflicting results [130, 131]. Rapamycin alleviated disease progression in ALS TDP-43 mice [132] but augmented motor neuron degeneration in SOD1 mutant mice [126], with an adverse effect on muscle performance [133]. Contradictory results were also reported when lithium was used to activate autophagy to treat ALS mouse models [134, 135]. The contradictory results could be due to many different reasons. One could be differential autophagy activities in non-neuron cells (such as muscle) that may affect whole body homeostasis and complicate the therapeutic outcomes. It is also worth noticing that beside its inhibitory effect on mTOR, Rapamycin also affects the gating properties of skeletal muscle ryanodine receptors by dissociating FK506-binding protein from them [136, 137]. Using live cell imaging, we quantified the autophagosome and lysosome in muscle fibers derived from G93A mice at three disease stages: 6 weeks (asymptomatic stage without axonal withdrawal reported [138]), 2–3 months (asymptomatic stage but axons have begun to pull away from muscle fibers] and 3–4 months (disease onset and ALS-like phenotype becomes well-established [71]). The formation of autophagosomes/lysosomes in G93A muscle was found to be increased at all stages when the mice were on regular diet [26]. Surprisingly, we found that autophagy flux in G93A muscle was significantly suppressed. The young G93A mice (6-week older) did not have the capacity to form more autophagosomes and lysosomes when challenged by starvation (to promote autophagy) plus intraperitoneal injection of colchicine (to block the fusion of autophagosomes to lysosomes), an established physiological autophagy induction procedure to evaluate the autophagy flux in mammalian skeletal muscle [139, 140]. The muscle derived from older G93A mice (> 2 months) even showed a significant reduction in autophagosome and lysosome formation following the autophagy induction procedure. In contrast, the muscle derived from age-matched wild type mice responded to the procedure with a robust increase in the number of autophagosome puncta as expected [139, 140]. A later biochemical study by Olivan et al (2015) also showed a significant increase in the ratio of LC3-II/LC3-I in muscle derived from G93A mice at asymptomatic and late stage [141]. Olivan et al (2015) also examined the autophagy flux in skeletal muscle derived from late stage G93A mice after the isolated muscles were chopped into small pieces and incubated with 100 μM chloroquine for 6 hours. They found a significant increase in the relative expression level of LC3-II. However, the chloroquine treatment was not performed under physiological conditions, and the chopped small pieces of muscle likely experienced unavoidable damage [141], thus the results are hard to be interpreted. In our experiments, we used an established physiological autophagy induction procedure and discovered a significant reduction in autophagy flux in G93A mice. The suppressed autophagy flux implies possible exhaustion of the reserved capacity to form autophagosome under stressed conditions in skeletal muscle of G93A mice upon disease progression [26]. In line with the dysregulation of mitochondrial dynamics and the excessive ROS generation during ALS progression, the reduced autophagy capacity, which occurs early and becomes severe in the later stage of ALS, could further handicap the clearance of damaged mitochondria and become an important player in the vicious cycle promoting mitochondrial damage.
4. Reduced mitochondrial Ca2+ uptake leads to cytosolic Ca2+ hyperactivity in G93A muscle fibers at the site of neuromuscular junction
4.1. Depolarized mitochondria at the neuromuscular junction (NMJ) of ALS muscle fibers
The first evaluation of mitochondrial lesion on live skeletal muscle fibers derived from adult ALS SOD1G93A mouse model was conducted by our group (Zhou et al, 2010) [15]. Oxidative phosphorylation creates a proton gradient across the inner mitochondrial member that establishes the mitochondrial inner membrane potential (ΔΨm) and acts as a driving force for ATP synthesis [142]. Using a mitochondrial membrane potential indicator (TMRE), we discovered that a portion of SOD1G93A muscle fibers derived from SOD1G93A mice at the age of ALS disease onset displayed localized loss of ΔΨm in fiber segments near the site of NMJ [15]. The depolarization of mitochondrial inner membrane potential would suggest reduced driving force for ATP synthesis, which is consistent with reports of reduced ATP level in skeletal muscle of ALS animal models [12, 143]. A major event during ALS progression is motor neuron axonal withdrawal from the NMJ. Those depolarized mitochondria at the site of NMJ could be a sole response of muscle fibers to motor neuron withdrawal (denervation). Interestingly, these types of mitochondrial defects also occur in young ALS mice even prior to the motor neuron axonal withdrawal reported in the same ALS mouse model [144]. Thus, this observed mitochondrial lesion could also result from intrinsic defects in ALS muscle.
4.2. Reduced mitochondrial Ca2+ uptake leads to cytosolic hyperactive Ca2+ transients in ALS muscle fibers near NMJ
Ca2+ is fundamental to normal cellular function. Dysregulated Ca2+ signaling plays critical roles in muscle degeneration in aging [145, 146] and muscular dystrophy [147–151]. Remarkably, the collapse of the inner mitochondrial membrane potential ΔΨm at NMJ is associated with hyperactive intracellular Ca2+ transients [14, 15]. With the driving force generated by the negative inner membrane potential ΔΨm, mitochondria in skeletal muscle could take up Ca2+ from cytosol during excitation-contraction (EC)-coupling [45, 152]. Since both Ca2+ transient and mitochondrial Ca2+ uptake in fiber regions with normal or defective mitochondria can be compared in the same ALS muscle fiber, in which other cytosolic Ca2+ removal processes likely remain the same, we were able to quantify the contribution of mitochondria-mediated Ca2+ uptake in shaping the cytosolic Ca2+ transients during EC-coupling. Applying live cell imaging with a mitochondrial targeted Ca2+ biosensor and the voltage-clamp technique, we have found that mitochondrial Ca2+ uptake decreases by ~14.6% in the fiber segment with depolarized mitochondria, accompanied by a ~15.6% elevation of cytosolic Ca2+ in ALS G93A muscle fibers. Our calculation estimates that about 10~18% of cytosolic Ca2+ removal at the peak of voltage-induced Ca2+ release is attributable to mitochondrial Ca2+ uptake in mouse skeletal muscle fiber [14]. Additionally, the elevation of cytosolic Ca2+ transient in the fiber region with depolarized mitochondria exhibits similar kinetics to the mitochondrial Ca2+ uptake profile in the fiber segment with normal mitochondria. Hence, the reduced Ca2+ buffering capacity of these depolarized mitochondria is considered as a major cause of the increased cytosolic Ca2+ transients during voltage-induced Ca2+ release at NMJ in ALS muscle fiber, which could constitute an important pathophysiological component of neuromuscular degeneration in ALS G93A mouse model [14]. Since NMJ is the primary site of neuron-muscle interactions, this type of Ca2+ hyperactivity at the NMJ may also imply a defective crosstalk between muscle and motor neuron in SOD1G93A mice.
In a separate study by Chin et al (2014), the muscle fibers derived from late stage ALS G93A mice showed a significant elevation of the resting intracellular Ca2+ level, and the Ca2+ transients had a slower decay to the resting level following 10-Hz electrical stimulation. The elevations in cytosolic Ca2+ could be linked to decreased protein level of ATPase SERCA1 and the Ca2+ buffer protein parvalbumin observed in skeletal muscle of ALS G93A mice [153]. There are also reports showing the protective effect of parvalbumin on Ca2+- related excitotoxicity in motor neurons of ALS animal models [154–156]. In addition, there are literatures suggesting a correlation between decreased SERCA efficiency (due to ATP deficiency) and elevated cytosolic Ca2+ in motor neuron degeneration [157–159]. The elevated intracellular Ca2+ could promote the aggregation of mutant SOD1 inside mitochondria and exacerbate the loss of mitochondria inner membrane potential in cultured motor neurons [160]. The same situation may happen in skeletal muscle. Indeed, overexpression of the GFP-tagged SOD1G93A in skeletal muscle of normal mice revealed mitochondrial accumulation of the mutant protein [15], and the GFP-SOD1G93A mutant protein formed aggregates inside mitochondria, leading to depolarized mitochondria and reduced mitochondrial dynamics [11]. It is possible that aggregation of ALS mutant protein triggers mitochondrial membrane depolarization that reduces mitochondrial Ca2+ uptake, slows down mitochondrial dynamics and promotes mitochondrial ROS production. Initially, the protein aggregation in mitochondria could instigate the unfolded protein response (UPR) aiming to restore protein homeostasis in mitochondria [161]. However, if mitochondrial damage persists during ALS progression, these events mentioned above could promote each other to further damage mitochondria, which could exhaust mitochondrial clearance pathways including autophagy/mitophagy in G93A skeletal muscle.
5. Loss of physiological Ca2+ transients following denervation initiates mPTP-related mitochondrial ROS production.
A major pathological event during ALS disease progression is the motor neuron axonal withdrawal from skeletal muscle. Denervation of skeletal muscle induces a dramatic increase in mitochondrial ROS production [162]. It is known that mitochondrial Ca2+ overload is a pathological stimulus of ROS generation [83]. Studies have shown that prolonged muscle denervation leads to an increased steady state resting Ca2+ level in cytosol, which could overload mitochondria, stimulating ROS production [163, 164]. However, the initial cause of the mitochondrial ROS production in denervated muscle fibers was elusive [165]. Following denervation, no action potential is initiated in muscle fibers, thus, no physiological Ca2+ transients occur in the cytosol and mitochondria. How do mitochondria respond to the absence of physiological Ca2+ transients in skeletal muscle? Through examining the mitoflash activity in live muscle fibers 24 hours after denervation, we (Karam et al, 2017) had a surprising finding. Mitochondria respond to this condition with a drastic increase in CypD-dependent mitoflash activity accompanied by an elevated fluorescence intensity of MitoSOX Red, indicating enhanced mitochondrial ROS production induced by the short term denervation. Remarkably, restoring physiological Ca2+ transients by a brief train of electric stimulation reduced mitochondrial ROS production, diminished the mitoflash activity, and stopped the repetitive mPTP opening in the denervated muscle fibers [85]. The alleviating effect of electric stimulation on mitochondrial ROS generation and mitoflash activity were diminished by application of RU360, an inhibitor of mitochondrial Ca2+ uptake through blocking the mitochondrial uniporter [15, 166]. Thus, loss of physiological Ca2+ uptake by mitochondria is likely an initial trigger for mPTP opening and excessive mitochondrial ROS production in muscle fibers following denervation. While mitochondrial Ca2+ overload is a pathological stimulus leading to mPTP opening and ROS generation, physiological intracellular Ca2+ transients are also critical for maintaining mitochondrial functional integrity in skeletal muscle. Our data support that application of the electric stimulation on skeletal muscle could be beneficial to patients suffering from denervation, including ALS. The molecular composition of mPTP is still not fully understood. It is unclear what are the molecular mechanisms underlying the different responses of mitochondria to a steady-state elevated intracellular Ca2+ level and to the absence of physiological Ca2+ transients following denervation. We speculate that there may be a biphasic dependence of mPTP on the cytosolic Ca2+ level or the dynamics of SR Ca2+ release. Future studies are needed to investigate these molecular basis [85].
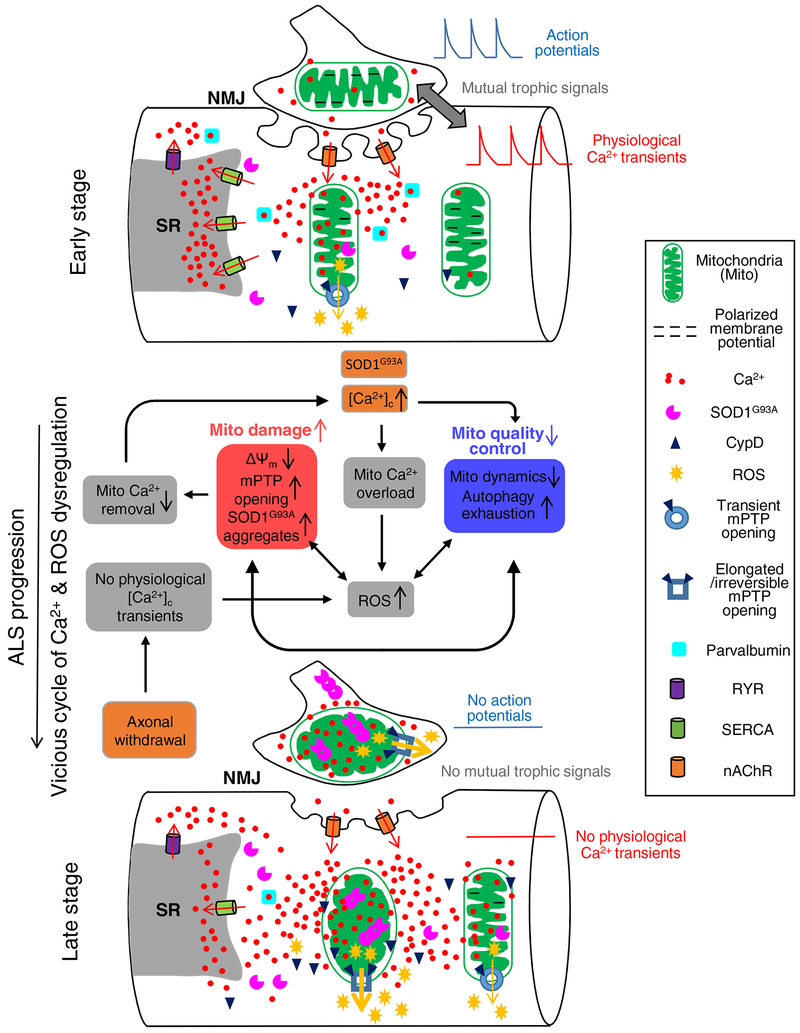
6. Proposed pathologic sequence of muscle degeneration in the ALS mouse model
Potential causes of muscle mitochondrial damage in ALS muscle include ALS-causing mutation (such as SOD1, TDP-43 mutations), denervation, or other unknown factors leading to SALS. Based on published studies from us and other research groups, we speculated dysfunctional mitochondria-related pathologic sequence of muscle degeneration in the ALS G93A mouse model during the disease progression (Fig 1). The factors initiating the vicious cycle of mitochondrial Ca2+ and ROS dysregulation in G93A muscle are SOD1G93A mutation and denervation, which are highlighted in orange boxes in Fig 1. At the early stage, motor neuron axonal withdrawal from the NMJ has not occurred yet. At the muscle side of the NMJ there are abundant nAChRs, which are known to have elevated Ca2+ permeability in adult mammalian muscle [167]. During repetitive muscle contraction, the repetitive opening of nAChR channels could lead to an elevated Ca2+ influx near NMJ area (micro-domain). Mitochondria at NMJ face elevated local [Ca2+]C and thereby become more susceptible to the deleterious effects of the ALS mutant aggregates as demonstrated in cultured motor neuron cells [168] and become depolarized [11]. The elevated [Ca2+]C may also reduce mitochondrial motility and promote mitochondrial fragmentation [169, 170], which could dampen the recovering capacity of damaged mitochondria. Both elevated mitochondrial Ca2+ overload and membrane depolarization are associated with increased CypD-related mPTP transient opening (revealed as the higher frequency of mitoflash events) and promote mitochondrial ROS production. At the early stage of ALS, this increased transient opening of mPTP may be a survival mechanism to eliminate mitochondrial Ca2+ overload [171, 172] in skeletal muscle. Upon the progression of ALS, accumulation of these defects would form a vicious cycle, leading to further mitochondrial damage with reduced mitochondrial Ca2+ removal and consequently enhanced cytosolic Ca2+ hyperactivity. This situation is exacerbated by the decreased level of SERCA1 and parvalbumin [153], resulting in further Ca2+ overload within adjacent “normal” mitochondria. Meanwhile the aggregation of SOD1G93A inside mitochondria further promotes mitochondrial damage and ROS production. These accumulated mitochondrial defects would also inhibit mitochondrial dynamics and exhaust the reserved capacity of autophagy, crippling mitochondrial quality control. When denervation becomes a major event in ALS skeletal muscle, mitochondria respond to the absence of physiological intracellular Ca2+ transients with excessive ROS production [85]. This excessive ROS production is accompanied by prolonged CypD-related mPTP opening (revealed as longer lasting mitoflash events) [91], leading to major consequences of abnormal energy metabolism and muscle cell death [173]. A similar Ca2+-mediated mechanism may also apply to the neuronal side of the NMJ. Earlier studies by Siklós et al (1996) showed that motor nerve terminals from ALS specimens contained a significantly higher level of calcium [52]. It has been demonstrated that repetitive action potentials increase cytosolic Ca2+ level rapidly, and heavily load local mitochondria in motor terminals of SOD1G93A mice [174, 175]. These alterations on both sides of NMJ lead to progressive withdrawal of the motor axonal terminal, and this can be a self-reinforcing consequence, including loss of mutual trophic signals between muscle and nerve cells.
Fig 1. Proposed pathologic sequence of muscle degeneration related to mitochondrial dysfunction in the ALS mouse model.
At the early stage, the integrity of NMJ is still intact. Muscle fibers could have elevated cytosolic Ca2+ near NMJ area, potentially due to Ca2+ permeability of nAChR during repetitive muscle contraction. This elevated cytosolic Ca2+ level at NMJ could lead to mitochondria Ca2+ overload and accumulation of SOD1G93A inside mitochondria, which causes mitochondrial membrane depolarization and promotes ROS production. The observed transient opening of mPTP at this stage could be an adaptive response attempting to relieve Ca2+ overload in mitochondria. Upon the progression of ALS, accumulation of these defects would form a vicious cycle, leading to further mitochondrial damage with reduced mitochondrial Ca2+ removal and consequently enhanced cytosolic Ca2+ hyperactivity, which is exacerbated by the decreased level of SERCA1 and parvalbumin. The aggregation of SOD1G93A inside mitochondria further promotes mitochondrial damage and ROS production, inhibiting mitochondrial dynamics and exhausting autophagy capacity. The axonal withdrawal event also promotes excessive ROS production, which is accompanied by prolonged CypD-related mPTP opening. These changes lead to major consequences on abnormal energy metabolism and muscle cell death.
Skeletal muscle degeneration in ALS is a complex scenario, and various molecular signaling pathways could be involved. Our speculation here could be oversimplified. In this current review, via discussion of current understanding of the dysregulated mitochondrial Ca2+ and ROS signaling during ALS muscle degeneration, we intend to attract more research efforts to explore the molecular mechanisms underlying the dysregulation of mitochondrial Ca2+ and ROS signaling in ALS muscle, and to develop potential alternative means to alleviate ALS progression through preserving mitochondrial function in skeletal muscle. In addition, motor neuron and skeletal muscle show similar abnormalities in mitochondrial structure and biochemical properties. Although many studies on mitochondrial function have been done on cultured motor neuron cell lines, it is difficult to isolate live motor neurons from the spinal cord of adult ALS rodent models. This limits the investigation of disease-stage-dependent mitochondrial changes in adult motor neurons during ALS disease progression. Studies of mitochondrial function in muscle cells may provide clues for unraveling potential mechanisms underlying motor neuron degeneration.
Acknowledgement
The work in JZ laboratory is supported by Muscular Dystrophy Association Grant MDA-4351, NIAMS/National Institutes of Health Grant R01 AR057404, the ALS Association (16-IIP-288), Victor E. Speas Foundation, McCown Gordon Gala Research Gift and Kansas City University Start-up Fund to JZ.
Abbreviations
- ΔΨm
mitochondrial inner membrane potential
- ADP
adenosine diphosphate
- ALS
myotrophic lateral sclerosis
- ATP
adenosine triphosphate
- COX
cytochrome c oxidase
- CypD
cyclophilin D
- FALS
familial amyotrophic lateral sclerosis
- G93A mice
transgenic mice with overexpression ALS-associated mutation SOD1G93A
- LC3
microtubule associated protein 1A/1B-light chain 3
- MLC
myosin light chain
- mPTP
mitochondrial permeability transition pore
- mt-cpYFP
mitochondrial targeted biosensor
- mTOR
mechanistic target of rapamycin kinase
- nAChRs
nicotinic acetylcholine receptor
- NADH
nicotinamide adenine dinucleotide (NAD) + hydrogen (H)
- NMJ
neuromuscular junctures
- PGC-1α
peroxisome proliferator-activated receptor gamma, coactivator 1 alpha
- ROS
reactive oxygen species
- RU360
oxygen-bridged dinuclear ruthenium amine complex selectively inhibit Ca2+ uniporters in the mitochondria
- SALS
sporadic amyotrophic lateral sclerosis
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- SOD1
superoxide dismutase type I
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare that they have no competing interests.
References
- [1].Marin B, Boumediene F, Logroscino G, Couratier P, Babron MC, Leutenegger AL, Copetti M, Preux PM, Beghi E, Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta-analysis, Int J Epidemiol, 46 (2017) 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Alonso A, Logroscino G, Jick SS, Hernan MA, Incidence and lifetime risk of motor neuron disease in the United Kingdom: a population-based study, European journal of neurology : the official journal of the European Federation of Neurological Societies, 16 (2009) 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jaiswal MK, Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs, Med Res Rev, (2018). [DOI] [PubMed] [Google Scholar]
- [4].Vucic S, Rothstein JD, Kiernan MC, Advances in treating amyotrophic lateral sclerosis: insights from pathophysiological studies, Trends Neurosci, 37 (2014) 433–442. [DOI] [PubMed] [Google Scholar]
- [5].Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z, van den Berg LH, Amyotrophic lateral sclerosis, Nat Rev Dis Primers, 3 (2017) 17071. [DOI] [PubMed] [Google Scholar]
- [6].Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC, Amyotrophic lateral sclerosis, Lancet, 377 (2011) 942–955. [DOI] [PubMed] [Google Scholar]
- [7].Pasinelli P, Brown RH, Molecular biology of amyotrophic lateral sclerosis: insights from genetics, Nature reviews. Neuroscience, 7 (2006) 710–723. [DOI] [PubMed] [Google Scholar]
- [8].Robberecht W, Philips T, The changing scene of amyotrophic lateral sclerosis, Nature reviews. Neuroscience, 14 (2013) 248–264. [DOI] [PubMed] [Google Scholar]
- [9].Boillee S, Vande Velde C, Cleveland DW, ALS: a disease of motor neurons and their nonneuronal neighbors, Neuron, 52 (2006) 39–59. [DOI] [PubMed] [Google Scholar]
- [10].Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, Belia S, Wannenes F, Nicoletti C, Del Prete Z, Rosenthal N, Molinaro M, Protasi F, Fano G, Sandri M, Musaro A, Skeletal muscle is a primary target of SOD1G93A-mediated toxicity, Cell Metab, 8 (2008) 425–436. [DOI] [PubMed] [Google Scholar]
- [11].Luo G, Yi J, Ma C, Xiao Y, Yi F, Yu T, Zhou J, Defective mitochondrial dynamics is an early event in skeletal muscle of an amyotrophic lateral sclerosis mouse model, PloS one, 8 (2013) e82112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wong M, Martin LJ, Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice, Human molecular genetics, 19 (2010) 2284–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu S, Yi J, Zhang YG, Zhou J, Sun J, Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model, Physiol Rep, 3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yi J, Ma C, Li Y, Weisleder N, Rios E, Ma J, Zhou J, Mitochondrial calcium uptake regulates rapid calcium transients in skeletal muscle during excitation-contraction (E-C) coupling, J Biol Chem, 286 (2011) 32436–32443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhou J, Yi J, Fu R, Liu E, Siddique T, Rios E, Deng HX, Hyperactive intracellular calcium signaling associated with localized mitochondrial defects in skeletal muscle of an animal model of amyotrophic lateral sclerosis, J Biol Chem, 285 (2010) 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhu K, Yi J, Xiao Y, Lai Y, Song P, Zheng W, Jiao H, Fan J, Wu C, Chen D, Zhou J, Xiao G, Impaired bone homeostasis in amyotrophic lateral sclerosis mice with muscle atrophy, J Biol Chem, 290 (2015) 8081–8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dupuis L, Loeffler JP, Neuromuscular junction destruction during amyotrophic lateral sclerosis: insights from transgenic models, Curr Opin Pharmacol, 9 (2009) 341–346. [DOI] [PubMed] [Google Scholar]
- [18].Loeffler JP, Picchiarelli G, Dupuis L, Gonzalez De Aguilar JL, The Role of Skeletal Muscle in Amyotrophic Lateral Sclerosis, Brain Pathol, 26 (2016) 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tsitkanou S, Della Gatta PA, Russell AP, Skeletal Muscle Satellite Cells, Mitochondria, and MicroRNAs: Their Involvement in the Pathogenesis of ALS, Frontiers in physiology, 7 (2016) 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pansarasa O, Rossi D, Berardinelli A, Cereda C, Amyotrophic lateral sclerosis and skeletal muscle: an update, Mol Neurobiol, 49 (2014) 984–990. [DOI] [PubMed] [Google Scholar]
- [21].Ilieva H, Polymenidou M, Cleveland DW, Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond, The Journal of cell biology, 187 (2009) 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Neel BA, Lin Y, Pessin JE, Skeletal muscle autophagy: a new metabolic regulator, Trends Endocrinol Metab, 24 (2013) 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ferri A, Coccurello R, What is “Hyper” in the ALS Hypermetabolism?, Mediators Inflamm, 2017 (2017) 7821672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nguyen QT, Son YJ, Sanes JR, Lichtman JW, Nerve terminals form but fail to mature when postsynaptic differentiation is blocked: in vivo analysis using mammalian nerve-muscle chimeras, J Neurosci, 20 (2000) 6077–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fitzsimonds RM, Poo MM, Retrograde signaling in the development and modification of synapses, Physiological reviews, 78 (1998) 143–170. [DOI] [PubMed] [Google Scholar]
- [26].Xiao Y, Ma C, Yi J, Wu S, Luo G, Xu X, Lin PH, Sun J, Zhou J, Suppressed autophagy flux in skeletal muscle of an amyotrophic lateral sclerosis mouse model during disease progression, Physiol Rep, 3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shadel GS, Horvath TL, Mitochondrial ROS signaling in organismal homeostasis, Cell, 163 (2015) 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cozzolino M, Carri MT, Mitochondrial dysfunction in ALS, Prog Neurobiol, 97 (2012) 54–66. [DOI] [PubMed] [Google Scholar]
- [29].Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E, Mitochondria, oxidative stress and neurodegeneration, Journal of the neurological sciences, 322 (2012) 254–262. [DOI] [PubMed] [Google Scholar]
- [30].Kawamata H, Manfredi G, Mitochondrial dysfunction and intracellular calcium dysregulation in ALS, Mechanisms of ageing and development, 131 (2010) 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen H, Chan DC, Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases, Human molecular genetics, 18 (2009) R169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kaus A, Sareen D, ALS Patient Stem Cells for Unveiling Disease Signatures of Motoneuron Susceptibility: Perspectives on the Deadly Mitochondria, ER Stress and Calcium Triad, Frontiers in cellular neuroscience, 9 (2015) 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ruffoli R, Bartalucci A, Frati A, Fornai F, Ultrastructural studies of ALS mitochondria connect altered function and permeability with defects of mitophagy and mitochondriogenesis, Frontiers in cellular neuroscience, 9 (2015) 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Smith EF, Shaw PJ, De Vos KJ, The role of mitochondria in amyotrophic lateral sclerosis, Neuroscience letters, (2017). [DOI] [PubMed] [Google Scholar]
- [35].Manfredi G, Kawamata H, Mitochondria and endoplasmic reticulum crosstalk in amyotrophic lateral sclerosis, Neurobiology of disease, 90 (2016) 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cozzolino M, Rossi S, Mirra A, Carri MT, Mitochondrial dynamism and the pathogenesis of Amyotrophic Lateral Sclerosis, Frontiers in cellular neuroscience, 9 (2015) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Musaro A, Understanding ALS: new therapeutic approaches, The FEBS journal, 280 (2013) 4315–4322. [DOI] [PubMed] [Google Scholar]
- [38].Martin LJ, Mitochondrial pathobiology in ALS, Journal of bioenergetics and biomembranes, 43 (2011) 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lennie P, The cost of cortical computation, Current biology : CB, 13 (2003) 493–497. [DOI] [PubMed] [Google Scholar]
- [40].Pysh JJ, Khan T, Variations in mitochondrial structure and content of neurons and neuroglia in rat brain: an electron microscopic study, Brain research, 36 (1972) 1–18. [DOI] [PubMed] [Google Scholar]
- [41].Eisenberg BR, Quantitative Ultrastructure of Mammalian Skeletal Muscle, American Physiological Society 1983. [Google Scholar]
- [42].Rolfe DF, Brown GC, Cellular energy utilization and molecular origin of standard metabolic rate in mammals, Physiological reviews, 77 (1997) 731–758. [DOI] [PubMed] [Google Scholar]
- [43].Kavanagh NI, Ainscow EK, Brand MD, Calcium regulation of oxidative phosphorylation in rat skeletal muscle mitochondria, Biochimica et biophysica acta, 1457 (2000) 57–70. [DOI] [PubMed] [Google Scholar]
- [44].Zhou J, Yi J, Royer L, Launikonis BS, Gonzalez A, Garcia J, Rios E, A probable role of dihydropyridine receptors in repression of Ca2+ sparks demonstrated in cultured mammalian muscle, American journal of physiology. Cell physiology, 290 (2006) C539–553. [DOI] [PubMed] [Google Scholar]
- [45].Zhou J, Dhakal K, Yi J, Mitochondrial Ca(2+) uptake in skeletal muscle health and disease, Sci China Life Sci, 59 (2016) 770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Feissner RF, Skalska J, Gaum WE, Sheu SS, Crosstalk signaling between mitochondrial Ca2+ and ROS, Front Biosci (Landmark Ed), 14 (2009) 1197–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].D’Autreaux B, Toledano MB, ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis, Nature reviews. Molecular cell biology, 8 (2007) 813–824. [DOI] [PubMed] [Google Scholar]
- [48].Zorov DB, Juhaszova M, Sollott SJ, Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release, Physiological reviews, 94 (2014) 909–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sasaki S, Iwata M, Ultrastructural study of synapses in the anterior horn neurons of patients with amyotrophic lateral sclerosis, Neuroscience letters, 204 (1996) 53–56. [DOI] [PubMed] [Google Scholar]
- [50].Sasaki S, Iwata M, Mitochondrial alterations in the spinal cord of patients with sporadic amyotrophic lateral sclerosis, Journal of neuropathology and experimental neurology, 66 (2007) 10–16. [DOI] [PubMed] [Google Scholar]
- [51].Atsumi T, The ultrastructure of intramuscular nerves in amyotrophic lateral sclerosis, Acta neuropathologica, 55 (1981) 193–198. [DOI] [PubMed] [Google Scholar]
- [52].Siklos L, Engelhardt J, Harati Y, Smith RG, Joo F, Appel SH, Ultrastructural evidence for altered calcium in motor nerve terminals in amyotropic lateral sclerosis, Annals of neurology, 39 (1996) 203–216. [DOI] [PubMed] [Google Scholar]
- [53].Afifi AK, Aleu FP, Goodgold J, MacKay B, Ultrastructure of atrophic muscle in amyotrophic lateral sclerosis, Neurology, 16 (1966) 475–481. [DOI] [PubMed] [Google Scholar]
- [54].Fidzianska A, Morphological differences between the atrophied small muscle fibres in amyotrophic lateral sclerosis and Werdnig-Hoffmann disease, Acta neuropathologica, 34 (1976) 321–327. [DOI] [PubMed] [Google Scholar]
- [55].Napoli L, Crugnola V, Lamperti C, Silani V, Di Mauro S, Bresolin N, Moggio M, Ultrastructural mitochondrial abnormalities in patients with sporadic amyotrophic lateral sclerosis, Archives of neurology, 68 (2011) 1612–1613. [DOI] [PubMed] [Google Scholar]
- [56].Chung MJ, Suh YL, Ultrastructural changes of mitochondria in the skeletal muscle of patients with amyotrophic lateral sclerosis, Ultrastruct Pathol, 26 (2002) 3–7. [DOI] [PubMed] [Google Scholar]
- [57].Wiedemann FR, Winkler K, Kuznetsov AV, Bartels C, Vielhaber S, Feistner H, Kunz WS, Impairment of mitochondrial function in skeletal muscle of patients with amyotrophic lateral sclerosis, Journal of the neurological sciences, 156 (1998) 65–72. [DOI] [PubMed] [Google Scholar]
- [58].Vielhaber S, Winkler K, Kirches E, Kunz D, Buchner M, Feistner H, Elger CE, Ludolph AC, Riepe MW, Kunz WS, Visualization of defective mitochondrial function in skeletal muscle fibers of patients with sporadic amyotrophic lateral sclerosis, Journal of the neurological sciences, 169 (1999) 133–139. [DOI] [PubMed] [Google Scholar]
- [59].Al-Sarraj S, King A, Cleveland M, Pradat PF, Corse A, Rothstein JD, Leigh PN, Abila B, Bates S, Wurthner J, Meininger V, Mitochondrial abnormalities and low grade inflammation are present in the skeletal muscle of a minority of patients with amyotrophic lateral sclerosis; an observational myopathology study, Acta Neuropathol Commun, 2 (2014) 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Crugnola V, Lamperti C, Lucchini V, Ronchi D, Peverelli L, Prelle A, Sciacco M, Bordoni A, Fassone E, Fortunato F, Corti S, Silani V, Bresolin N, Di Mauro S, Comi GP, Moggio M, Mitochondrial respiratory chain dysfunction in muscle from patients with amyotrophic lateral sclerosis, Archives of neurology, 67 (2010) 849–854. [DOI] [PubMed] [Google Scholar]
- [61].Echaniz-Laguna A, Zoll J, Ponsot E, N’Guessan B, Tranchant C, Loeffler JP, Lampert E, Muscular mitochondrial function in amyotrophic lateral sclerosis is progressively altered as the disease develops: a temporal study in man, Exp Neurol, 198 (2006) 25–30. [DOI] [PubMed] [Google Scholar]
- [62].Soraru G, Vergani L, Fedrizzi L, D’Ascenzo C, Polo A, Bernazzi B, Angelini C, Activities of mitochondrial complexes correlate with nNOS amount in muscle from ALS patients, Neuropathol Appl Neurobiol, 33 (2007) 204–211. [DOI] [PubMed] [Google Scholar]
- [63].Vielhaber S, Kunz D, Winkler K, Wiedemann FR, Kirches E, Feistner H, Heinze HJ, Elger CE, Schubert W, Kunz WS, Mitochondrial DNA abnormalities in skeletal muscle of patients with sporadic amyotrophic lateral sclerosis, Brain : a journal of neurology, 123 (Pt 7) (2000) 1339–1348. [DOI] [PubMed] [Google Scholar]
- [64].Artuso L, Zoccolella S, Favia P, Amati A, Capozzo R, Logroscino G, Serlenga L, Simone I, Gasparre G, Petruzzella V, Mitochondrial genome aberrations in skeletal muscle of patients with motor neuron disease, Amyotroph Lateral Scler Frontotemporal Degener, 14 (2013) 261–266. [DOI] [PubMed] [Google Scholar]
- [65].Ro LS, Lai SL, Chen CM, Chen ST, Deleted 4977-bp mitochondrial DNA mutation is associated with sporadic amyotrophic lateral sclerosis: a hospital-based case-control study, Muscle & nerve, 28 (2003) 737–743. [DOI] [PubMed] [Google Scholar]
- [66].Richter C, Park JW, Ames BN, Normal oxidative damage to mitochondrial and nuclear DNA is extensive, Proceedings of the National Academy of Sciences of the United States of America, 85 (1988) 6465–6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Indo HP, Davidson M, Yen HC, Suenaga S, Tomita K, Nishii T, Higuchi M, Koga Y, Ozawa T, Majima HJ, Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage, Mitochondrion, 7 (2007) 106–118. [DOI] [PubMed] [Google Scholar]
- [68].Wiedemann FR, Manfredi G, Mawrin C, Beal MF, Schon EA, Mitochondrial DNA and respiratory chain function in spinal cords of ALS patients, Journal of neurochemistry, 80 (2002) 616–625. [DOI] [PubMed] [Google Scholar]
- [69].Bernardini C, Censi F, Lattanzi W, Barba M, Calcagnini G, Giuliani A, Tasca G, Sabatelli M, Ricci E, Michetti F, Mitochondrial network genes in the skeletal muscle of amyotrophic lateral sclerosis patients, PloS one, 8 (2013) e57739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Russell AP, Wada S, Vergani L, Hock MB, Lamon S, Leger B, Ushida T, Cartoni R, Wadley GD, Hespel P, Kralli A, Soraru G, Angelini C, Akimoto T, Disruption of skeletal muscle mitochondrial network genes and miRNAs in amyotrophic lateral sclerosis, Neurobiology of disease, 49 (2013) 107–117. [DOI] [PubMed] [Google Scholar]
- [71].Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. , Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation, Science, 264 (1994) 1772–1775. [DOI] [PubMed] [Google Scholar]
- [72].McGoldrick P, Joyce PI, Fisher EM, Greensmith L, Rodent models of amyotrophic lateral sclerosis, Biochimica et biophysica acta, 1832 (2013) 1421–1436. [DOI] [PubMed] [Google Scholar]
- [73].Joyce PI, Fratta P, Fisher EM, Acevedo-Arozena A, SOD1 and TDP-43 animal models of amyotrophic lateral sclerosis: recent advances in understanding disease toward the development of clinical treatments, Mammalian genome : official journal of the International Mammalian Genome Society, 22 (2011) 420–448. [DOI] [PubMed] [Google Scholar]
- [74].Turner MR, Bowser R, Bruijn L, Dupuis L, Ludolph A, McGrath M, Manfredi G, Maragakis N, Miller RG, Pullman SL, Rutkove SB, Shaw PJ, Shefner J, Fischbeck KH, Mechanisms, models and biomarkers in amyotrophic lateral sclerosis, Amyotroph Lateral Scler Frontotemporal Degener, 14 Suppl 1 (2013) 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Carri MT, Cozzolino M, SOD1 and mitochondria in ALS: a dangerous liaison, Journal of bioenergetics and biomembranes, 43 (2011) 593–599. [DOI] [PubMed] [Google Scholar]
- [76].Carri MT, D’Ambrosi N, Cozzolino M, Pathways to mitochondrial dysfunction in ALS pathogenesis, Biochemical and biophysical research communications, (2016). [DOI] [PubMed] [Google Scholar]
- [77].Ivanova MI, Sievers SA, Guenther EL, Johnson LM, Winkler DD, Galaleldeen A, Sawaya MR, Hart PJ, Eisenberg DS, Aggregation-triggering segments of SOD1 fibril formation support a common pathway for familial and sporadic ALS, Proceedings of the National Academy of Sciences of the United States of America, 111 (2014) 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Philips T, Rothstein JD, Rodent Models of Amyotrophic Lateral Sclerosis, Curr Protoc Pharmacol, 69 (2015) 5 67 61–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL, An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria, Neuron, 14 (1995) 1105–1116. [DOI] [PubMed] [Google Scholar]
- [80].Kong J, Xu Z, Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1, J Neurosci, 18 (1998) 3241–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Deng HX, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, Gorrie GH, Khan MS, Hung WY, Bigio EH, Lukas T, Dal Canto MC, O’Halloran TV, Siddique T, Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria, Proceedings of the National Academy of Sciences of the United States of America, 103 (2006) 7142–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wong M, Gertz B, Chestnut BA, Martin LJ, Mitochondrial DNMT3A and DNA methylation in skeletal muscle and CNS of transgenic mouse models of ALS, Frontiers in cellular neuroscience, 7 (2013) 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS, Calcium ATP, and ROS: a mitochondrial love-hate triangle, American journal of physiology. Cell physiology, 287 (2004) C817–833. [DOI] [PubMed] [Google Scholar]
- [84].Fang H, Chen M, Ding Y, Shang W, Xu J, Zhang X, Zhang W, Li K, Xiao Y, Gao F, Shang S, Li JC, Tian XL, Wang SQ, Zhou J, Weisleder N, Ma J, Ouyang K, Chen J, Wang X, Zheng M, Wang W, Cheng H, Imaging superoxide flash and metabolism-coupled mitochondrial permeability transition in living animals, Cell research, 21 (2011) 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Karam C, Yi J, Xiao Y, Dhakal K, Zhang L, Li X, Manno C, Xu J, Li K, Cheng H, Ma J, Zhou J, Absence of physiological Ca2+ transients is an initial trigger for mitochondrial dysfunction in skeletal muscle following denervation, Skeletal muscle, 7 (2017) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Halter B, Gonzalez de Aguilar JL, Rene F, Petri S, Fricker B, Echaniz-Laguna A, Dupuis L, Larmet Y, Loeffler JP, Oxidative stress in skeletal muscle stimulates early expression of Rad in a mouse model of amyotrophic lateral sclerosis, Free radical biology & medicine, 48 (2010) 915–923. [DOI] [PubMed] [Google Scholar]
- [87].Leclerc N, Ribera F, Zoll J, Warter JM, Poindron P, Lampert E, Borg J, Selective changes in mitochondria respiratory properties in oxidative or glycolytic muscle fibers isolated from G93AhumanSOD1 transgenic mice, Neuromuscular disorders : NMD, 11 (2001) 722–727. [DOI] [PubMed] [Google Scholar]
- [88].St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM, Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators, Cell, 127 (2006) 397–408. [DOI] [PubMed] [Google Scholar]
- [89].Jones AW, Yao Z, Vicencio JM, Karkucinska-Wieckowska A, Szabadkai G, PGC-1 family coactivators and cell fate: roles in cancer, neurodegeneration, cardiovascular disease and retrograde mitochondria-nucleus signalling, Mitochondrion, 12 (2012) 86–99. [DOI] [PubMed] [Google Scholar]
- [90].Capitanio D, Vasso M, Ratti A, Grignaschi G, Volta M, Moriggi M, Daleno C, Bendotti C, Silani V, Gelfi C, Molecular signatures of amyotrophic lateral sclerosis disease progression in hind and forelimb muscles of an SOD1(G93A) mouse model, Antioxidants & redox signaling, 17 (2012) 1333–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Xiao Y, Karam C, Yi J, Zhang L, Li X, Yoon D, Wang H, Dhakal K, Ramlow P, Yu T, Mo Z, Ma J, Zhou J, ROS-related mitochondrial dysfunction in skeletal muscle of an ALS mouse model during the disease progression, Pharmacol Res, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ding Y, Fang H, Shang W, Xiao Y, Sun T, Hou N, Pan L, Sun X, Ma Q, Zhou J, Wang X, Zhang X, Cheng H, Mitoflash altered by metabolic stress in insulin-resistant skeletal muscle, Journal of molecular medicine, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Wang W, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H, Superoxide flashes in single mitochondria, Cell, 134 (2008) 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wei L, Salahura G, Boncompagni S, Kasischke KA, Protasi F, Sheu SS, Dirksen RT, Mitochondrial superoxide flashes: metabolic biomarkers of skeletal muscle activity and disease, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 25 (2011) 3068–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wang W, Zhang H, Cheng H, Mitochondrial flashes: From indicator characterization to in vivo imaging, Methods, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Wei L, Dirksen RT, Perspectives on: SGP symposium on mitochondrial physiology and medicine: mitochondrial superoxide flashes: from discovery to new controversies, The Journal of general physiology, 139 (2012) 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD, Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death, Nature, 434 (2005) 658–662. [DOI] [PubMed] [Google Scholar]
- [98].Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y, Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death, Nature, 434 (2005) 652–658. [DOI] [PubMed] [Google Scholar]
- [99].Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P, Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D, J Biol Chem, 280 (2005) 18558–18561. [DOI] [PubMed] [Google Scholar]
- [100].Wang W, Gong G, Wang X, Wei-LaPierre L, Cheng H, Dirksen R, Sheu SS, Mitochondrial Flash: Integrative Reactive Oxygen Species and pH Signals in Cell and Organelle Biology, Antioxidants & redox signaling, 25 (2016) 534–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Pouvreau S, Superoxide flashes in mouse skeletal muscle are produced by discrete arrays of active mitochondria operating coherently, PloS one, 5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Muller FL, A critical evaluation of cpYFP as a probe for superoxide, Free radical biology & medicine, 47 (2009) 1779–1780. [DOI] [PubMed] [Google Scholar]
- [103].Schwarzlander M, Logan DC, Fricker MD, Sweetlove LJ, The circularly permuted yellow fluorescent protein cpYFP that has been used as a superoxide probe is highly responsive to pH but not superoxide in mitochondria: implications for the existence of superoxide ‘flashes’, The Biochemical journal, 437 (2011) 381–387. [DOI] [PubMed] [Google Scholar]
- [104].Schwarzlander M, Wagner S, Ermakova YG, Belousov VV, Radi R, Beckman JS, Buettner GR, Demaurex N, Duchen MR, Forman HJ, Fricker MD, Gems D, Halestrap AP, Halliwell B, Jakob U, Johnston IG, Jones NS, Logan DC, Morgan B, Muller FL, Nicholls DG, Remington SJ, Schumacker PT, Winterbourn CC, Sweetlove LJ, Meyer AJ, Dick TP, Murphy MP, The ‘mitoflash’ probe cpYFP does not respond to superoxide, Nature, 514 (2014) E12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wei-LaPierre L, Gong G, Gerstner BJ, Ducreux S, Yule DI, Pouvreau S, Wang X, Sheu SS, Cheng H, Dirksen RT, Wang W, Respective contribution of mitochondrial superoxide and pH to mitochondria-targeted circularly permuted yellow fluorescent protein (mt-cpYFP) flash activity, J Biol Chem, 288 (2013) 10567–10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Fu ZX, Tan X, Fang H, Lau PM, Wang X, Cheng H, Bi GQ, Dendritic mitoflash as a putative signal for stabilizing long-term synaptic plasticity, Nature communications, 8 (2017) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Gong G, Liu X, Zhang H, Sheu SS, Wang W, Mitochondrial flash as a novel biomarker of mitochondrial respiration in the heart, American journal of physiology. Heart and circulatory physiology, 309 (2015) H1166–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Liu X, Weaver D, Shirihai O, Hajnoczky G, Mitochondrial ‘kiss-and-run’: interplay between mitochondrial motility and fusion-fission dynamics, The EMBO journal, 28 (2009) 3074–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Westermann B, Mitochondrial fusion and fission in cell life and death, Nature reviews. Molecular cell biology, 11 (2010) 872–884. [DOI] [PubMed] [Google Scholar]
- [110].Lu B, Mitochondrial dynamics and neurodegeneration, Curr Neurol Neurosci Rep, 9 (2009) 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cho DH, Nakamura T, Lipton SA, Mitochondrial dynamics in cell death and neurodegeneration, Cell Mol Life Sci, 67 (2010) 3435–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Debattisti V, Gerencser AA, Saotome M, Das S, Hajnoczky G, ROS Control Mitochondrial Motility through p38 and the Motor Adaptor Miro/Trak, Cell reports, 21 (2017) 1667–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Fan X, Hussien R, Brooks GA, H2O2-induced mitochondrial fragmentation in C2C12 myocytes, Free radical biology & medicine, 49 (2010) 1646–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Munoz JP, Ivanova S, Sanchez-Wandelmer J, Martinez-Cristobal P, Noguera E, Sancho A, Diaz-Ramos A, Hernandez-Alvarez MI, Sebastian D, Mauvezin C, Palacin M, Zorzano A, Mfn2 modulates the UPR and mitochondrial function via repression of PERK, The EMBO journal, 32 (2013) 2348–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Tang S, Le PK, Tse S, Wallace DC, Huang T, Heterozygous mutation of Opa1 in Drosophila shortens lifespan mediated through increased reactive oxygen species production, PloS one, 4 (2009) e4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Song W, Song Y, Kincaid B, Bossy B, Bossy-Wetzel E, Mutant SOD1G93A triggers mitochondrial fragmentation in spinal cord motor neurons: neuroprotection by SIRT3 and PGC-1alpha, Neurobiology of disease, 51 (2013) 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Magrane J, Hervias I, Henning MS, Damiano M, Kawamata H, Manfredi G, Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities, Human molecular genetics, 18 (2009) 4552–4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Jezek J, Cooper KF, Strich R, Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression, Antioxidants (Basel), 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kim B, Song YS, Mitochondrial dynamics altered by oxidative stress in cancer, Free radical research, 50 (2016) 1065–1070. [DOI] [PubMed] [Google Scholar]
- [120].Hara H, Kuwano K, Araya J, Mitochondrial Quality Control in COPD and IPF, Cells, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Rodney GG, Pal R, Abo-Zahrah R, Redox regulation of autophagy in skeletal muscle, Free radical biology & medicine, 98 (2016) 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Banerjee R, Beal MF, Thomas B, Autophagy in neurodegenerative disorders: pathogenic roles and therapeutic implications, Trends in neurosciences, 33 (2010) 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Li L, Zhang X, Le W, Altered macroautophagy in the spinal cord of SOD1 mutant mice, Autophagy, 4 (2008) 290–293. [DOI] [PubMed] [Google Scholar]
- [124].Morimoto N, Nagai M, Ohta Y, Miyazaki K, Kurata T, Morimoto M, Murakami T, Takehisa Y, Ikeda Y, Kamiya T, Abe K, Increased autophagy in transgenic mice with a G93A mutant SOD1 gene, Brain research, 1167 (2007) 112–117. [DOI] [PubMed] [Google Scholar]
- [125].Sasaki S, Autophagy in spinal cord motor neurons in sporadic amyotrophic lateral sclerosis, Journal of neuropathology and experimental neurology, 70 (2011) 349–359. [DOI] [PubMed] [Google Scholar]
- [126].Zhang X, Li L, Chen S, Yang D, Wang Y, Zhang X, Wang Z, Le W, Rapamycin treatment augments motor neuron degeneration in SOD1(G93A) mouse model of amyotrophic lateral sclerosis, Autophagy, 7 (2011) 412–425. [DOI] [PubMed] [Google Scholar]
- [127].Crippa V, Boncoraglio A, Galbiati M, Aggarwal T, Rusmini P, Giorgetti E, Cristofani R, Carra S, Pennuto M, Poletti A, Differential autophagy power in the spinal cord and muscle of transgenic ALS mice, Frontiers in cellular neuroscience, 7 (2013) 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH, XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy, Genes & development, 23 (2009) 2294–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Ikenaka K, Kawai K, Katsuno M, Huang Z, Jiang YM, Iguchi Y, Kobayashi K, Kimata T, Waza M, Tanaka F, Mori I, Sobue G , dnc-1/dynactin 1 knockdown disrupts transport of autophagosomes and induces motor neuron degeneration, PloS one, 8 (2013) e54511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Nassif M, Hetz C, Targeting autophagy in ALS: a complex mission, Autophagy, 7 (2011) 450–453. [DOI] [PubMed] [Google Scholar]
- [131].Chen S, Zhang X, Song L, Le W, Autophagy dysregulation in amyotrophic lateral sclerosis, Brain pathology, 22 (2012) 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Wang IF, Guo BS, Liu YC, Wu CC, Yang CH, Tsai KJ, Shen CK, Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43, Proceedings of the National Academy of Sciences of the United States of America, 109 (2012) 15024–15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Bhattacharya A, Bokov A, Muller FL, Jernigan AL, Maslin K, Diaz V, Richardson A, Van Remmen H, Dietary restriction but not rapamycin extends disease onset and survival of the H46R/H48Q mouse model of ALS, Neurobiology of aging, 33 (2012) 1829–1832. [DOI] [PubMed] [Google Scholar]
- [134].Fornai F, Longone P, Cafaro L, Kastsiuchenka O, Ferrucci M, Manca ML, Lazzeri G, Spalloni A, Bellio N, Lenzi P, Modugno N, Siciliano G, Isidoro C, Murri L, Ruggieri S, Paparelli A, Lithium delays progression of amyotrophic lateral sclerosis, Proceedings of the National Academy of Sciences of the United States of America, 105 (2008) 2052–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Pizzasegola C, Caron I, Daleno C, Ronchi A, Minoia C, Carri MT, Bendotti C, Treatment with lithium carbonate does not improve disease progression in two different strains of SOD1 mutant mice, Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases, 10 (2009) 221–228. [DOI] [PubMed] [Google Scholar]
- [136].Ahern GP, Junankar PR, Dulhunty AF, Subconductance states in single-channel activity of skeletal muscle ryanodine receptors after removal of FKBP12, Biophysical journal, 72 (1997) 146–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Lamb GD, Stephenson DG, Effects of FK506 and rapamycin on excitation-contraction coupling in skeletal muscle fibres of the rat, The Journal of physiology, 494 (Pt 2) (1996) 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P, Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases, J Neurosci, 20 (2000) 2534–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Ju JS, Varadhachary AS, Miller SE, Weihl CC, Quantitation of “autophagic flux” in mature skeletal muscle, Autophagy, 6 (2010) 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M, FoxO3 controls autophagy in skeletal muscle in vivo, Cell Metab, 6 (2007) 458–471. [DOI] [PubMed] [Google Scholar]
- [141].Olivan S, Calvo AC, Gasco S, Munoz MJ, Zaragoza P, Osta R, Time-Point Dependent Activation of Autophagy and the UPS in SOD1G93A Mice Skeletal Muscle, PloS one, 10 (2015) e0134830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Huttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW, Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease, Journal of bioenergetics and biomembranes, 40 (2008) 445–456. [DOI] [PubMed] [Google Scholar]
- [143].Dupuis L, di Scala F, Rene F, de Tapia M, Oudart H, Pradat PF, Meininger V, Loeffler JP, Up-regulation of mitochondrial uncoupling protein 3 reveals an early muscular metabolic defect in amyotrophic lateral sclerosis, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 17 (2003) 2091–2093. [DOI] [PubMed] [Google Scholar]
- [144].Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD, Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man, Exp Neurol, 185 (2004) 232–240. [DOI] [PubMed] [Google Scholar]
- [145].Weisleder N, Brotto M, Komazaki S, Pan Z, Zhao X, Nosek T, Parness J, Takeshima H, Ma J, Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release, The Journal of cell biology, 174 (2006) 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Payne AM, Jimenez-Moreno R, Wang ZM, Messi ML, Delbono O, Role of Ca2+, membrane excitability, and Ca2+ stores in failing muscle contraction with aging, Experimental gerontology, 44 (2009) 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].DiFranco M, Woods CE, Capote J, Vergara JL, Dystrophic skeletal muscle fibers display alterations at the level of calcium microdomains, Proceedings of the National Academy of Sciences of the United States of America, 105 (2008) 14698–14703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Hopf FW, Turner PR, Denetclaw WF Jr., Reddy P, Steinhardt RA, A critical evaluation of resting intracellular free calcium regulation in dystrophic mdx muscle, The American journal of physiology, 271 (1996) C1325–1339. [DOI] [PubMed] [Google Scholar]
- [149].De Backer F, Vandebrouck C, Gailly P, Gillis JM, Long-term study of Ca(2+) homeostasis and of survival in collagenase-isolated muscle fibres from normal and mdx mice, The Journal of physiology, 542 (2002) 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Wang X, Weisleder N, Collet C, Zhou J, Chu Y, Hirata Y, Zhao X, Pan Z, Brotto M, Cheng H, Ma J, Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle, Nature cell biology, 7 (2005) 525–530. [DOI] [PubMed] [Google Scholar]
- [151].Han R, Grounds MD, Bakker AJ, Measurement of sub-membrane [Ca2+] in adult myofibers and cytosolic [Ca2+] in myotubes from normal and mdx mice using the Ca2+ indicator FFP-18, Cell calcium, 40 (2006) 299–307. [DOI] [PubMed] [Google Scholar]
- [152].Rudolf R, Mongillo M, Magalhaes PJ, Pozzan T, In vivo monitoring of Ca(2+) uptake into mitochondria of mouse skeletal muscle during contraction, The Journal of cell biology, 166 (2004) 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Chin ER, Chen D, Bobyk KD, Mazala DA, Perturbations in intracellular Ca2+ handling in skeletal muscle in the G93A*SOD1 mouse model of amyotrophic lateral sclerosis, American journal of physiology. Cell physiology, 307 (2014) C1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Elliott JL, Snider WD, Parvalbumin is a marker of ALS-resistant motor neurons, Neuroreport, 6 (1995) 449–452. [DOI] [PubMed] [Google Scholar]
- [155].Reiner A, Medina L, Figueredo-Cardenas G, Anfinson S, Brainstem motoneuron pools that are selectively resistant in amyotrophic lateral sclerosis are preferentially enriched in parvalbumin: evidence from monkey brainstem for a calcium-mediated mechanism in sporadic ALS, Experimental neurology, 131 (1995) 239–250. [DOI] [PubMed] [Google Scholar]
- [156].Van Den Bosch L, Schwaller B, Vleminckx V, Meijers B, Stork S, Ruehlicke T, Van Houtte E, Klaassen H, Celio M, Missiaen L, Protective effect of parvalbumin on excitotoxic motor neuron death, Experimental neurology, 174 (2002) 150–161. [DOI] [PubMed] [Google Scholar]
- [157].Barrett EF, Barrett JN, David G, Dysfunctional mitochondrial Ca2+ handling in mutant SOD1 mouse models of fALS: integration of findings from motor neuron somata and motor terminals, Frontiers in cellular neuroscience, 8 (2014) 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Faes L, Callewaert G, Mitochondrial dysfunction in familial amyotrophic lateral sclerosis, Journal of bioenergetics and biomembranes, 43 (2011) 587–592. [DOI] [PubMed] [Google Scholar]
- [159].Le Masson G, Przedborski S, Abbott L, A computational model of motor neuron degeneration, Neuron, 83 (2014) 975–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Kim HJ, Im W, Kim S, Kim SH, Sung JJ, Kim M, Lee KW, Calcium-influx increases SOD1 aggregates via nitric oxide in cultured motor neurons, Exp Mol Med, 39 (2007) 574–582. [DOI] [PubMed] [Google Scholar]
- [161].Bozzo F, Mirra A, Carri MT, Oxidative stress and mitochondrial damage in the pathogenesis of ALS: New perspectives, Neuroscience letters, 636 (2017) 3–8. [DOI] [PubMed] [Google Scholar]
- [162].Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, Van Remmen H, Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production, American journal of physiology. Regulatory, integrative and comparative physiology, 293 (2007) R1159–1168. [DOI] [PubMed] [Google Scholar]
- [163].Ingalls CP, Warren GL, Armstrong RB, Intracellular Ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading, Journal of applied physiology, 87 (1999) 386–390. [DOI] [PubMed] [Google Scholar]
- [164].Tischler ME, Rosenberg S, Satarug S, Henriksen EJ, Kirby CR, Tome M, Chase P, Different mechanisms of increased proteolysis in atrophy induced by denervation or unweighting of rat soleus muscle, Metabolism: clinical and experimental, 39 (1990) 756–763. [DOI] [PubMed] [Google Scholar]
- [165].Powers SK, Wiggs MP, Duarte JA, Zergeroglu AM, Demirel HA, Mitochondrial signaling contributes to disuse muscle atrophy, American journal of physiology. Endocrinology and metabolism, 303 (2012) E31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, Phillips R, Altschuld R, Katsube Y, Sperelakis N, Bers DM, Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes, J Biol Chem, 273 (1998) 10223–10231. [DOI] [PubMed] [Google Scholar]
- [167].Fucile S, Ca2+ permeability of nicotinic acetylcholine receptors, Cell calcium, 35 (2004) 1–8. [DOI] [PubMed] [Google Scholar]
- [168].Kim J, Lee H, Lee JH, Kwon DY, Genovesio A, Fenistein D, Ogier A, Brondani V, Grailhe R, Dimerization, oligomerization, and aggregation of human Amyotrophic lateral sclerosis Cu/Zn-superoxide dismutase 1 mutant forms in live cells, J Biol Chem, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnoczky G, Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase, Proceedings of the National Academy of Sciences of the United States of America, 105 (2008) 20728–20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Yi M, Weaver D, Hajnoczky G, Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit, The Journal of cell biology, 167 (2004) 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Barsukova A, Komarov A, Hajnoczky G, Bernardi P, Bourdette D, Forte M, Activation of the mitochondrial permeability transition pore modulates Ca2+ responses to physiological stimuli in adult neurons, Eur J Neurosci, 33 (2011) 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Bernardi P, von Stockum S, The permeability transition pore as a Ca(2+) release channel: new answers to an old question, Cell calcium, 52 (2012) 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Rasola A, Sciacovelli M, Pantic B, Bernardi P, Signal transduction to the permeability transition pore, FEBS letters, 584 (2010) 1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Nguyen KT, Garcia-Chacon LE, Barrett JN, Barrett EF, David G, The Psi(m) depolarization that accompanies mitochondrial Ca2+ uptake is greater in mutant SOD1 than in wild-type mouse motor terminals, Proceedings of the National Academy of Sciences of the United States of America, 106 (2009) 2007–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Vila L, Barrett EF, Barrett JN, Stimulation-induced mitochondrial [Ca2+] elevations in mouse motor terminals: comparison of wild-type with SOD1-G93A, The Journal of physiology, 549 (2003) 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]