Abstract
Objective:
To describe engagement along the HIV continuum of care using a large network of clinics in southern Africa.
Methods:
We employed a practical framework to describe retention along the HIV treatment cascade, using routinely collected clinical data available in resource-constrained settings. We included health facilities in four Zambian provinces with more than 300 enrolled patients over the age of 5 years. We described attrition at each step, from HIV enrollment to 720 days after ART initiation. The population was further stratified by year of enrollment to describe temporal trends in patient engagement.
Results:
From January 2004 to December 2014, 444,439 individuals over the age of 5 years sought HIV care at 75 eligible health facilities. Among those enrolled into HIV care, 82.1% (95% confidence interval [CI]: 79.4–84.5%) were fully assessed for ART eligibility within 180 days of enrollment and 63.6% (95%CI: 61.7–65.3) were found to be eligible for ART based on the HIV treatment guidelines at the time. Of those patients eligible for ART, 81.1% (95%CI: 79.5–82.7%) initiated ART within 180 days. Patient retention in ART program was 81.2% (95%CI: 80.4–81.9%) at 90 days, 70.0% (95%CI: 68.7–71.2%) at 360 days, and 61.6% (95%CI: 60.0–63.2%) at 720 days. We noted a steady decline in proportions assessed for ART eligibility and deemed eligible for ART in the timeframe. Proportions that started ART and remained in care remained relatively consistent.
Conclusion:
We describe a simple approach for assessing patient engagement after enrollment into HIV care. Using limited types of data routinely available, we demonstrate an important and replicable approach to monitoring programs in resource-constrained settings.
Keywords: HIV, treatment, cascade, continuum of care, Zambia, Africa
Introduction
Antiretroviral therapy (ART) has become available to an increasingly large number of patients in resource-constrained settings. In 2014, the Joint United Nations Programme on HIV/AIDS reported that as many as 7.5 million people in sub-Saharan Africa had initiated ART,1 a 50-fold increase over the past decade. Access to HIV treatment, however, must be accompanied by long-term retention in care if the expected public health gains of ART are to be fully realized. Interruptions in HIV care may lead to antiretroviral drug resistance, horizontal transmission, and disease progression. To minimize such adverse outcomes, HIV-infected individuals must enroll in a health facility, initiate ART in a timely fashion, and adhere to long-term treatment.2–4 Routine monitoring of such outcomes is the first step to optimizing individual and health systems level interventions to enhance program outcomes.5,6
The “HIV treatment cascade” is a conceptual framework that systematically characterizes patient engagement along the HIV care continuum. Developed initially to describe HIV services in the U.S.,7,8 the concept of the cascade has gained prominence in the global HIV literature and has been used to evaluate program performance in various settings. Many studies have examined individual steps along this cascade in the high HIV prevalence, resource-constrained settings where ART expansion has been greatest.9,10 Missing from the current literature, however, is a practical approach to the HIV treatment cascade for resource-constrained settings, where the types of available data are limited compared to U.S. and other developed countries.11,12 In this report, we propose such a construct, using programmatic data from a large HIV care and treatment program in Zambia.
Methods
Clinical Care
In this analysis, we describe the HIV treatment cascade among patients seeking care in the Lusaka, Southern, Eastern, and Western Provinces of Zambia from 2004 to 2014. We have previously described the clinical care provided at these public health facilities.13,14 HIV treatment services are guided by national guidelines set forth by the Zambian Ministry of Health, which closely reflect recommendations made by the World Health Organization (WHO). As such, eligibility criteria for initiating ART have evolved over time. In 2004, for example, ART eligibility was reserved for HIV-infected individuals with CD4 cell counts < 200 cells/mm3, with CD4 cell counts < 350 cells/mm3 and WHO stage 3, or with WHO stage 4 disease. Starting in January 2011, the eligibility criteria were updated so that all HIV-infected individuals with CD4 cell counts less than 350 cells/mm3 or with WHO stage 3 or stage 4 were to initiate ART. Since April 2014, new policies from the Zambia Ministry have further raised the CD4 threshold for ART eligibility to 500 cells/mm3. We have observed a similar evolution in first-line ART regimens. At present, HIV-infected adults initiating ART receive a combination regimen of tenofovir, lamivudine or emtricitabine, and efavirenz. Protease inhibitors are reserved for second-line regimens and rarely used at time of ART initiation. Treatment response has been primarily monitored by clinical and immunological status, though virological testing is available in certain settings and based on specific circumstances.15 HIV care for much of the country is provided by mid-level clinical practitioners, with complicated cases referred to the few advanced treatment centers nationwide.
HIV Treatment Cascade
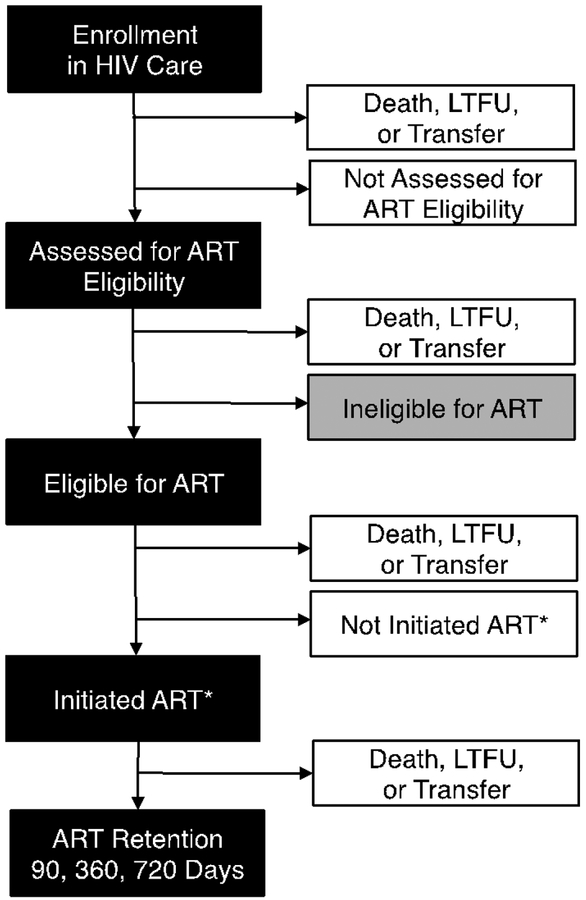
The HIV treatment cascade helps monitor patient outcomes and overall program performance.7,8,16 For a number of reasons (e.g., anonymous and unlinked HIV testing, poor access to viral load monitoring), we modified the prevalent treatment cascade framework for HIV clinical care in Zambia. Specifically, we used available programmatic data to show the following steps: (a) enrolling in HIV services, (b) being assessed for ART eligibility, (c) being eligible for ART, (d) initiating ART, (e) alive and remaining in care for 90 or more days, (f) alive and remaining in care for 360 or more days, (g) alive and remaining in care for 720 or more days. The first two steps (steps a-b) apply to all HIV-infected individuals seeking care at government-approved ART programs. The remaining five steps of this cascade (steps c-g) focus only on ART-eligible patients. Noting that the days in ART program have been described by applying thresholds (90, 360, and 720), further research on critical thresholds for long-term retention may provide evidence for alternative divisions. Nonetheless, our choices provide diverse look at short- to long-term retention. Because virologic monitoring is only available on a very limited basis, we used patient retention at approximately two years (i.e., 720 days) as our primary indicator of long-term program performance. These steps are shown in greater detail in Figure 1.
Figure 1. Diagram of HIV treatment cascade.
We defined steps along the HIV treatment cascade based on data available in the Zambian context. ART = antiretroviral therapy. *Initiation of ART within 180 days of eligibility screening.
Data Collection and Statistical Analysis
To construct the HIV treatment cascade, we included data from ART program sites across four Zambian provinces. These longitudinal, individual-level data were originally collected in the SmartCare electronic medical record supported by the Zambian Ministry of Health,17 but de-identified and exported for analysis. All clinics that are electronically tracked in the SmareCare database are considered in this analysis. We considered HIV-infected children and adolescents (6 to 16 years) and adults (17 or older). Note that we analyzed these two groups separately and altogether. Because of their different ART eligibility criteria, those under five years of age were excluded. To be included in this analysis, a facility had to have at least 300 HIV-infected patients (>5 years old at enrollment) by the data freeze date; this was deemed the minimum number required to have reasonable site-level precision. At the same time, this criteria eventually removes 102 patients (0.022% of total), that do not influence our overall estimates.
ART eligibility was based on the prevailing guidelines at the time of enrollment into HIV care. Severity of HIV disease was assessed by CD4 counts and WHO clinical stage around the time of ART eligibility assessment. When a record of CD4 count did not exist on the day of staging, a nearest record of CD4 count, within a window of 30 days before and after the day of staging, was used to assess eligibility. Assessment of ART eligibility was defined as having such records within 180 days of enrollment. In cases where data about CD4 or WHO clinical stage was missing – but the health provider initiated ART – we deferred to the clinician’s judgment and discretion. Again, initiation of ART had to occur within 180 days of eligibility determination in order to be considered. We recognize that these 180 days represent a broad interval for capturing ART eligibility and initiation. However, due to the linear nature of our continuum framework – where individuals classified as lost to follow-up cannot “re-enter” the cascade – we adopted this more inclusive threshold to minimize misclassification.
When calculating the percentage of patients retained at each step along the treatment cascade, we ensured that all patients included in the denominator were active 180 days after the threshold date. Using the same approach, the ART program retention at t = 90, 360, or 720 days was calculated using patients who started ART early enough (data freeze date – t) and had 180 days to make the follow-up appointment (additional 180 days). For example, given our data freeze date of December 31, 2014, the percentages of patients who remained in ART program at 90, 360, and 720 days were calculated among patients who enrolled prior to April 5, 2014, July 9, 2013, and July 14, 2012, respectively. We considered clinical, laboratory, and pharmacy visits in determining program retention.
Attrition at each step of the cascade could be due to a number of reasons, including death, transfer to different health facility, withdrawal from care, or loss to follow-up (LTFU). Because of the inherent limitations to these data, we were not able to delineate the separate causes of attrition, as has been proposed by others.19 According to the national guidelines, facility staff should contact patients who are over 60 days late for a missed appointment,20 but in reality local practices varied greatly. Given the breadth and scope of this analysis, we were unable to collect more detailed information about contact tracing across different sites and over different time periods.
To characterize patient engagement over time, the patient population from all facilities was stratified into “annual cohorts” based on individual patients’ enrollment dates. We grouped patients who had enrolled in HIV care during a given calendar year and followed each annual cohort onwards for each step of the cascade. Multiple annual cohorts were visualized over time and compared to reveal temporal trends of program performance. We included a pooled estimate for each cascade step, as well as site-level estimates to illustrate variation from facility to facility. We also conducted stratified analysis based on sex, geographic location (i.e., provinces), and CD4 count at enrollment. 95% confidence intervals (CI) for each point estimate were calculated using a generalized linear model with a logit link function that accounted for clustered sampling at the health facility level. To minimize uncertainty in our facility estimates, we include only those sites with at least 30 new enrollments for a given calendar year. Statistical analyses were conducted using the R statistical programming language (Version 3.1.1, The R Foundation for Statistical Computing, Vienna, Austria).
Ethical Approvals
These data are collected for routine medical care as part of the SmartCare electronic medical record; as such, individual patient informed consent was not required. Analysis of de-identified program data was approved by the University of Zambia Biomedical Research Ethics Committee (Lusaka, Zambia) the University of North Carolina Institutional Review Board (Chapel Hill, NC, USA), and the Zambian Ministry of Health (Lusaka, Zambia).
Results
From January 1, 2004 to December 31, 2014, a total of 444,541 individuals over the age of five years sought HIV care and treatment across 78 health facilities in Lusaka, Southern, Eastern, and Western Provinces. Of these, 444,439 (>99%) patients received care in the 75 health facilities that met minimum enrollment volume criteria for this analysis. In this cohort, 276,914 (62.3%) patients were female. The median age of enrollment was 33 years (interquartile range [IQR]: 27–40); the median time in HIV care was 394 days (Q1-Q3: 29–1347); and the median time on ART was 613 days (IQR: 128–1535). Other clinical and demographic characteristics are shown in Table 1.
Table 1.
Demographic and clinical care characteristics of HIV-infected individuals included in our HIV treatment cascade
| Male | Female | Total | ||
|---|---|---|---|---|
| N | 167525 | 276914 | 444439 | |
| Province | Lusaka, n (%) | 101855 (60.8%) | 172139 (62.2%) | 273994 (61.6%) |
| Eastern, n (%) | 19835 (11.8%) | 31794 (11.5%) | 51629 (11.6%) | |
| Western, n (%) | 19171 (11.4%) | 31250 (11.3%) | 50421 (11.3%) | |
| Southern, n (%) | 26664 (15.9%) | 41731 (15.1%) | 68395 (15.4%) | |
| Age at enrollment | Median (Q1, Q3) | 35 (30 – 42) | 31 (26 – 38) | 33 (27 – 40) |
| 6–16 years, n (%) | 6871 (4.1%) | 8430 (3.0%) | 15301 (3.4%) | |
| ≥ 16 years, n (%) | 160654 (95.9%) | 268484 (97.0%) | 429138 (96.6%) | |
| Days in Care* | Median (Q1, Q3) | 347 (28 – 1251) | 425 (33 – 1404) | 394 (29 – 1347) |
| < 90 days, n (%) | 57735 (34.5%) | 87743 (31.7%) | 145478 (32.7%) | |
| 90–360 days, n (%) | 26874 (16.0%) | 43319 (15.6%) | 70193 (15.8%) | |
| 360–720 days, n (%) | 20219 (12.1%) | 33250 (12.0%) | 53469 (12.0%) | |
| ≥ 720 days, n (%) | 62654 (37.4%) | 112518 (40.6%) | 175172 (39.4%) | |
| Days on ART** | Median (Q1, Q3) | 558 (106 – 1448) | 647 (140 – 1587) | 613 (128 – 1535) |
| % < 90 days, n (%) | 28443 (23.0%) | 40356 (20.3%) | 68799 (21.3%) | |
| % 90–360 days, n (%) | 22350 (18.1%) | 34935 (17.6%) | 57285 (17.8%) | |
| % 360–720 days, n (%) | 18244 (14.8%) | 29000 (14.6%) | 47244 (14.7%) | |
| % ≥ 720 days, n (%) | 54399 (44.1%) | 94488 (47.5%) | 148887 (46.2%) | |
| WHO stage at enrollment | Stage I, n (%) | 40120 (23.9%) | 100323 (36.2%) | 140443 (31.6%) |
| Stage II, n (%) | 30922 (18.5%) | 51917 (18.7%) | 82839 (18.6%) | |
| Stage III, n (%) | 63913 (38.1%) | 74188 (26.8%) | 138101 (31.1%) | |
| Stage IV, n (%) | 9187 (5.5%) | 10174 (3.7%) | 19361 (4.4%) | |
| Not Available, n (%) | 23383 (14.0%) | 40312 (14.6%) | 63695 (14.3%) | |
| CD4 counts at enrollment | < 200 cells/mm3, n (%) | 64065 (38.2%) | 82467 (29.8%) | 146532 (33.0%) |
| 200 – 350 cells/mm3, n (%) | 29818 (17.8%) | 52529 (19.0%) | 82347 (18.5%) | |
| 350 – 500 cells/mm3, n (%) | 15045 (9.0%) | 31789 (11.5%) | 46834 (10.5%) | |
| ≥ 500 cells/mm3, n (%) | 11916 (7.1%) | 32000 (11.6%) | 43916 (9.9%) | |
| Not Available, n (%) | 46681 (27.9%) | 78129 (28.2%) | 124810 (28.1%) | |
from time of enrollment,
from time of antiretroviral therapy initiation. ART = antiretroviral therapy, WHO = World Health Organization
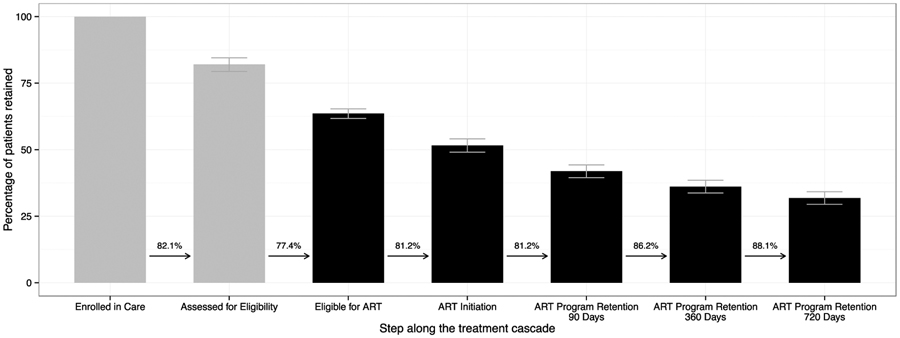
We constructed the HIV treatment cascade based on pooled data for all health facilities (Figure 2). In the first step of the cascade, which considered all HIV-infected patients enrolled in care, 82.1% (95% CI: 79.4–84.5%) were fully assessed for ART eligibility within 180 days of enrollment. Of these, 271,608 (63.6%, 95%CI: 61.7–65.3%) were found to be eligible for ART based on the HIV treatment guidelines at the time. Of those, 81.1% (95%CI: 79.5–82.7%) initiated ART within 180 days.
Figure 2. HIV treatment cascade in Zambia, 2004–2014.
The percentage of Zambian patients retained at each step along the treatment cascade is depicted in a treatment cascade framework. The first two steps (grey) represent all HIV-infected patients over 5 years of age seeking care; the last five steps (black) include only those who were eligible for antiretroviral therapy, based on the prevailing guidelines at the time of assessment. The numeric value above the arrow between steps is the percentage of patients conditional on the immediately preceding step. Error bars represent 95% confidence intervals.
ART program retention was calculated at 90, 360, and 720 days. We restricted the analysis to those patients who had adequate periods of follow-up time (i.e. 180 days) after each respective threshold. Among 145,437 patients who met this minimum follow-up time for 90 days, 81.2% (95%CI: 80.4–81.9%) remained in care. Among 134,950 patients who met this minimum follow-up time for 360 days, 70.0% (95%CI: 68.7–71.2%) remained in care. Among 120,393 patients who met this minimum follow-up time for 720 days, 61.6% (95%CI: 60.0–63.2%) remained in care Overall, approximately one-half of patients who were eligible for ART started HIV treatment within 6 months and remained in care at two years (Figure 2). We then performed stratified analyses based on geographic region, age, sex, and enrollment CD4 count (Supplementary Figure 1–4). When stratifying HIV facilities based on 4 provinces (Lusaka, Eastern, Southern, and Western), we found that a greater proportion of patients treated in the Lusaka Province were assessed for ART eligibility, compared to other provinces (Supplementary Figure 1). Children appeared to have better long-term engagement in HIV care than adults (Supplementary Figure 3).
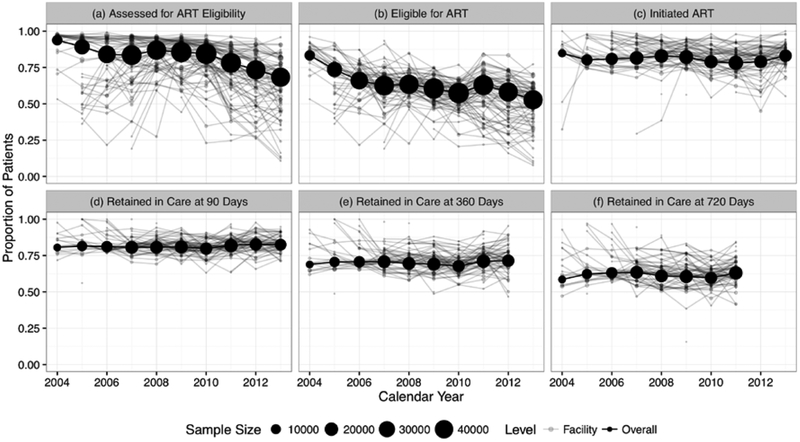
We then examined the temporal trends for each step for the HIV treatment cascade. We examined each annual cohort, both in pool analysis and by facility (Figure 3). The sizes of annual cohorts are presented as proportional black circles that demonstrate substantial growth since the program launch in 2004. Interestingly, we noted a steady decline in 2011–2014 for those assessed for ART eligibility and those deemed eligible for ART. This may have been related to CD4 count at enrollment, which appeared to increase steadily over the observation period (Supplementary Figure 5). The proportions that started ART and remained in care over time remained relatively consistent.
Figure 3. Temporal trends of patient engagement.
We grouped patients enrolled in the same calendar year as an “annual cohort” and described their progression through the HIV treatment cascade. We characterize programmatic temporal trends for each of the following cascade steps: (a) being assessed for ART eligibility, (b) becoming eligible for ART, (c) starting ART within 180 days, (d) retained in care at 90 days, (e) retained in care at 360 days, and (f) retained in care at 720 days.
Discussion
In this report, we characterize the HIV treatment cascade across 75 facilities over an 11-year period in Zambia. We show key points of attrition along the continuum of HIV care, identifying areas in need of intervention and further program focus. Our evaluation of temporal trends provides insight into program performance in the context of changing HIV treatment guidelines and increasing health systems demands. This simple approach to program evaluation can be replicated in other settings.
Results from our pooled analysis are consistent with other studies from sub-Saharan Africa. McNairy and colleagues reported comparable point estimates for ART initiation (70%) and ART retention at 12 months (78%) when they constructed an analogous HIV treatment cascade using data from Kenya, Mozambique, Rwanda, and Tanzania.19 In systematic reviews of African cohorts, Rosen and Fox reported a median of 59% (range 35%−88%) among 10 studies from HIV testing to eligibility assessment and a median of 68% (range 14%−84%) among 14 studies from ART eligibility determination to ART initiation.9 In a separate systematic review of 32 studies, Rosen and colleagues estimated a two-year retention of 62%, similar to our results here.10
One key feature of our analysis was the stratification of our patient population by year of enrollment and longitudinal follow-up of these “annual cohorts.” We observed relatively stable estimates over time for ART initiation, 90-day retention, 360-day retention, and 720-day retention. More concerning was the declining proportion of patients assessed for eligibility over time, which dropped to under 75% for the 2013 cohort. Given its temporal relationship with increasing clinics and patient volumes, it could be related to an overburdened health infrastructure. This may be particularly true in the case of CD4 screening, where the expansion of laboratory services (including timely results reporting) may have lagged behind an increasing demand for HIV services. Tracking of these temporal trends should continue, particularly in the face of evolving national HIV guidelines. Results from the START trial suggest that the initiation of ART may have significant health benefits, even for asymptomatic patients with high CD4 counts.21 Following the WHO recommendation of ART initiation irrespective of clinical disease stage or immunologic status,22 the Zambian Ministry of Health announced its own policy for universal ART. The increased volume of patients initiating ART could threaten program performance and negatively affect the HIV continuum of care for all HIV-infected patients.
Ascertainment of transfers, withdrawals, and deaths varied between the sites included in our analysis. As programmatic data typically do not include reasons for attrition, greater investments in contact tracing and vital registries could have a direct and positive impact on our ability to classify those who drop out of care.35–37 Intensive, sampling-based approaches also hold promise in this regard. Geng and colleagues have implemented such a methodology in numerous African settings,38–40 including an ongoing study in Zambia.41 Our practical approach to evaluating patient engagement contrasts the “comprehensive HIV care cascade” proposed by McNairy, et al. Although both use a cohort-based approach, the comprehensive HIV care cascade classifies different outcomes as optimal, suboptimal, and poor to describe program performance.19 The inclusion of patients not yet eligible for ART is a key addition of the comprehensive cascade. As HIV policy moves toward universal HIV treatment,22 this distinction could lose its prominence, since all HIV-infected individuals would be eligible to initiate ART.
Our focus on simplicity and routinely available data may have inherent limitations. First, the scope of our treatment cascade was constrained by the types of data available in a routine programmatic setting. We were unable to describe linkages between HIV testing and HIV care at our target facilities. Although this has been an area of emphasis for the Zambian Ministry of Health, data systems are not fully aligned to capture individual-level data across different health venues. Similarly, because of the poor accessibility of HIV RNA PCR testing nationwide, we were unable to describe the proportion of individuals with virologic suppression, an objective measure of ART adherence. Second, we conceived the HIV treatment cascade as a linear process, but this may not reflect the reality in the field. Hallett and Eaton described possible “side doors” to ART initiation that might sidestep the traditional progression (e.g., presentation with advanced clinical symptoms, previously dropping out of clinical care).42 Such dynamics were not considered in this framework because of their added complexity. Instead, we permitted a wide window period (i.e., 180 days) after each specific visit in which patients could return. This approach minimized wrongful categorization of active patients as lost to follow-up; however, it may have incrementally elevated our retention estimates as well. We also recognize that a proportion of patients who re-engage in HIV care may do so at new facilities – with newly assigned medical record numbers – which may be difficult to capture with routinely captured observational data. Unfortunately, we were unable to account for these individuals in our analysis. Finally, challenges to data quality that often accompany electronic medical records could lead to biases. For example, poor operational linkages between service providers and data entry staff at the health facility could lead to incomplete/missing data, which in turn could overestimate the proportions that dropped out of care.
In summary, patient engagement in HIV care remains a top priority for sustainably managing the global HIV epidemic. We present programmatic data from a large HIV treatment program in Zambia, demonstrating temporal trends for each step of HIV treatment cascade. The development of such tools can be of great assistance to frontline providers and program managers seeking to improve the quality of clinical care at health facilities. In order to be effective, however, such data must be integrated in routine care and used to guide program improvement efforts.
Supplementary Material
Acknowledgements
This work was supported by the International Epidemiologic Databases to Evaluate AIDS Southern Africa collaboration, the UJMT Fogarty Global Health Fellows Program Consortium, and the UNC Center for AIDS Research, all funded by the National Institutes of Health.
References
- 1.UNAIDS. Access to Antiretroviral Therapy in Africa: Status Report on Progress Towards the 2015 Targets. Geneva: Joint United Nations Programme on HIV/AIDS; 2014. [Google Scholar]
- 2.Berg MB, Safren SA, Mimiaga MJ, Grasso C, Boswell S, Mayer KH. Nonadherence to medical appointments is associated with increased plasma HIV RNA and decreased CD4 cell counts in a community-based HIV primary care clinic. AIDS care. 2005;17:902–907. [DOI] [PubMed] [Google Scholar]
- 3.Giordano TP, Gifford AL, White AC, et al. Retention in care: a challenge to survival with HIV infection. Clinical Infectious Diseases. 2007;44:1493–1499. [DOI] [PubMed] [Google Scholar]
- 4.Mugavero MJ, Lin H-Y, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harries AD, Zachariah R, Lawn SD, Rosen S. Strategies to improve patient retention on antiretroviral therapy in sub-Saharan Africa. Tropical medicine & international health: TM & IH. June 2010;15 Suppl 1:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horstmann E, Brown J, Islam F, Buck J, Agins BD. Retaining HIV-infected patients in care: Where are we? Where do we go from here? Clinical Infectious Diseases. 2010;50:752–761. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg AE, Hader SL, Masur H, Young AT, Skillicorn J, Dieffenbach CW. Fighting HIV/AIDS in Washington, D.C. Health affairs. Nov-Dec 2009;28(6):1677–1687. [DOI] [PubMed] [Google Scholar]
- 8.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. March 15 2011;52(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. July 2011;8(7):e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. October 16 2007;4(10):e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haber N, Pillay D, Porter K, Barnighausen T. Constructing the cascade of HIV care: methods for measurement. Curr Opin HIV AIDS. January 2016;11(1):102–108. [DOI] [PubMed] [Google Scholar]
- 12.Medland NA, McMahon JH, Chow EP, Elliott JH, Hoy JF, Fairley CK. The HIV care cascade: a systematic review of data sources, methodology and comparability. J Int AIDS Soc. 2015;18:20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. August 16 2006;296(7):782–793. [DOI] [PubMed] [Google Scholar]
- 14.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. October 24 2007;298(16):1888–1899. [DOI] [PubMed] [Google Scholar]
- 15.Goldman JD, Cantrell RA, Mulenga LB, et al. Simple adherence assessments to predict virologic failure among HIV-infected adults with discordant immunologic and clinical responses to antiretroviral therapy. AIDS Res Hum Retroviruses. August 2008;24(8):1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox MP, Shearer K, Maskew M, et al. Treatment outcomes after 7 years of public-sector HIV treatment. Aids. September 10 2012;26(14):1823–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muyunda G Zambia leads the way in SmartCare electronic health records system, a benefit to both providers and patients. http://www.jhpiego.org/content/zambia-leads-way-smartcare-electronic-health-records-system-benefit-both-providers-and-patie. Accessed on December 22, 2015.
- 18.Chi BH, Yiannoutsos CT, Westfall AO, et al. Universal definition of loss to follow-up in HIV treatment programs: A statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Medicine. October 2011;8(10):e1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNairy ML, Lamb MR, Abrams EJ, et al. Use of a Comprehensive HIV Care Cascade for Evaluating HIV Program Performance: Findings From 4 Sub-Saharan African Countries. J Acquir Immune Defic Syndr. October 1 2015;70(2):e44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi BH, Cantrell, R.A., Mwango, A., Westfall, A.O., Mutale, W. An empirical approach to defining loss to follow-up among patients enrolled in antiretroviral treatment programs. Am J Epidemiol. 2010;171:924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Insight Start Study Group, Lundgren JD, Babiker AG, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. August 27 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV, September 2015. http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1. Accessed on October 9, 2015. [PubMed]
- 23.Stringer EM, Chi BH, Chintu N, et al. Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bull World Health Organ. January 2008;86(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNairy ML, Teasdale CA, El-Sadr WM, Mave V, Abrams EJ. Mother and child both matter: reconceptualizing the prevention of mother-to-child transmission care continuum. Curr Opin HIV AIDS. September 8 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Risher K, Mayer KH, Beyrer C. HIV treatment cascade in MSM, people who inject drugs, and sex workers. Curr Opin HIV AIDS. September 8 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanser F, Barnighausen T, Vandormael A, Dobra A. HIV treatment cascade in migrants and mobile populations. Curr Opin HIV AIDS. September 8 2015. [DOI] [PubMed] [Google Scholar]
- 27.Lessells RJ, Swaminathan S, Godfrey-Faussett P. HIV treatment cascade in tuberculosis patients. Curr Opin HIV AIDS. September 8 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bobat R, Archary M, Lawler M. An update on the HIV treatment cascade in children and adolescents. Curr Opin HIV AIDS. September 8 2015. [DOI] [PubMed] [Google Scholar]
- 29.Morris MB, Chapula BT, Chi BH, et al. Use of task-shifting to rapidly scale-up HIV treatment services: experiences from Lusaka, Zambia. BMC Health Serv Res. 2009;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes CB, Sanne I. Changing models of care to improve progression through the HIV treatment cascade in different populations. Curr Opin HIV AIDS. September 8 2015. [DOI] [PubMed] [Google Scholar]
- 31.Forster M, Bailey C, Brinkhof MW, et al. Electronic medical record systems, data quality and loss to follow-up: survey of antiretroviral therapy programmes in resource-limited settings. Bull World Health Organ. December 2008;86(12):939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gimbel S, Voss J, Mercer MA, et al. The prevention of mother-to-child transmission of HIV cascade analysis tool: supporting health managers to improve facility-level service delivery. BMC Res Notes. 2014;7(1):743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forrest JI, Wiens M, Kanters S, Nsanzimana S, Lester RT, Mills EJ. Mobile health applications for HIV prevention and care in Africa. Curr Opin HIV AIDS. September 8 2015. [DOI] [PubMed] [Google Scholar]
- 34.Finocchario-Kessler S, Gautney BJ, Khamadi S, et al. If you text them, they will come: using the HIV infant tracking system to improve early infant diagnosis quality and retention in Kenya. AIDS. July 2014;28 Suppl 3:S313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krebs DW, Chi BH, Mulenga Y, et al. Community-based follow-up for late patients enrolled in a district-wide programme for antiretroviral therapy in Lusaka, Zambia. AIDS Care. March 2008;20(3):311–317. [DOI] [PubMed] [Google Scholar]
- 36.Cornell M, Lessells R, Fox MP, et al. Mortality among adults transferred and lost to follow-up from antiretroviral therapy programmes in South Africa: a multicenter cohort study. J Acquir Immune Defic Syndr. October 1 2014;67(2):e67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Cutsem G, Ford N, Hildebrand K, et al. Correcting for mortality among patients lost to follow up on antiretroviral therapy in South Africa: a cohort analysis. PLoS One. 2011;6(2):e14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. August 6 2008;300(5):506–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geng EH, Glidden DV, Bwana MB, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: estimation via a sampling-based approach. PLoS One. 2011;6(7):e21797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geng EH, Odeny TA, Lyamuya RE, et al. Estimation of Mortality among HIV-infected people on antiretroviral therapy treatment in east Africa: a sampling based approach in an observational, multisite, cohort study. The lancet HIV. March 1 2015;2(3):e107–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CIDRZ. CIDRZ awarded grant from the Bill & Melinda Gates Foundation. [Press Release]. http://www.cidrz.org/cidrz-awarded-grant-from-the-bill-melinda-gates-foundation/. Accessed July 8, 2015.
- 42.Hallett TB, Eaton JW. A side door into care cascade for HIV-infected patients? J Acquir Immune Defic Syndr. July 2013;63 Suppl 2:S228–232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.