Abstract
Dravet syndrome (DS) is a form of epilepsy with a high incidence of sudden unexpected death in epilepsy (SUDEP). Respiratory failure is a leading cause of SUDEP, and DS patients’ frequently exhibit disordered breathing. Despite this, mechanisms underlying respiratory dysfunction in DS are unknown. We found that mice expressing a DS-associated Scn1a missense mutation (A1783V) conditionally in inhibitory neurons (Slc32a1cre/+::Scn1aA1783V fl/+; defined as Scn1aΔE26) exhibit spontaneous seizures, die prematurely and present a respiratory phenotype including hypoventilation, apnea, and a diminished ventilatory response to CO2. At the cellular level in the retrotrapezoid nucleus (RTN), we found inhibitory neurons expressing the Scn1a A1783V variant are less excitable, whereas glutamatergic chemosensitive RTN neurons, which are a key source of the CO2/H+-dependent drive to breathe, are hyper-excitable in slices from Scn1aΔE26 mice. These results show loss of Scn1a function can disrupt respiratory control at the cellular and whole animal levels.
Research organism: Mouse
Introduction
Dravet syndrome (DS) (aka. severe myoclonic epilepsy of infancy) is a severe form of early-onset epilepsy that is resistant to anti-epileptic drugs and has a high incidence of sudden unexpected death in epilepsy (SUDEP) (Kalume, 2013; Kearney, 2013; Shmuely et al., 2016). Mechanisms contributing to SUDEP involve disruption of cardiac and/or respiratory function (Massey et al., 2014); the relative contribution of each may vary depending on multiple pathological factors including severity of symptoms and underlying cause of epilepsy. With regard to SUDEP in DS, most work implicates cardiac failure caused by seizure-induced parasympathetic suppression of cardiac activity (Kearney, 2013; Kalume et al., 2013; Gataullina and Dulac, 2017). However, recent evidence suggests respiratory dysfunction precipitates cardiac failure and contributes to mortality in DS. For example, DS patients showed peri-ictal breathing problems including hypoventilation and apnea prior to the manifestation of bradycardia, a slower than normal heart rate (Kim et al., 2018). Patients with DS also exhibited a blunted ventilatory response to CO2 (Kim et al., 2018). This finding suggests that respiratory dysfunction, possibly at the level of respiratory chemoreceptors (neurons that regulate breathing in response to changes in tissue CO2/H+), contributes to the pathology of DS. Despite this physiological significance, mechanisms underlying respiratory dysfunction in DS or epilepsy in general are not well understood. Leading hypotheses propose that seizure activity disrupts respiratory control by a feed-forward mechanisms involving spreading depolarization (Aiba and Noebels, 2015) or activation of inhibitory subcortical projections to brainstem respiratory centers (Dlouhy et al., 2015; Lacuey et al., 2017). Consistent with the latter possibility, there is evidence that activity of serotonergic neurons in the dorsal and medullary raphe regions in rats are suppressed during ictal and post-ictal periods (Zhan et al., 2016). Serotonin is a potent modulator of breathing and arousal (Richerson, 2004; Buchanan and Richerson, 2010); therefore, it is possible that loss of this drive during seizures contributes to SUDEP. This possibility is supported by evidence that pharmacological augmentation of serotonergic signaling can prevent seizure-induced respiratory arrest in a mouse model of epilepsy and may improve seizure control in DS patients (Tupal and Faingold, 2019). However, some epilepsy patients show breathing abnormalities under baseline inter-ictal conditions including a reduced ventilatory response to CO2 (i.e., chemoreflex) (Sainju et al., 2019), suggesting factors other than seizure activity compromise respiratory control. Based on this, we consider a yet unexplored possibility that epilepsy-associated mutations may directly affect brainstem respiratory centers to compromise breathing under inter-ictal conditions, and thus serve as a common substrate for both seizures and respiratory dysfunction.
Most DS cases (70–95%) are caused by mutations in the Scn1a gene (MIM#182389), which encodes the pore-forming subunit of a voltage-gated Na+ channel (Nav1.1) (Meisler and Kearney, 2005; Fujiwara, 2006; Catterall et al., 2010; Akiyama et al., 2012). Approximately 700 different Scn1a pathological variants have been identified in DS patients, the majority of which are missense or frameshift mutations that result in loss of function (Parihar and Ganesh, 2013). Consistent with this, conventional Scn1a knockout mouse models (on a mixed C57B/6 background) recapitulate characteristic features of DS, including motor problems, seizures and premature death, in a remarkably titratable manner. For example, homozygous Scn1a knockout mice develop ataxia and die at 15 days postnatal, whereas heterozygous Scn1a deficient mice show seizure activity and early mortality starting at 3 weeks of age (Yu et al., 2006; Ogiwara et al., 2007). The cellular basis for many features of DS including seizures and premature death appears to involve disinhibition, as global deletion of Scn1a suppresses activity of inhibitory but not excitatory neurons in the cortex and hippocampus (Yu et al., 2006; Dutton et al., 2013), and conditional deletion of Scn1a from forebrain inhibitory neurons results in a DS-like phenotype similar to global Scn1a deletion (Cheah et al., 2012). For these reasons, most studies have used global or inhibitory neuron-specific Scn1a deletions to model DS (Catterall, 2012), with few studies focusing on other high-priority genetic risk factors like Scn1a missense mutations, which represent ~40% of DS-associated mutations (Depienne et al., 2009; Parihar and Ganesh, 2013). Thus, the extent to which expression of Scn1a loss-of-function mutations recapitulate features of DS remains unclear. Furthermore, despite the lethality associated with Scn1a mutations, nothing is known regarding how loss of Scn1a affects brainstem respiratory centers.
The main goal of this study was to provide the first detailed characterization of breathing in a Scn1a missense mutation mouse model of DS. We modeled DS by expressing a loss-of-function missense mutation (A1783V) conditionally in inhibitory neurons (referred to as Scn1aΔE26 mice). The A1783V variant is a DS mutation (Marini et al., 2007; Lossin, 2009; Klassen et al., 2014) predicted to result in loss of function by increasing Nav1.1 voltage-dependent inactivation. We found that Scn1aΔE26 mice (on a 90% C57BL6/J: : 10% 129/SvJ background) exhibited spontaneous seizure activity and premature death starting at ~2 weeks of age, thus confirming this is a model of SUDEP in DS. At this same developmental time point, Scn1aΔE26 mice hypoventilate, exhibit frequent apneas under baseline conditions, and show a reduced ventilatory response to CO2. This respiratory phenotype is similar to what has been described DS patients (Kim et al., 2018). At the cellular level in a key brainstem respiratory chemoreceptor region known as the retrotrapezoid nucleus (RTN), we found that inhibitory neurons expressing the A1783V pathological variant show less spontaneous activity and a diminished ability to maintain firing during sustained depolarization. This is consistent with the possibility that the A1783V channel mutant disrupts channel expression or function by increasing voltage dependent inactivation. Also consistent with a brainstem disinhibition mechanism, we found that basal activity and CO2/H+-sensitivity of excitatory chemosensitive RTN neurons was enhanced in slices from Scn1aΔE26 mice. These results show that RTN chemoreceptor function is altered in this DS model and may contribute to premature death.
Results
Scn1aΔE26 mice have spontaneous seizures and die prematurely
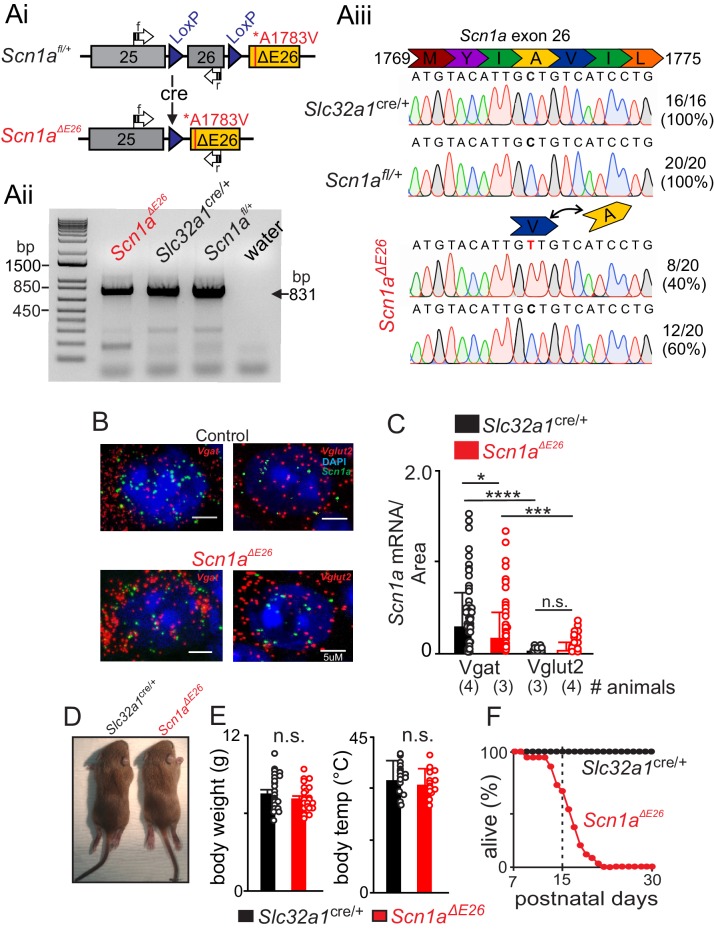
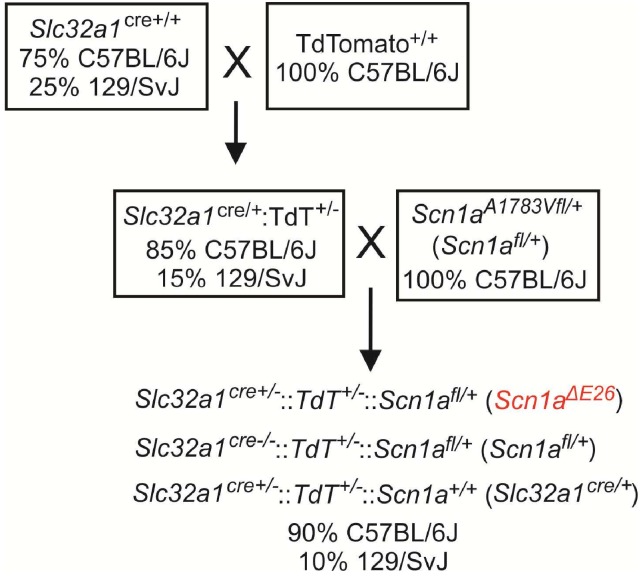
To generate mice that heterologously express the Scn1a A1783V pathological variant conditionally in inhibitory neurons (Scn1aΔE26 mice), we crossed floxed stop Scn1aA1783Vfl/+ mice (Scn1afl/+) with those that express Cre recombinase targeted to Slc32a1, the gene that encodes the vesicular GABA transporter Vgat, to generate a Slc32a1cre/+:TdT+/- (Slc32a1cre/+) line (see Figure 1—figure supplement 1). To determine whether transcript containing the A1783V variant is expressed in control or experimental animals, we isolated brainstem tissue from 13 day old pups of each genotype for subsequent cDNA amplification and sequencing. We were able to detect the expected amplicon product size of 831 base pairs in tissue from each genotype (Figure 1Ai–Aii). Importantly, we also were able to detect the alanine to valine single nucleotide substitution at position 1772 (analogous to position 1783 in human) in 8 of 20 (40%) samples of Scn1aΔE26 tissue (Figure 1Aiii), indicating this missense mutation is expressed at the mRNA level. As expected, only wild type sequence was detected in Slc32a1cre/+ and Scn1afl/+ control tissue (Figure 1Aiii), suggesting there is minimal leaky expression of A1783V in the absence of Cre.
Figure 1. Conditional expression of Scn1a A1783V in inhibitory neurons results in premature death.
(A) Construct design and validation of A1783V variant transcript expression. Note that this line was developed by Ana Mingorance (Chief Development Officer of the Loulou Foundation) and is available at JAX (sock # 026133). Ai, schematic shows loxP sites flanking wild type exon 26 followed by an edited version of exon 26 that contains the human A1783V pathological variant (ΔE26). When Cre recombinase is expressed, wild type exon 26 is removed, thus allowing transcription of ΔE26. Aii, Agarose gel shows detectable levels of Scn1a transcript (expected size of 831 bp) in brainstem tissue isolated from each genotype (primers span between exon 25 and 26, including residue 1783 of exon 26). Water was used as a no template negative control. Aiii, PCR products were sequenced to confirm that transcript containing A1783V is detectable in 40% of samples from Scn1aΔE26 tissue but was not detectable in samples from Slc32a1cre/+ and Scn1afl/+ control tissue. (B–C), fluorescent in situ hybridization (RNAScope) was performed to characterize expression of Scn1a transcript in inhibitory (Slc32a1+, Slc32a1) and glutamatergic (Vglut2+, Slc17a6) neurons in the RTN region in brainstem sections from control and Scn1aΔE26mice. (B) brainstem sections from Slc32a1cre/+ and Scn1aΔE26 mice containing the RTN show Scn1a labeling (green puncta) of both Vgat+ and Vglut2 +neurons. (C), summary data show Scn1a transcript expression (normalized to cell size) in Vgat+ and Vglut2 +RTN neurons from each genotype; channel transcript was reduced in Vgat+ cells from Scn1aΔE26 mice (0.43 ± 0.7 mRNA/area, n = 94 cells) compared to control (0.73 ± 0.9 mRNA/area, n = 82 cells) (p<0.05), whereas Vglut2 +cells showed low channel transcript across both genotypes. (D–E) Scn1aΔE26 mice did not show any obvious differences gross morphology (A) body weight (D) or temperature (E) compared to age-matched litter mate control mice. (F) (Figure 1—source data 1), survival curve shows that control mice (n = 57) survive to adulthood (30 days postnatal) while Scn1aΔE26 mice (n = 41) die prematurely starting at 9 days postnatal and reaching 100% lethality by 25 days (χ2 = 63.9, p<0.0001). These results were compared using a two-way ANOVA and Sidak multiple comparison test. *, p<0.05; ***p<0.001; ****p<0.0001.
Figure 1—figure supplement 1. Breeding strategy to generate mice that heterologously express the Scn1a A1783V pathological variant conditionally in inhibitory neurons (Scn1aΔE26 mice).
To characterize the cellular distribution of Scn1a in the RTN, we prepared brainstem sections containing the RTN from Slc32a1cre/+ and Scn1aΔE26 mice (15 days old) for fluorescent in situ hybridization using probes for (1) Scn1a, which does not distinguish Scn1a channel variants; (2) Slc32a1 gene which encodes Vgat to identify GABAergic and glycinergic inhibitory neurons; and (3) Slc17a6 gene which encodes the vesicular glutamate transporter 2 (Vglut2) to identify excitatory glutamatergic neurons, including chemosensitive RTN neurons. We labeled all cell nuclei with DAPI. Inhibitory Vgat+ cells were present in the RTN region and were in close proximity to excitatory Vglut2+ neurons (i.e., putative RTN chemoreceptors). Both genotypes showed similar relative distributions of Vgat+ cells (T172 = 0.142, p=0.88). We also observed numerous bright fluorescent puncta, which corresponded to Scn1a transcript in the soma of Vgat+ cells and, to a lesser extent, in Vglut2+ cells in slices from control mice (F3,321 = 24.07, p<0.0001). In slices from Scn1aΔE26 mice, we found a modest reduction in Scn1a transcript in Vgat+ but not Vglut2+ cells (F3,321 = 24.07, p<0.05; see Figure 1B–C). Together with our sequencing data (Figure 1Aiii), these results suggest that the A1783V pathogenic variant is expressed by brainstem inhibitory neurons but possibly at slightly reduced levels compared to control. Therefore, cellular and behavioral phenotypes associated with Scn1aΔE26 mice (see below) may involve either reduced expression, impaired channel function, or both. In a separate experiment to validate cell-type-specific Cre expression, we confirmed that all TdT+ cells expressed Vgat, but not Vglut2, mRNA (not shown).
Based on previous evidence showing that heterozygous deletion mutations can give rise to severe forms of DS (Yu et al., 2006; Miller et al., 2014) and since Scn1aΔE26 mice express Scn1a transcript, we expect Scn1aΔE26 mice to exhibit a mild epilepsy-like phenotype. Contrary to this expectation, Scn1aΔE26 mice show a severe SUDEP-like phenotype. Scn1aΔE26 mice were born in the expected ratios, were viable, and by ~15 days postnatal, were similar in terms of body weight (T46 = 1.62, p=0.11) and temperature (T26 = 0.77, p=0.44) as their Slc32a1cre/+ control littermates (Figure 1D–F). However, Scn1aΔE26 pups show seizure-like behavior by ~2 weeks of age (Table 1). More specifically, based on the Racine seizure-behavior scoring paradigm, only 22.7% of Slc32a1cre/+ control mice (N = 22) showed seizure-like behavior, which mainly manifested as head-bobbing (category 1). By contrast, 77.3% of Scn1aΔE26 mice (N = 22) showed severe seizure behavior, including forelimb tremor (category 3), rearing alone (category 4) or in conjunction with falling over, and full-body tonic-clonic seizure (category 5). Unlike Slc32a1cre/+control animals, several of the mutant mice exhibited behavioral arrest that may reflect absence seizure-like activity. Furthermore, since febrile seizures are considered a hallmark of DS, we also characterized the susceptibility of Slc32a1cre/+control and Scn1aΔE26 pups (mixed sex, 12–14 days old) to heat-induced seizures. When core body temperature was increased from 37°C to 42.5°C (0.5°C increments every 2 min) all Scn1aΔE26 mice (N = 9) developed tonic-clonic seizures (category 5) at an average body temperature of 41.1 ± 0.2°C (Table 2). Conversely, none of the Slc32a1cre/+- litter mate controls (N = 10) showed seizure activity up to the cut-off temperature of 42.5°C (Table 2). These results differ somewhat from previous work that showed heterozygous Scn1a knockout mice do not develop temperature-induced seizures until ~18 days of age (Oakley et al., 2009). In addition to increased febrile seizure propensity, Scn1aΔE26 also begin to die at ~12 days of age, reaching 100% lethality by 23 days postnatal (Figure 1F and Figure 1—source data 1). This early onset of premature death in Scn1aΔE26 mice occurs ~1 week prior to mortality in heterozygous Scn1a knockout mice on a pure C57BL/6J background (Yu et al., 2006; Catterall, 2012), suggesting Scn1aΔE26 mice (90% C57BL/6J background) have a particularly severe phenotype.
Table 1. Behavioral Assessment of Seizure activity.
| Racine score | N | 0 | 1 | 2 | 3 | 4 | 5 | Behavioral arrest |
|---|---|---|---|---|---|---|---|---|
| Slc32a1cre/+ | 22 | 17 | 5 | 0 | 0 | 0 | 0 | 0 |
| Scn1aΔE26 | 22 | 0 | 3 | 3 | 6 | 2 | 3 | 5 |
Table 2. Febrile seizure propensity.
| Genotype | N | Weight | Induced seizure (%) |
|---|---|---|---|
| Slc32a1cre/+ | 10 | 6.65 ± 0.3 | 0 |
| Scn1aΔE26 | 9 | 6.71 ± 0.3 | 100**** |
****Fisher’s exact test p<0.000.
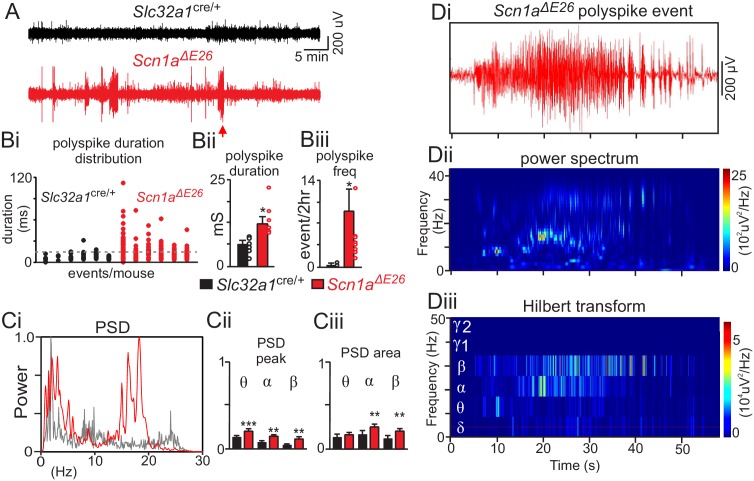
To determine whether Scn1aΔE26 mice exhibit abnormal brain activity, we made video electrocorticogram (ECoG) recordings from Slc32a1cre/+control and Scn1aΔE26 mice. We allowed mice 12 hr to recover after implanting them with the ECoG head stage. We continuously recorded animal behavior and ECoG activity over a two-hour period, between the hours of 9:00 AM – 2:00 PM. Consistent with frequent polyspike activity observed in the ECoG recordings of DS patients (Bender et al., 2012), Scn1aΔE26 mice showed large amplitude (at least twice baseline) polyspike activity that lasted for an average duration of 15.6 ± 0.8 s (Figure 2A–Bi). These events always occurred in conjunction with seizure activity (category 4–5) but were frequently preceded by brief behavioral arrest. The duration of these polyspike events were considerably shorter than spike wave discharges associated with absence epilepsy that typically last for >1 s (Letts et al., 2014). Conversely, Slc32a1cre/+ littermate control animals showed minimal large amplitude spike activity; any detectable events were of a short duration 7.55 ± 0.6 s (Figure 2A–Bi) and occurred when the animal was exhibiting exploratory behavior and less obvious seizure like activity (category 0–2) or freezing behavior. Based on this, we define epileptic spike activity for this model as abrupt onset polyspiking events with greater than twice baseline amplitude, minimum duration of 14 ms, and that occur in conjunction with seizure activity (category 3–5). Based on this criteria, Scn1aΔE26 and Slc32a1cre/+ control mice show epileptic spike activity with a frequency 9.167 ± 3.9 events/2 hr and of 0.25 ± 0.1 events/2 hr, respectively (T5 = 2.28, p<0.05) (Figure 2Bii). Note that three Slc32a1cre/+ animals showed a polyspike event that lasted longer than 14 ms and occurred with noticeable forelimb shaking and so were included in our analysis (Figure 2Biii). Power spectral analysis of poly-spike events in Slc32a1cre/+ and epileptic events in Scn1aΔE26 mice show that epileptic events were composed of high alpha and beta frequencies (F4, 840 = 5.605, p<0.001) (Figure 2C–D and Figure 2—source data 1). These results suggest Scn1aΔE26 mice are phenotypically similar to global and inhibitory neuron-specific Scn1a haploinsufficient models of DS (Yu et al., 2006; Kalume et al., 2013; Kim et al., 2018), but with an accelerated time course for manifestation of pathological features including spontaneous and heat-induced seizures as well as premature death. Based on the above results, we consider the Scn1aΔE26 mouse model to be useful for dissecting the mechanisms that underlie respiratory failure in DS.
Figure 2. Scn1aΔE26 exhibit frequent spontaneous seizures.
(A) traces of raw EcoG activity show that Scn1aΔE26 mice but not Slc32a1cre/+ mice exhibit frequent spontaneous burst of high amplitude poly-spike activity. The arrow identifies a typical seizure-like poly-spike event that was analyzed further by power spectral analysis in panel D. Polyspike events with a minimum duration of 14 ms were accompanied by seizure-like behavior and so were considered epileptic activity. (B), Scn1aΔE26 mice showed more frequent epileptic poly-spike bursts of activity (Bi, dotted line designates duration threshold for epileptic activity); poly-spike bursts (>14 ms) occurred more frequently in Scn1aΔE26 mice (Bi-Bii) (control 0.13 ± 0.1 events/2 hr, n = 6; Scn1aΔE26 0.37 ± 0.05 events/2 hr, n = 6; T10 = 3.009, p<0.01) and lasted for a longer duration (Biii) (control 7.6 ± 0.6 ms, n = 6; Scn1aΔE26 15.6 ± 0.8 ms, n = 6, T10 = 2.268, p<0.05) compared to control animals. Ci, representative power spectrum density (PSD) plots of spontaneous poly-spike burst events show typical strong activity in the theta-, alpha and beta frequency range in Scn1aΔE26 but not control mice. Cii-Ciii (Figure 2—source data 1), summary data (normalized to the maximum value at each event) show PSD peak (Cii) and PSD area under the curve (Ciii) of each frequency range for each genotype. Note that poly-spike burst events measured in Scn1aΔE26 mice show increased activity in the theta, alpha and beta range. Di-iii, poly-spike burst events recorded from a Scn1aΔE26 mouse (arrow in panel A) plotted on an expanded time scale (Di) and corresponding time frequency distribution (Dii) and deconstructed spectrum into its various frequency domains (Diii). These results were compared using a two-way ANOVA and the Sidak multiple comparison test. *, p<0.05; **, p<0.01; ***p<0.001.
Scn1aΔE26 mice hypoventilate under baseline conditions and have a reduced CO2/H+ ventilatory response
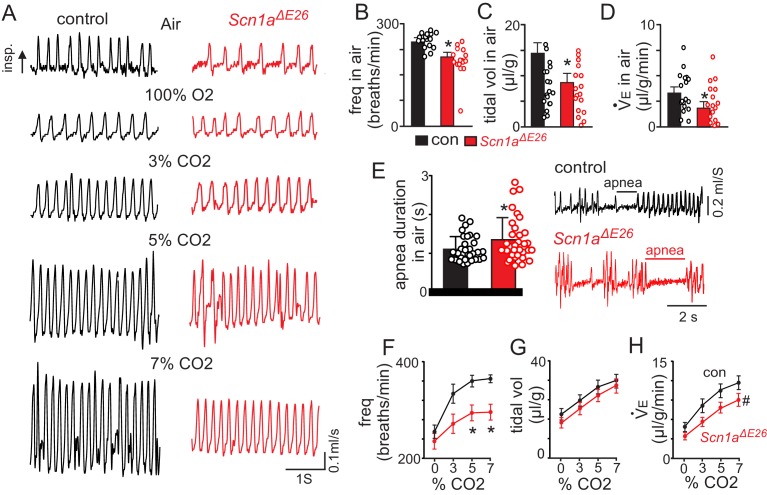
Recent evidence (Kim et al., 2018) showed that DS patients have post-ictal respiratory abnormalities, including hypoventilation, apnea and impaired CO2 chemoreception. These symptoms can last for several hours after seizure, which indicates that respiratory problems contribute to SUDEP in DS. Therefore, to determine whether Scn1aΔE26 mice exhibit respiratory problems, we used whole-body plethysmography to measure baseline breathing and the ventilatory response to CO2 in 15-day-old control and Scn1aΔE26 mice. Note that there were no measureable differences in respiratory activity between Slc32a1cre/+ (n = 16) and Scn1afl/+ (N = 5) mice (p=0.267). Therefore, these genotypes were pooled as controls for this set of experiments. We found that compared to control animals, Scn1aΔE26 mice show diminished respiratory output under room air conditions. Specifically, Scn1aΔE26 exhibit suppressed frequency (256 ± 11 bpm for control compared to 211 ± 16 bpm for Scn1aΔE26; T33 = 2.43; p<0.05); tidal volume (14.8 ± 2.0 μl/g for control compared to 8.9 ± 1.9 μl/g for Scn1aΔE26, T33 = 2.02, p<0.05); and minute ventilation (3.6 ± 0.5 μl/min/g for control compared to 2.7 ± 0.5 μl/min/g for Scn1aΔE26; T33 = 2.01, p<0.05; Figure 3A–D). Although both control and Scn1aΔE26 mice exhibit apneic events at similar frequencies (0.23 ± 0.1/min for control and 0.11 ± 0.04/min for Scn1aΔE26; p=0.6), the duration of these events were longer in Scn1aΔE26 mice (Figure 3E; 1,104 ± 58.6 ms for controls versus 1,350 ± 99.2 ms Scn1aΔE26; T51 = 2.135; p<0.05). We also found that Scn1aΔE26 mice had a diminished capacity to increase respiratory frequency in response to graded increases in CO2 (Figure 3F). Specifically, respiratory frequency in 7% CO2 (balance O2) was higher in controls (363.1 ± 7.7 bpm; N = 22) versus Scn1aΔE26 (300.7 ± 17.4 bpm; N = 17; F1,37 = 5.69, p<0.05). Although tidal volume responses to CO2/H+ are similar between genotypes (p=0.47), total respiratory output, as measured by minute ventilation— the product of respiratory frequency and tidal volume—was diminished in Scn1aΔE26 mice compared to controls (F3,111 = 3.167, p<0.05; Figure 3G–H and Figure 3—source data 1). Specifically, increasing inspired CO2 from 0% to 3% increased minute ventilation in control mice by 3.3 ± 0.5 μl/min/g (p<0.0001). These same conditions, however, led to a much smaller and non-significant increase in minute ventilation among Scn1aΔE26 mice (increase of 1.5 ± 0.5 μl/min/g; p=0.07). These results show that Scn1aΔE26 mice exhibit a respiratory phenotype similar to that observed in DS patients, and further supports the possibility that respiratory problems may contribute to mortality in this DS model.
Figure 3. Scn1aΔE26 mice show reduced respiratory output under control conditions and during exposure to high CO2.
For these experiments Scn1afl/+ and Slc32a1cre/+ were used as control. (A) traces of respiratory activity from a control and Scn1aΔE26 mouse during exposure to room air, 100% O2 and 3–7% CO2 (balance O2). (B–D), summary data (n = 22 control; n = 17 Scn1aΔE26) show respiratory frequency (B), tidal volume (C) and minute ventilation (D) are reduced in Scn1aΔE26 mice compared to control under room air conditions. (E), traces of respiratory activity (left) and summary data (right) show that under room air conditions both control and Scn1aΔE26 mice exhibit periods of apnea; the frequency of these events were similar between genotypes, however, they lasted for a longer duration in Scn1aΔE26 mice compared to control. F-H (Figure 3—source data 1), summary data shows the respiratory frequency (F), tidal volume (G) and minute ventilation response of control and Scn1aΔE26 mice to graded increases in CO2 (balance O2). Scn1aΔE26 mice showed a blunted respiratory frequency to 5% and 7% CO2 which resulted in a diminished CO2/H+-dependent increase in minute ventilation. These results were compared using either unpaired t test (panels B-E) or two-way ANOVA followed by the Holm-Sidak multiple comparison test (panels F-H). *, difference between means p<0.05, #, different interaction factor, p<0.05.
Disinhibition and altered RTN chemoreception in Scn1aΔE26 mice
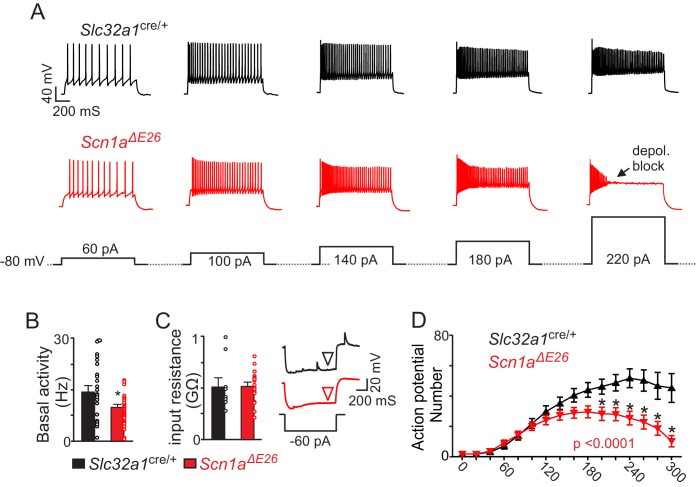
The RTN regulates several aspects of breathing including chemoreception (Guyenet and Bayliss, 2015) and since the ventilatory response to CO2 is disrupted in DS patients (Kim et al., 2018), we wanted to determine whether activity of chemosensitive RTN neurons are disrupted in Scn1aΔE26 mice. Furthermore, evidence also suggests that inhibitory neurons in the RTN region contribute to respiratory drive (Ott et al., 2011). Therefore, we first sought to determine whether loss of Scn1a function in inhibitory neurons decreases inhibitory neuron activity and disinhibits excitatory, chemosensitive, neurons. Inhibitory neurons were identified by Cre-dependent TdT labeling of Vgat+ cells in both control and Scn1aΔE26 lines. Consistent with other Scn1a knockout (Tai et al., 2014) or missense knockin (Ogiwara et al., 2007; Mashimo et al., 2010; Hedrich et al., 2014) DS models, we found that loss of Scn1a function in inhibitory neurons suppressed inhibitory neural activity. For example, whole-cell current-clamp recordings from inhibitory neurons in the RTN region in slices from Slc32a1cre/+ or Scn1aΔE26 mice show that inhibitory neurons from Scn1aΔE26 mice have lower basal activity than those of Slc32a1cre/+ control mice (14.39 ± 1.5 Hz for Slc32a1cre/+ vs. 9.902 ± 0.64 Hz for Scn1aΔE26; T60 = 2.97, p<0.01; Figure 4A–B). Furthermore, Scn1aΔE26 inhibitory neurons fired fewer action potentials in response to depolarizing current steps (0–300 pA; Δ 20 pA) from a holding potential of −80 mV. This activity deficit became more pronounced during large (200–300 pA) sustained (1,000 ms) current injections where inhibitory neurons from Scn1aΔE26 mice showed pronounced spike amplitude and frequency decrement (Figure 4A,D and Figure 4—source data 1). That is, the number of spikes elicited by a + 300 pA current step (1,000 ms) was 53.7 ± 11 for Slc32a1cre/+ controls (N = 13) compared to 13.9 ± 6.4 for Scn1aΔE26 (N = 20; F15,465 = 9.536; p<0.0001). We also found that inhibitory neurons from each genotype had similar input resistance (517.6 ± 82.2 MΩ for Slc32a1cre/+ control vs. 519.2 ± 38.9 MΩ for Scn1aΔE26; T25 = 0.02; p=0.3; Figure 4C). These results show that inhibitory neurons in slices from Scn1aΔE26 mice have diminished spontaneous activity and a reduced ability to respond to a range of excitatory inputs.
Figure 4. Brainstem inhibitory neurons in slices from Scn1aΔE26 show diminished basal activity and repetitive firing behavior during sustained depolarization.
(A) segments of membrane potential from inhibitory neurons in the RTN region in slices from control and Scn1aΔE26 mice during depolarizing current injections (40 to 220 pA; 1 s duration) from a membrane potential of –80 mV. (B) summary data shows inhibitory neurons in slices from Scn1aΔE26 mice (n = 36) are less active under resting conditions (0 pA holding current) compared to inhibitory neurons in slices form Slc32a1cre/+ control mice (n = 26 cells). (C), summary data and representative voltage responses to a −60 pA current injection show that inhibitory neurons from each genotype had similar input resistance. (D) (Figure 4—source data 1), input-output relationship show that inhibitory neurons from Scn1aΔE26 mice generate fewer action potentials in response to moderate depolarizing current injections (1 s duration) and at more positive steps go into depolarizing block. Results were compared using t-test (B–C) and two-way ANOVA and Sidak multiple comparison test (D). *, p<0.05; **, p<0.01; ***, p<0.001.
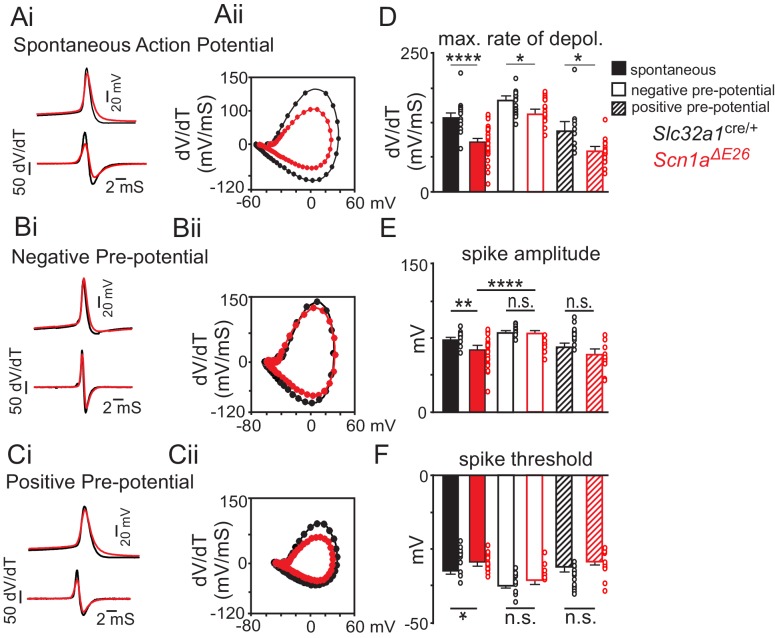
The A1783V pathological variant is located in the S6 segment of domain 4 (Marini et al., 2007; Lossin, 2009), a region thought to regulate voltage-dependent inactivation (Catterall, 2000). Based on our evidence that A1783V is expressed in tissue form Scn1aΔE26 mice (Figure 1Aiii) and since inhibitory neurons from these animals show reduced excitability (Figure 4A–B), we hypothesized that the Scn1a A1783V variant results in loss of function, in part, by causing Nav1.1 channels to inactivate at more negative voltages. Consistent with this hypothesis, when examining spontaneous action potentials (as measured under resting conditions with a 0 pA holding current) in inhibitory neurons in slices of Scn1aΔE26 and Slc32a1cre/+ control mice, the latter showed a higher amplitude (73.5 ± 1.9 mV) than Scn1aΔE26 (61.9 ± 2.6 mV; F1,95 = 9.931, p<0.001). The maximum rate of depolarization was higher for Slc32a1cre/+ controls (134.4 ± 5.0 mV/mS) compared to Scn1aΔE26 (89.4 ± 5.3 mV/mS; F1,96 = 35.2, p<0.0001; see Figure 5A,D–F and Figure 5—source data 1). Action potential threshold was also higher in inhibitory neurons in slices from Scn1aΔE26 (−29.2 ± 0.9 mV) compared to Slc32a1cre/+ controls (−32.4 ± 0.6 mV; F1,95 = 7.403, p<0.05; Figure 5A,F).
Figure 5. The Scn1a A1783V pathological variant may result in loss of channel function by increased voltage-dependent inactivation.
(A) average spontaneous action (Slc32a1cre/+ control n = 24 spikes, Scn1aΔE26 n = 29 spikes (top) and first time derivative of average action potentials (bottom) recorded from inhibitory neurons in slices from control and Scn1aΔE26 mice (Ai) and corresponding phase plot (Aii) (dV/dt; Y-axis vs mV; X-axis) of the traces in panel Ai show that cells expressing Scn1a A1783V depolarize slower compared to control cells (Figure 5—source data 1). (B), average first action potential following a hyperpolarizing pre-potential (−100 pA; 1 s) (Slc32a1cre/+ control n = 13 spikes, Scn1aΔE26 n = 16 spikes) (top) and first time derivative of average action potentials (bottom) recorded from inhibitory neurons in slices from control and Scn1aΔE26 mice (Bi) and corresponding phase plot (Bii) of traces in panel Bi show that holding cells at a negative pre-potential to remove sodium channel inactivation improved the depolarization kinetics of subsequent spikes (Figure 5—source data 1). (C), average first action potential following a depolarizing pre-potential (+180 pA; 1 s) (control n = 9 spikes, Scn1aΔE26 n = 11 spikes (top) and first time derivative of average action potentials (bottom) recorded from inhibitory neurons in slices from control and Scn1aΔE26 mice (Ci) and corresponding phase plot (Cii) of traces in panel Ci show that holding cells at a depolarized pre-potential to increase sodium channel inactivation diminished genotype differences in action potential kinetics (Figure 5—source data 1). (D–F), summary data showing the maximum rate of depolarization (D), action potential amplitude (E) and action potential threshold (F) of spontaneous action potentials and first spikes following positive or negative pre-potentials recorded in slices from control and Scn1aΔE26 mice. Results were compared by two-way ANOVA and the Sidak multiple comparison test.. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001.
Next, we characterized the properties of the first action potential elicited after holding cells at potentials that either remove or enhance Nav1.1 channel inactivation. We found that differences in action potential waveform properties between genotypes were minimized when cells are held at a negative voltage to remove Na+ channel inactivation. For example, holding inhibitory neurons in slices from Scn1aΔE26 mice at a negative pre-potential by injecting a hyperpolarizing current (−100 pA; 1,000 ms) increased action potential amplitude (78.01 ± 2.0 mV; F1, 95 = 9.931, p<0.0001) to an amount similar to spikes from Slc32a1cre/+ control cells (81.05 ± 1.2 mV; p=0.83) (Figure 5B,E). Under these conditions, the maximum rate of depolarization also increased 140.3 ± 5.441 mV/ms (F 1, 96 = 35.21, p<0.0001) (Figure 5B,D and Figure 5—source data 1); this rate was similar to that measured in spontaneous spikes from Slc32a1cre/+ control animals (p>0.99) but slower than spikes from Slc32a1cre/+ control cells following a negative pre-potential (166.6 ± 7.3 mV/mS, F1,96 = 35.21, p<0.05). Holding inhibitory neurons in slices from Scn1aΔE26 mice at a negative pre-potential also lowered the threshold for spike initiation (−35.68 ± 0.7 mV; F1, 95 = 7.403, p<0.001) to a level similar to Slc32a1cre/+ control cells (−37.2 ± 1.2 mV; F1, 95 = 7.403, p=0.06) (Figure 5B,F). We also found that delivering a + 180 pA current for 1,000 ms to enhance Na+ channel inactivation in inhibitory neurons in slices from Slc32a1cre/+ control mice resulted in similar action potential amplitude (F1, 95 = 9.931, p>0.99), rate of depolarization (F 1, 96 = 35.21, p=0.58) and spike threshold (F1, 95 = 7.403, p=0.97) as spikes measured in inhibitory neurons from Scn1aΔE26 slices under resting conditions (holding current = 0 pA) (Figure 5C,D–F and Figure 5—source data 1). Although it is possible that diminished expression of channel containing A1783V (Figure 1C) may also contribute to this electrophysiological phenotype; since transcript containing A1783V appears to be abundantly expressed (Figure 1Aiii), we speculate that loss of Scn1a function involves enhanced Nav1.1 inactivation.
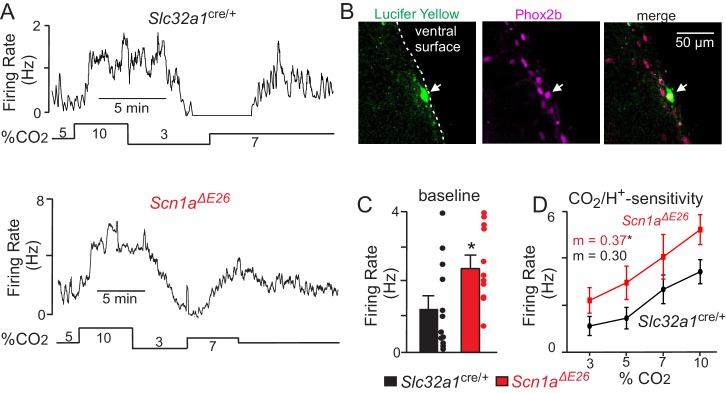
Based on previous evidence suggests inhibitory neurons in the RTN region can regulate activity of chemosensitive neurons (Ott et al., 2011), we predict that loss of inhibitory tone by expression of Scn1a A1783V would enhance basal activity and CO2/H+ sensitivity of glutamatergic chemosensitive neurons. To test this, we characterized the firing activity of chemosensitive RTN neurons in slices from Slc32a1cre/+ control and Scn1aΔE26 mice during exposure to CO2 levels ranging from 3% to 10%. We initially identified chemosensitive RTN neurons in each genotype by their firing response to CO2. We considered neurons that are spontaneously active in 5% CO2 and responded to 10% CO2 with at least a 1.0 Hz increase in firing rate to be chemosensitive. Chemosensitive RTN neurons also have been shown to express the transcription factor Phox2b; therefore, at the end of each experiment, we filled all recorded cells with Lucifer yellow for later immunohistochemical confirmation of Phox2b expression. Chemosensitive RTN neurons in slices from Slc32a1cre/+ control mice had an average basal activity of 1.3 ± 0.4 Hz under control conditions (5% CO2; pH 7.3). These cells were strongly inhibited by decreasing CO2 to 3% (pHo = 7.6) (1.02 ± 0.3 Hz) and showed a linear firing increase in response to 7% (pHo = 7.2) (2.4 ± 0.5 Hz) and 10% CO2 (pHo = 7.0) (2.8 ± 0.4 Hz) (Figure 6A,B–C). This CO2 response profile is consistent with type I chemoreceptors (pH50 = 7.3), which were described previously in a Phox2b mouse reporter line (Lazarenko et al., 2009). Consistent with our hypothesis, chemosensitive RTN neurons in slices from Scn1aΔE26 mice were more active under control conditions (5% CO2) (2.4 ± 0.35 Hz) (Figure 6C) (T21 = 2.223, p<0.05) and showed an enhanced firing response to high CO2/H+ (Figure 6D and Figure 6—source data 1) (slope: 0.3 ± 0.01 Slc32a1cre/+ vs. 0.37 ± 0.01 Scn1aΔE26, F1,4 = 8.04, p<0.05). These results show that loss of Scn1a function in inhibitory neurons disrupts activity of RTN chemoreceptors.
Figure 6. Chemosensitive RTN neurons in slices from Scn1aΔE26 mice are hyper-excitable.
(A) firing rate traces from chemosensitive neurons in slices from control (top) and Scn1aΔE26 mice (bottom) show that neurons from both genotypes respond to changes in CO2/H+; RTN neurons are spontaneously active under control conditions (5% CO2; pHo 7.3) and respond to 7% CO2 (pHo 7.2) and 10% CO2 (pHo 7.0) with a linear increase in activity, whereas exposure to 3% CO2 (pHo 7.6) decreases neural activity. However, basal activity and CO2/H+-dependent output of RTN chemoreceptors from Scn1aΔE26 tissue is enhanced compared to RTN neurons in slices from Slc32a1cre/+control mice. (B) double-immunolabeling shows that a Lucifer Yellow-filled CO2/H+-sensitive RTN neuron (green) is immunoreactive for phox2b (magenta), the merged image is shown to the right. We confirmed that all CO2/H+-sensitive neurons (Slc32a1cre/+ control n = 12; Scn1aΔE26 n = 11) included in this study were phox2b-positive. (C–D) (Figure 6—source data 1), summary data shows that RTN chemoreceptors in slices from Scn1aΔE26 mice have higher basal activity (C) and enhanced CO2/H+-dependent output between 3–10% CO2 (D). Results were compared by t-test (C) or ANCOVA test (D). *, p<0.05.
Discussion
Epilepsy patients have a 20-fold higher mortality rate than the general population (Massey et al., 2014). The most common cause of death for this patient population is SUDEP, a leading cause of which is respiratory failure (Surges et al., 2009; Ryvlin et al., 2013; Kennedy and Seyal, 2015; Dlouhy et al., 2016). However, mechanisms underlying respiratory dysfunction in epilepsy and SUDEP are largely unknown. This is particularly true in the context of DS, where patients have an exceedingly high mortality rate and commonly exhibit life-threatening respiratory problems (Kim et al., 2018), yet little is known regarding how loss of Scn1a function impacts brainstem respiratory centers. The results presented here address this knowledge gap by showing that expression of the a DS-associated Scn1a variant A1783V in inhibitory neurons resulted in both spontaneous (Figure 2) and heat-induced seizures (Table 2) as well as pre-mature death (Figure 1E). Moreover, this mouse model presents with a respiratory phenotype reminiscent of that exhibited by DS patients (Figure 3). Perhaps not surprising, we found that loss of Scn1a function in inhibitory neurons in the RTN diminished activity in a cell-autonomous manner (Figures 4–5) but, importantly, also enhanced baseline activity and CO2/H+ sensitivity of glutamatergic chemosensitive neurons (Figure 6). These results suggest that disruption of Scn1a in inhibitory neurons can alter normal activity of brainstem respiratory centers and so may contribute to pathological features of DS including disordered breathing associated with SUDEP.
By ~2 weeks of age, Scn1aΔE26 mice show respiratory abnormalities characterized by hypoventilation, increased apneas and diminished ventilatory response to CO2/H+ (Figure 3). This phenotype is similar to that observed in DS patients (Kim et al., 2018). Furthermore, these breathing problems occurred in conjunction with a marked increase in mortality (Figure 1F and Figure 1—source data 1), thus correlatively supporting the possibility that respiratory dysfunction contributes to premature death in DS. Although mechanisms contributing to respiratory dysfunction in DS are unknown, previous work showed that loss of Scn1a from inhibitory neurons in the forebrain, but not the brainstem where respiratory control centers are located, resulted in premature death (Cheah et al., 2012). These results suggest respiratory dysfunction in DS is a secondary consequence of cortical seizure activity propagating to and disrupting brainstem function.
There are numerous direct and indirect projections from the cortex to brainstem respiratory centers (Shea, 1996) that may serve as the anatomical substrate for seizure-induced respiratory dysfunction. For example, recent work in humans showed that apnea and arterial oxygen desaturation occurred when cortical seizure activity spread to the amygdala (Dlouhy et al., 2015) and presumably activated descending inhibitory projections to brainstem respiratory centers. However, SUDEP can also occur in epilepsy patients in the absence of an overt seizure or outside the peri-ictal period (Lhatoo and Shorvon, 1998) and some epilepsy patients exhibit breathing problems including a suppressed ventilatory response to CO2 (Sainju et al., 2019), thus suggesting factors other than acute seizures disrupt respiratory control and predispose individuals to SUDEP. For example, it is possible that repeated bombardment of brainstem respiratory centers by frequent cortical seizure events alters cellular or neural network function, leading to progressive respiratory disruption and increased SUDEP propensity. Consistent with this possibility, patients with temporal lobe epilepsy (a common type of focal epilepsy) show widespread alterations in neural network activity including at the level of the brainstem (Englot et al., 2018). However, it remains unclear whether elements of respiratory control are compromised by repeated seizure activity in a similar manner.
Our results show that Scn1a transcript is highly expressed by brainstem inhibitory neurons and to a lesser extent by glutamatergic neurons (Figure 1A–C); therefore, loss of Scn1a function conceivably will also directly impact brainstem inhibitory neurons independent of descending seizure activity. Consistent with this possibility and analogous to cortical inhibitory neurons in Scn1a-/+ knockout models (Cheah et al., 2012) and induced pluripotent stem cells derived from DS patients with an Scn1a truncation mutation (Higurashi et al., 2013), we found that inhibitory neurons in the RTN region expressed the Scn1a A1783V pathological variant produced fewer action potentials in response to sustained depolarizing current injection and were more prone to depolarization block compared to inhibitory neurons from control mice (Figure 4A–D). These results suggest that loss of Scn1a might suppress inhibitory tone in brainstem respiratory centers including the RTN where inhibitory neurons appear to interact with and regulate the activity of excitatory chemosensitive neurons (Ott et al., 2011).
We confirmed this possibility at the cellular level by showing that baseline activity and CO2/H+-dependent output of RTN chemoreceptors in slices from Scn1aΔE26 mice was enhanced compared to RTN chemoreceptors in slices from Slc32a1cre/+ control mice, thus demonstrating that RTN chemoreceptor function is potentiated in this DS model. Although increasing RTN chemoreceptor drive is expected to increase baseline breathing and the ventilatory response to CO2, this response is dependent in inhibitory neurotransmission. For example, the frequency response elicited by photo-activation of RTN chemoreceptors in vitro was eliminated by systemically blocking inhibition with picrotoxin and strychnine (Cregg et al., 2017). Therefore, although RTN chemoreceptor function is perturbed in Scn1aΔE26 mice, it is likely that other respiratory elements also contribute to the observed hypoventilation phenotype. For example, evidence suggests serotonergic neurons in the dorsal and medullary raphe, which modulate breathing in response to changes in CO2 and arousal (Richerson, 2004; Buchanan and Richerson, 2010) are inhibited during and after seizures (Zhan et al., 2016). Furthermore, loss of serotonergic signaling has been shown to increase the likelihood of seizure-induced respiratory arrest in a mouse model of epilepsy, whereas administration of serotonin reuptake inhibitors does the opposite (Tupal and Faingold, 2019). Therefore, disruption of serotonergic signaling may contribute to breathing problems in Scn1aΔE26 mice. Another possibility worth noting is that loss of Scn1a function may compromise inspiratory rhythm generation by the pre-bötzinger complex. This is notable because loss of inhibitory tone within this region has been shown to decrease respiratory frequency (Del Negro et al., 2018; Baertsch et al., 2018) and the breathing phenotype in Scn1aΔE26 mice under high CO2 conditions preferentially involves diminished respiratory frequency but otherwise normal tidal volume (Figure 3F–H). There are certainly several other possible mechanisms by which loss of Scn1a may contribute to breathing problems in DS. Results presented here represent a first step towards understanding the cellular basis of disordered breathing in this disease.
Despite the prevalence of Scn1a missense mutations in DS (Parihar and Ganesh, 2013), few studies have characterized the pathophysiology associated with specific mutant alleles. This is particularly important for the development of patient-directed therapies because the aberrant products of Scn1a missense mutations are potentially expressed, thus representing a novel therapeutic target that is absent from haploinsufficient models of DS. Here, we show that expression of the Scn1a pathological variant A1783V in inhibitory neurons results in seizures and premature death on an accelerated time scale compared to haploinsufficient DS models (Catterall, 2012). Inhibitory neurons from Scn1aΔE26 mice showed a modest reduction in channel transcript (Figure 1C) that may contribute to loss of inhibitory tone (Figures 4–5); however, transcript containing the A1783V variant was expressed in tissue from Scn1aΔE26 mice (Figure 1A) and the repetitive firing characteristics of inhibitory neurons from Scn1aΔE26 mice is consistent with loss of function due to increased Nav1.1 channel inactivation. For example, genotype differences in the action potential amplitude and rate of depolarization were diminished under experimental conditions designed to remove Na+ channel inactivation. Therefore, an effective treatment for Scn1a A1783V-associated pathology might be to selectively potentiate Nav1.1 channel activity by slowing voltage-dependent inactivation. However, future experiments are required to test this possibility.
In sum, our results show that expression of Scn1a A1783V in inhibitory neurons results in clinical features of DS including spontaneous seizures and respiratory dysfunction. At the cellular level, brainstem inhibitory neurons in the RTN of slices from Scn1aΔE26 are less excitable whereas glutamatergic chemosensitive neurons are more excitable. Thus, our findings indicate that RTN chemoreceptors are a potential substrate for respiratory dysfunction in DS.
Materials and methods
Key resources table.
| Reagent type | Designation | Source or reference | Identifiers | Adtl. info |
|---|---|---|---|---|
| Strain, strain background (M. musculus, Scn1a A1783V, C57BL6/J background) |
B6(Cg)-Scn1atm1.1Dsf/J | Jackson Laboratories | RRID:IMSR_JAX:026133 | unpublished model |
| Strain, strain background (M. musculus, Vgat-iris-Cre, mixed 129/SvJ and C57BL6/J background) |
Slc32a1tm2(cre)Lowl/J | PMID: 21745644 | RRID:IMSR_JAX:016962 | |
| Strain, strain background (M. musculus, tdTomato reporter Ai14, C57BL6/J background) |
B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J | PMID: 20023653 | RRID:IMSR_JAX:007914 | |
| Genetic reagent (M-MLV Reverse Transcriptase (200 U/µL)) | MMLV RT first-strand reagent | ThermoFisher Scientific | 28025013 | |
| Genetic reagent (GoTaq Flexi DNA Polymerase) | Taq polymerase | Promega | M8291 | |
| Antibody | goat anti-Phox2b antibody | R and D Systems | AF4940; RRID:AB_10889846 | used on fixed tissue, 1:500 dilution |
| Antibody | rabbit anti-Lucifer Yellow antibody | Invitrogen | A-5750; RRID:AB_2536190 | used on fixed tissue, 1:2000 dilution |
| Sequence based reagent | RNAscope Probe- Mm-Scn1a | ACDBio | 434181 | 1:50 |
| Sequence based reagent | RNAscope Probe- Mm-Slc32a1-C2 | ACDBio | 319191-C2 | 1:50 |
| Sequence based reagent | RNAscope Probe- Mm-Slc17a6-C2 | ACDBio | 319171-C2 | 1:50 |
| Commercial assay or kit | RNAscope Fresh Frozen Multiplex Fluorescent Kit | ACDBio | 320851 | |
| Commercial assay or kit |
NEB PCR Cloning Kit | New England BioLabs | E1202S | |
| Commercial assay or kit | QIAprep Gel Extraction Kit | Qiagen | 28704 | |
| Commercial assay or kit | QIAprep Spin Miniprep Kit | Qiagen | 27104 | |
| Commercial assay or kit |
Direct-zol RNA MicroPrep | Zymo Research | R2061 | |
| Chemical compound, drug | Lucifer Yellow | Sigma | B4261 | 0.10% |
| Software, algorithm | SnapGene Viewer | SnapGene | RRID:SCR_015053 | |
| Software, algorithm | Ponemah | DSI | RRID:SCR_017107 | Version 5.20 |
| Software, algorithm | Spike | Cambridge Electronic Design |
RRID:SCR_000903 | Version 5.0 |
| Software, algorithm | Sirenia | Pinnacile Technology | RRID:SCR_016183 | |
| Software, algorithm | Matlab | Mathworks | RRID:SCR_001622 | Version R2018 |
| Software, algorithm | Brainstorm | Tadel et al., 2011 | RRID:SCR_001761 | Version 3.0 |
| Software, algorithm | Prism 7 | GraphPad | RRID:SCR_002798 | Version 7.03 |
| Software, algorithm | pCLAMP 10 | Molecular Devices | RRID:SCR_011323 | Version 10 |
| Software, algorithm | ImageJ | NIH | RRID:SCR_003070 | Version 2.0.0 |
Ethics statement
All experiments were performed according to the guidelines described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Connecticut, Storrs (Protocols A16-034 and A17-002).
Animals
Scn1aΔE26 mice were generated by crossing offspring of Slc32a1cre+/+ (RRID:IMSR_JAX:016962) and homozygous Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J reporter mice (Ai14; RRID:IMSR_JAX:007914) with heterozygous Scn1aA1783Vfl/+ mice (RRID:IMSR_JAX:026133) to introduce the Scn1a variant A1783V conditionally in inhibitory neurons. Experimental animals heterologously express both the reporter and the Scn1a A1783V pathological variant (Scn1aΔE26 mice) and litter mate controls and litter mate controls used for experiments were Vgacre-/-::Tdt+/-:: Scn1aA1783Vfl/+ and Slc32a1cre+/-::Tdt+/-::Scn1a+/+ (on a common background of 90% C57BL/6J and 10% 129/SvJ). The proportion of each background stain was determined by Genome Scan Analysis performed by the Jackson Laboratory. Aged matched mice of each genotype and sex were used for all experiments included in this study.
PCR and sequencing
Somatosensory cortex and brainstem tissue was isolated from pups of each genotype (Scn1aΔE26, Scn1afl/+, and Slc32a1cre/+) and triturated to make a single cell suspension for RNA isolation using the Zymo RNA Microprep kit (Zymo, Cat # R2061). The RNA was converted to cDNA using the MMLV RT first-strand reagent (ThermoFisher, Cat. # 28025013). The cDNA was then amplified using Scn1a gene primers (both 5’ to 3’, exon 25 forward: GCATTATGTGACAAGCATTTTGTCACGC, exon 26 reverse: GCGCTCTAGAACCCCCTCTCATTTGCCAC) in a 24.5 uL reaction volume per sample containing: 5 uL of 5X buffer with loading dye (Promega M891A), 4 uL of MgCl2 (25 mM), 1 uL of dNTPs (10 mM), 12.3 uL of DEPC H2O, 0.2 uL of Taq polymerase (Promega M8291), 1 uL of each primer (30 pM/uL) and 0.5 uL of cDNA. The cycling protocol was 95°C for 2 min, 95°C for 30 s/58°C for 1 min/72°C for 1 min (repeated for 30 cycles total), 72°C for 5 min, 12°C hold. The PCR product was run on a 1.5% EtBr gel at 90 mV for 30 min.
The amplified 831 bp product was excised and placed into a new DNase/RNase free micro-centrifuge tube. To extract the amplified cDNA we used the Qiagen Gel Extraction Kit (Cat 28704). The purified sequence was inserted into the linearized pMiniT 2.0 vector using the NEB PCR cloning kit and NEB 10-beta competent cells (Cat E1202S). The resulting transformed competent cells were then streaked out onto LB agar plates with ampicillin and grown out overnight at 37°C. Single colonies were selected and placed into 2 mL of LB broth with ampicillin in a 5 mL polystyrene round bottom tube and grown out for 16 hr/overnight, shaken at 250 rpm at 37°C. 1.5 mL of each bacterial sample was used for plasmid purification using the Qiagen Spin Miniprep Kit (Cat 27104) according to manufacture instructions. Samples were then sequenced at Eurofins Genomics using the forward or reverse primers for the pMiniT 2.0 vector provided in the NEB PCR cloning kit. Once sequences were returned from Eurofins Genomics, the SnapGene Viewer (RRID:SCR_015053) was used to map the sequence to the pMini.T 2.0 vector map; the sequence was then searched in NCBI BLAST to yield similar sequences from the mouse genome. All samples mapped back to the Scn1a gene, between exon 25 and 26. After mapping the inserted sequence to Scn1a, the A to V single nucleotide mutation was localized and identified at nucleotide 1772 mapped to the mouse genome. In total, a minimum of 15 samples were collected from each genotype for sequencing.
Fluorescent in situ hybridization (FISH)
To prepare fresh frozen slice, postnatal week 2 mice of both genotypes were anesthetized with isoflurane, decapitated, and brainstem tissues were rapidly frozen with dry ice and embedded with OCT compound. Brainstem slices (14 um thick) containing the RTN were crysectioned and collected onto SuperFrost Plus microscope slides. Slices were fixed with 4% paraformaldehyde and dehydrated with 50%,70% and 100% ethanol. FISH was processed with the instruction of RNAscope Multiplex Fluorescent Assay (ACD, 320850), the probes used in our study were designed and validated by ACD (Table 3). Confocal images of FISH experiments were obtained using a Leica TSC Sp8 and confocal image files containing image stacks were loaded into ImageJ (version 2.0.0, NIH, RRID:SCR_003070).
Table 3. Probes used for FISH.
| Gene name | Probe cat no. | Target region |
|---|---|---|
| Scn1a | 434181 | 1624–2967 |
| Slc32a1 | 319191-C2 | 894–2037 |
| Slc17a6 | 319171-C2 | 1986–2998 |
Unrestrained whole-body plethysmography
Respiratory activity was measured using a whole-body plethysmograph system (Data Scientific International; DSI), utilizing animal chamber (600 mL volume) maintained at room temperature and ventilated with air (1.3 L/min) using a small animal bias flow generator. Fifteen day old mice (~7 g) were individually placed into a chamber and allowed 2 hr to acclimate prior to the start of an experiment. Respiratory activity was recorded using Ponemah 5.20 software (DSI) for a period of 15 min in room air followed by exposure to graded increases in CO2 from 0% to 7% CO2 (balance O2). Body temperature was measured before and after each experiment and although body temperature tended to drop ~1 °C by the end of an experiment, there were no genotype difference in the degree of cooling (p=0.37). Parameters of interests include respiratory frequency (FR, breaths per minute), tidal volume (VT, measured in mL; normalized to body weight and corrected to account for chamber and animal temperature, humidity, and atmospheric pressure), and minute ventilation (VE, mL/min/g). A 20 s period of relative quiescence after 2 min of exposure to each condition was selected for analysis. Spontaneous apneic events, conservatively defined as three or more missed breaths not preceded by a sigh or augmented breath, were analyzed off-line. All experiments were performed between 9 a.m. and 6 p.m. to minimize potential circadian effects.
Acute slice preparation and in vitro electrophysiology
Slices containing the RTN were prepared as previously described (Mulkey et al., 2007). In short, rats were anesthetized by administration of ketamine (375 mg/kg, I.P.) and xylazine (25 mg/kg; I.P.) and rapidly decapitated; brainstems were removed and transverse brain stem slices (300 μm) were cut using a microslicer (DSK 1500E; Dosaka) in ice-cold substituted Ringer solution containing the following (in mM): 260 sucrose, 3 KCl, 5 MgCl2, 1 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, and one kynurenic acid. Slices were incubated for 30 min at 37°C and subsequently at room temperature in a normal Ringer’s solution containing (in mM): 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose. Both substituted and normal Ringer’s solutions were bubbled with 95% O2 and 5% CO2 (pH = 7.30).
Individual slices containing the RTN were transferred to a recording chamber mounted on a fixed-stage microscope (Olympus BX5.1WI) and perfused continuously (~2 ml/min) with a bath solution containing (in mM): 140 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, 10 glucose (equilibrated with 5% CO2; pH = 7.3). All recordings were made with an Axopatch 200B patch-clamp amplifier, digitized with a Digidata 1322A A/D converter, and recorded using pCLAMP 10.0 software (RRID:SCR_011323). Recordings were obtained at room temperature (~22° C) with patch electrodes pulled from borosilicate glass capillaries (Harvard Apparatus, Molliston, MA) on a two-stage puller (P-97; Sutter Instrument, Novato, CA) to a DC resistance of 5–7 MΩ when filled with a pipette solution containing the following (in mM): 125 K-gluconate, 10 HEPES, 4 Mg-ATP, 3 Na-GTP, 1 EGTA, 10 Na-phosphocreatine (uM), 0.2% Lucifer yellow (pH 7.30). Electrode tips were coated with Sylgard 184 (Dow Corning, Midland, MI).
The firing response of chemosensitive RTN neurons to CO2(3–10% CO2) was assessed in the cell-attached voltage-clamp configuration (seal resistance >1 GΩ) with holding potential matched to the resting membrane potential (Vhold = −60 mV) and with no current generated by the amplifier (Iamp = 0 pA). Firing rate histograms were generated by integrating action potential discharge in 10 to 20 s bins using Spike 5.0 software (RRID:SCR_000903). We confirmed that all chemosensitive RTN neurons included in this study were immunoreactive for the transcription factor Phox2b.
To characterize action potential properties and repetitive firing behavior of inhibitory neurons, we made whole-cell current-clamp recordings from fluorescently labeled neurons located in the region of the RTN in slices from Vgat::TdTomato mice. Repetitive firing responses to 1 s depolarizing current steps from 0 to 300 pA (Δ 20 pA increments) were characterized from an initial holding potential of −80 mV. Action potential amplitude, threshold (dV/dT > 10 mV/mS) and the maximum rate of depolarization obtained from the peak of the first time derivative of the action potential were characterized for spontaneous spikes measured under resting conditions (holding current = 0 pA) and for the first spike elicited after delivering a positive (+200 pA) or negative (−100 pA) 1 s current step. All whole-cell recordings had an access resistance (Ra) <20 MΩ, recordings were discarded if Ra varied >10% during an experiment. A liquid junction potential of −14 mV was accounted for during each experiment.
Immunofluorescence staining
Slices were fixed in 4% PFA over night after recording, and blocked with 5% normal horse serum in 1X PBS with 2.5% triton for 1 hr. Slices were incubated in goat anti-phox2b antibody (RRID:AB_10889846) and rabbit anti-lucifer yellow antibodies (RRID:AB_2536190) mixed in blocking solution under 4°C overnight. After washing the primary antibody a secondary antibody was applied for 2 hr followed by an additional wash and mounting with ProLong Gold Antifade Reagent (Invitrogen, P36934). Slices were imaged using a Leica SP8 confocal microscope (40x/1.3 HC oil objective) to identify cells that co-express Alex Fluor 647 for phox2b and Alex Fluor 488 for lucifer yellow.
Electrocorticography recording
Subdural EcoG electrodes were implanted in 15 day old Slc32a1cre/+ and Scn1aΔE26 mouse pups. To minimize damage we used stainless steel wire electrodes (diameter = 0.003 in) (A-M system, 790900) inserted just under the skull for a length of 2 mm into each hemisphere near the fontal cortex. A reference wire electrode was placed in the posterior cortex. Each electrode was connected to a Mill-MAX miniature socket (digikey, ED11265-ND) and secured to the skull with super glue. Differential voltage signals were amplified 1000 × with a DAM-50 differential amplifier (1 Hz low filter, 10 kHz high filter), digitized at 5 kHz and recorded using Sirenia Software (RRID:SCR_016183).
Mice were allowed to 12 hr to recover from surgery before recording EcoG activity for a period of 2 hr. We also video recorded all experiments to correlate animal behavior with EcoG recordings. Only spike wave discharge (SWD) activity that occurred in conjunction with observable seizure behavior was included in the analysis. Any data including movement artifacts were excluded from analysis. The same criteria for seizure events were used for both mutant mice and control group. The full duration of each seizure event was segmented and then down sampled from 600 Hz to 100 Hz to focus on the frequency range of interest (0–50 Hz) prior to performing the power spectral analysis in Matlab (RRID:SCR_001622). Frequency ranges of EcoG signals are defined as follows: delta, δ (1–5 Hz), theta, Θ (6–8 Hz), alpha, α (9–16 Hz), beta, β (17–36 Hz), and gamma, γ (37–50 Hz). Frequency analysis results were normalized to the maximum frequency amplitude at each event. For each frequency range, maximal amplitude and area under each frequency range were calculated to report the spectral power. To show the time-varying frequency distribution, time frequency analysis using Hilbert and Morlet transformations were also performed using Brainstorm 3.0 (Tadel et al., 2011, RRID:SCR_001761).
Seizure behavior scoring
We video monitored mice for 1 hr after placing them individually in a cage and giving them access to food and water ad lib. Seizure behavior during this time was evaluated using the Racine scoring system as follows: score 1, mouth and facial movements; score 2, head nodding; score 3, forelimb clonus; score 4, rearing with limb clonus; score 5, full body clonus, rearing and falling.
Thermal seizure induction
To record febrile seizures mice were placed in a Plexiglas cylindrical chamber and we continuously monitor animal body temperature using a Type T thermocouple rectal probe connected to a feedback temperature controller and a heating lamp (Physitemp) that was positioned directly above the chamber. This system allowed us to maintain body temperature to within ±0.4°C of command temperature. Mice were held at 37°C for 10 min before body temperature was increased in 0.5°C increments every 2 min until a tonic-clonic seizure occurs or 42°C is reached. All experiments were video recorded for later conformation of seizure behavior.
Statistical analysis
Data are reported as mean ± SE. All experiments were performed blind to genotype and all statistical analysis was performed using Prism 7 (RRID:SCR_002798). Power analysis was used to determine sample size, all data sets were tested for normality using Shapiro-Wilk test, and comparisons were made using t-test, Chi Square test, Fisher’s exact test, one-way or two-way ANOVA followed by multiple comparison tests as appropriate. The specific test used for each comparison is reported in the figure legend and all relevant values used for statistical analysis are included in the results section.
Acknowledgements
We thank Drs. Ana Mingorance (Chief Development Officer of the Loulou Foundation), Rahul Kanadia and Anastasios Tzingounis (Dept. Physiology and Neurobiology, Univ. Connecticut) for their constructive suggestions regarding this project. This work was supported by funds from the National Institutes of Health Grants HL104101 (DKM), HL137094 (DKM) NS104999 (JLL) and F31HL142227 (CMC). Additional funds were also provided by the Dravet syndrome Foundation Grant AG180243 (DKM) and American Epilepsy Society (F-SK).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Daniel K Mulkey, Email: daniel.mulkey@uconn.edu.
Jan-Marino Ramirez, Seattle Children's Research Institute and University of Washington, United States.
Ronald L Calabrese, Emory University, United States.
Funding Information
This paper was supported by the following grants:
National Institutes of Health HL104101 to Daniel K Mulkey.
Dravet Syndrome Foundation AG180243 to Daniel K Mulkey.
American Epilepsy Society to Fu-Shan Kuo.
National Institutes of Health NS104999 to Joseph J LoTurco.
National Institutes of Health F31HL142227 to Colin M Cleary.
National Institutes of Health HL137094 to Daniel K Mulkey.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Writing—original draft, Project administration, Writing—review and editing.
Data curation, Formal analysis, Funding acquisition, Writing—review and editing.
Supervision, Methodology, Writing—review and editing.
Formal analysis, Methodology, Writing—review and editing.
Conceptualization, Supervision, Funding acquisition, Writing—original draft, Project administration, Writing—review and editing.
Ethics
Animal experimentation: All animal use was in accordance with guidelines approved by the University of Connecticut Institutional Animal Care and Use Committee. (Protocols A16-034 and A17-002).
Additional files
Data availability
We have included source data files for all summary figures that do not include individual data points.
References
- Aiba I, Noebels JL. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Science Translational Medicine. 2015;7:282ra46. doi: 10.1126/scitranslmed.aaa4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama M, Kobayashi K, Ohtsuka Y. Dravet syndrome: a genetic epileptic disorder. Acta Medica Okayama. 2012;66:369–376. doi: 10.18926/AMO/48961. [DOI] [PubMed] [Google Scholar]
- Baertsch NA, Baertsch HC, Ramirez JM. The interdependence of excitation and inhibition for the control of dynamic breathing rhythms. Nature Communications. 2018;9:843. doi: 10.1038/s41467-018-03223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AC, Morse RP, Scott RC, Holmes GL, Lenck-Santini PP. SCN1A mutations in dravet syndrome: impact of interneuron dysfunction on neural networks and cognitive outcome. Epilepsy & Behavior. 2012;23:177–186. doi: 10.1016/j.yebeh.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. PNAS. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/S0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Kalume F, Oakley JC. NaV1.1 channels and epilepsy. The Journal of Physiology. 2010;588:1849–1859. doi: 10.1113/jphysiol.2010.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Sodium Channel Mutations and Epilepsy. In: Bethesda M. D, Noebels J. L, Avoli M, Rogawski M. A, Olsen R. W, Delgrado-Escueta A. V, editors. Jasper's Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information; 2012. [DOI] [PubMed] [Google Scholar]
- Cheah CS, Yu FH, Westenbroek RE, Kalume FK, Oakley JC, Potter GB, Rubenstein JL, Catterall WA. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. PNAS. 2012;109:14646–14651. doi: 10.1073/pnas.1211591109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, Chu KA, Dick TE, Landmesser LT, Silver J. Phasic inhibition as a mechanism for generation of rapid respiratory rhythms. PNAS. 2017;114:12815–12820. doi: 10.1073/pnas.1711536114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nature Reviews Neuroscience. 2018;19:351–367. doi: 10.1038/s41583-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depienne C, Trouillard O, Saint-Martin C, Gourfinkel-An I, Bouteiller D, Carpentier W, Keren B, Abert B, Gautier A, Baulac S, Arzimanoglou A, Cazeneuve C, Nabbout R, LeGuern E. Spectrum of SCN1A gene mutations associated with dravet syndrome: analysis of 333 patients. Journal of Medical Genetics. 2009;46:183–191. doi: 10.1136/jmg.2008.062323. [DOI] [PubMed] [Google Scholar]
- Dlouhy BJ, Gehlbach BK, Kreple CJ, Kawasaki H, Oya H, Buzza C, Granner MA, Welsh MJ, Howard MA, Wemmie JA, Richerson GB. Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. Journal of Neuroscience. 2015;35:10281–10289. doi: 10.1523/JNEUROSCI.0888-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlouhy BJ, Gehlbach BK, Richerson GB. Sudden unexpected death in epilepsy: basic mechanisms and clinical implications for prevention. Journal of Neurology, Neurosurgery & Psychiatry. 2016;87:402–413. doi: 10.1136/jnnp-2013-307442. [DOI] [PubMed] [Google Scholar]
- Dutton SB, Makinson CD, Papale LA, Shankar A, Balakrishnan B, Nakazawa K, Escayg A. Preferential inactivation of Scn1a in parvalbumin interneurons increases seizure susceptibility. Neurobiology of Disease. 2013;49:211–220. doi: 10.1016/j.nbd.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Gonzalez HFJ, Reynolds BB, Konrad PE, Jacobs ML, Gore JC, Landman BA, Morgan VL. Relating structural and functional brainstem connectivity to disease measures in epilepsy. Neurology. 2018;91:e67–e77. doi: 10.1212/WNL.0000000000005733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T. Clinical spectrum of mutations in SCN1A gene: severe myoclonic epilepsy in infancy and related epilepsies. Epilepsy Research. 2006;70:223–230. doi: 10.1016/j.eplepsyres.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Gataullina S, Dulac O. From genotype to phenotype in dravet disease. Seizure. 2017;44:58–64. doi: 10.1016/j.seizure.2016.10.014. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron. 2015;87:946–961. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich UB, Liautard C, Kirschenbaum D, Pofahl M, Lavigne J, Liu Y, Theiss S, Slotta J, Escayg A, Dihné M, Beck H, Mantegazza M, Lerche H. Impaired action potential initiation in GABAergic interneurons causes hyperexcitable networks in an epileptic mouse model carrying a human na(V)1.1 mutation. Journal of Neuroscience. 2014;34:14874–14889. doi: 10.1523/JNEUROSCI.0721-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higurashi N, Uchida T, Lossin C, Misumi Y, Okada Y, Akamatsu W, Imaizumi Y, Zhang B, Nabeshima K, Mori MX, Katsurabayashi S, Shirasaka Y, Okano H, Hirose S. A human dravet syndrome model from patient induced pluripotent stem cells. Molecular Brain. 2013;6:19. doi: 10.1186/1756-6606-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F. Sudden unexpected death in dravet syndrome: respiratory and other physiological dysfunctions. Respiratory Physiology & Neurobiology. 2013;189:324–328. doi: 10.1016/j.resp.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Westenbroek RE, Cheah CS, Yu FH, Oakley JC, Scheuer T, Catterall WA. Sudden unexpected death in a mouse model of dravet syndrome. Journal of Clinical Investigation. 2013;123:1798–1808. doi: 10.1172/JCI66220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. Sudden unexpected death in dravet syndrome. Epilepsy Currents. 2013;13:264–265. doi: 10.5698/1535-7597-13.6.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy JD, Seyal M. Respiratory pathophysiology with seizures and implications for sudden unexpected death in epilepsy. Journal of Clinical Neurophysiology. 2015;32:10–13. doi: 10.1097/WNP.0000000000000142. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bravo E, Thirnbeck CK, Smith-Mellecker LA, Kim SH, Gehlbach BK, Laux LC, Zhou X, Nordli DR, Richerson GB. Severe peri-ictal respiratory dysfunction is common in dravet syndrome. Journal of Clinical Investigation. 2018;128:1141–1153. doi: 10.1172/JCI94999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen TL, Bomben VC, Patel A, Drabek J, Chen TT, Gu W, Zhang F, Chapman K, Lupski JR, Noebels JL, Goldman AM. High-resolution molecular genomic autopsy reveals complex sudden unexpected death in epilepsy risk profile. Epilepsia. 2014;55:e6–e12. doi: 10.1111/epi.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacuey N, Zonjy B, Londono L, Lhatoo SD. Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology. 2017;88:701–705. doi: 10.1212/WNL.0000000000003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. The Journal of Comparative Neurology. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letts VA, Beyer BJ, Frankel WN. Hidden in plain sight: spike-wave discharges in mouse inbred strains. Genes, Brain and Behavior. 2014;13:519–526. doi: 10.1111/gbb.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhatoo SD, Shorvon SD. Relevance of the first seizure. The Lancet. 1998;352:1003–1004. doi: 10.1016/S0140-6736(05)60070-0. [DOI] [PubMed] [Google Scholar]
- Lossin C. A catalog of SCN1A variants. Brain and Development. 2009;31:114–130. doi: 10.1016/j.braindev.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Marini C, Mei D, Temudo T, Ferrari AR, Buti D, Dravet C, Dias AI, Moreira A, Calado E, Seri S, Neville B, Narbona J, Reid E, Michelucci R, Sicca F, Cross HJ, Guerrini R. Idiopathic epilepsies with seizures precipitated by fever and SCN1A abnormalities. Epilepsia. 2007;48:1678–1685. doi: 10.1111/j.1528-1167.2007.01122.x. [DOI] [PubMed] [Google Scholar]
- Mashimo T, Ohmori I, Ouchida M, Ohno Y, Tsurumi T, Miki T, Wakamori M, Ishihara S, Yoshida T, Takizawa A, Kato M, Hirabayashi M, Sasa M, Mori Y, Serikawa T. A missense mutation of the gene encoding voltage-dependent sodium channel (Nav1.1) confers susceptibility to febrile seizures in rats. Journal of Neuroscience. 2010;30:5744–5753. doi: 10.1523/JNEUROSCI.3360-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nature Reviews Neurology. 2014;10:271–282. doi: 10.1038/nrneurol.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler MH, Kearney JA. Sodium channel mutations in epilepsy and other neurological disorders. Journal of Clinical Investigation. 2005;115:2010–2017. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AR, Hawkins NA, McCollom CE, Kearney JA. Mapping genetic modifiers of survival in a mouse model of dravet syndrome. Genes, Brain and Behavior. 2014;13:163–172. doi: 10.1111/gbb.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. Journal of Neuroscience. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. PNAS. 2009;106:3994–3999. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, Furuichi T, Hensch TK, Yamakawa K. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. Journal of Neuroscience. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott MM, Nuding SC, Segers LS, Lindsey BG, Morris KF. Ventrolateral medullary functional connectivity and the respiratory and central chemoreceptor-evoked modulation of retrotrapezoid-parafacial neurons. Journal of Neurophysiology. 2011;105:2960–2975. doi: 10.1152/jn.00262.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar R, Ganesh S. The SCN1A gene variants and epileptic encephalopathies. Journal of Human Genetics. 2013;58:573–580. doi: 10.1038/jhg.2013.77. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nature Reviews Neuroscience. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Høgenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. The Lancet Neurology. 2013;12:966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- Sainju RK, Dragon DN, Winnike HB, Nashelsky MB, Granner MA, Gehlbach BK, Richerson GB. Ventilatory response to CO2 in patients with epilepsy. Epilepsia. 2019;60:508–517. doi: 10.1111/epi.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SA. Behavioural and arousal-related influences on breathing in humans. Experimental Physiology. 1996;81:1–26. doi: 10.1113/expphysiol.1996.sp003911. [DOI] [PubMed] [Google Scholar]
- Shmuely S, Sisodiya SM, Gunning WB, Sander JW, Thijs RD. Mortality in dravet syndrome: a review. Epilepsy & Behavior. 2016;64:69–74. doi: 10.1016/j.yebeh.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Surges R, Thijs RD, Tan HL, Sander JW. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nature Reviews Neurology. 2009;5:492–504. doi: 10.1038/nrneurol.2009.118. [DOI] [PubMed] [Google Scholar]
- Tadel F, Baillet S, Mosher JC, Pantazis D, Leahy RM. Brainstorm: a user-friendly application for MEG/EEG analysis. Computational Intelligence and Neuroscience. 2011;2011:1–13. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai C, Abe Y, Westenbroek RE, Scheuer T, Catterall WA. Impaired excitability of somatostatin- and parvalbumin-expressing cortical interneurons in a mouse model of dravet syndrome. PNAS. 2014;111:E3139–E3148. doi: 10.1073/pnas.1411131111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupal S, Faingold CL. Fenfluramine, a serotonin-releasing drug, prevents seizure-induced respiratory arrest and is anticonvulsant in the DBA/1 mouse model of SUDEP. Epilepsia. 2019;60:485–494. doi: 10.1111/epi.14658. [DOI] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nature Neuroscience. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- Zhan Q, Buchanan GF, Motelow JE, Andrews J, Vitkovskiy P, Chen WC, Serout F, Gummadavelli A, Kundishora A, Furman M, Li W, Bo X, Richerson GB, Blumenfeld H. Impaired serotonergic brainstem function during and after seizures. The Journal of Neuroscience. 2016;36:2711–2722. doi: 10.1523/JNEUROSCI.4331-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]