Abstract
Background:
Chronic stress exposure causes neuronal atrophy and synaptic deficits in the medial prefrontal cortex (PFC), contributing to development of anxiety-and depressive-like behaviors. Concomitantly microglia in the PFC undergo morphological and functional changes following stress exposure, suggesting that microglia contribute to synaptic deficits underlying behavioral consequences.
Methods:
Male and female mice were exposed to chronic unpredictable stress (CUS) to examine the role of neuron-microglia interactions in the medial PFC during development of anxiety- and depressive-like behaviors. Thy1-GFP-M mice were used to assess microglia-mediated neuronal remodeling and dendritic spine density in the medial PFC. Viral-mediated knockdown of neuronal colony stimulating factor-1 (CSF1) was used to modulate microglia function and behavioral consequences after CUS.
Results:
CUS promoted anxiety- and depressive-like behaviors that were associated with increased mRNA levels of CSF1 in the PFC. Increased CSF1 mRNA levels were also detected in postmortem dorsolateral PFC of depressed individuals. Moreover, frontal cortex microglia in mice exposed to CUS show elevated CSF1 receptor expression and increased phagocytosis of neuronal elements. Notably, functional alterations in microglia were more pronounced in male mice compared to female mice. These functional changes in microglia corresponded with reduced dendritic spine density on pyramidal neurons in layer I of the medial PFC. Viral-mediated knockdown of neuronal CSF1 in the medial PFC attenuated microglia-mediated neuronal remodeling and prevented behavioral deficits caused by CUS.
Conclusions:
These findings revealed that stress-induced elevations in neuronal CSF1 provokes microglia-mediated neuronal remodeling in the medial PFC, contributing to synaptic deficits and development of anxiety- and depressive-like behavior.
Keywords: Stress, Depression, Neuroimmune, Microglia, Prefrontal Cortex, Colony Stimulating Factor-1
Introduction:
Affective disorders are a major source of disability and cause significant social and economic burdens (1, 2). Clinical and basic research show that anxiety and depressive symptoms arise from synaptic deficits in the medial prefrontal cortex (PFC) and hippocampus (HPC)(3–5). Similarly, the rodent chronic unpredictable stress (CUS) model causes synaptic loss on medial PFC pyramidal neurons that lead to development of depressive-like behaviors (6–8). Other studies report that repeated stress exposure causes morphological and molecular changes in brain-resident macrophages, termed microglia (9, 10). This is pertinent because recent studies show microglia modulate neuroplasticity, raising the possibility that stress-induced ‘microglia activation’ contributes to synaptic deficits underlying depressive-like behaviors.
Microglia are acutely sensitive to neuronal homeostasis and influence neuroplasticity by release of neuromodulators (i.e., cytokines) and by activity-dependent elimination of dendrites and synapses (4, 11, 12). Notably, dysregulation of neuronal activity and neuronal atrophy in the medial PFC following stress (13, 14), may provoke functional alterations in microglia. Indeed neuronal dystrophy or neuronal damage causes fluctuations in neuron-derived factors that regulate microglia function, such as colony stimulating factor 1 (CSF1)(15), fractalkine (CX3CL1) (16), or transforming growth factor-β (TGFβ) (17). For example, kainic acid-induced neuronal hyperactivity caused up-regulation of neuron-derived CSF1, which caused microglia activation and neuronal loss in the hippocampus (18). In another study sensory neuron injury increased neuronal CSF1 resulting in microglia activation that contributed to neuropathic pain. (19). In the context of stress, neuronal atrophy may initiate release of factors that alter microglia function, which may contribute to maladaptive neuronal responses and synaptic deficits underlying depressive-like behaviors (4).
The primary objective of the present studies was to determine the role of microglia in neuronal remodeling and development of depressive-like behavior in mice exposed to CUS. These studies showed that up-regulation of neuronal CSF1 provoked microglia-mediated dendritic remodeling in the medial PFC leading to development of anxiety- and depressive-like behaviors. These findings revealed a novel cellular mechanism by which microglia contribute to the neurobiology of affective disorders.
Materials and Methods:
Animals.
Transgenic (and wild-type littermate) male and female Thy1-GFP-M mice were obtained from in-house breeders (Jackson Laboratories; Tg(Thy1-EGFP)MJrs/J; #007788). For CSF1 knockdown studies, male wild-type C57BL/6 mice were purchased (Jackson Laboratories; C57BL/6J; #000664). Studies were performed with mice 6–12 weeks old. Mice were group- housed (3/cage) in 11.5”× 7.5”× 6” polypropylene cages under a 12 h light-dark cycle with ad libitum access to water and rodent chow.
Chronic unpredictable stress (CUS).
CUS was performed as previously described (6, 20). In brief, mice were exposed to random intermittent stressors over 14 days, including: cage rotation, isolation, radio noise, food or water deprivation, light on overnight, light off during day, rat odor, stroboscope overnight, crowding, wet bedding, no bedding, or tilted cage.
Behavioral Testing.
Open-field (OF), forced swim test (FST), sucrose consumption test (SCT), and novelty-suppressed feeding test were conducted as previously described (7, 20, 21). Further details are provided in supplemental information.
RNA isolation and real-time PCR.
RNA was extracted from whole brain regions (PFC) and N2a cells using TRIzol Reagent according to manufacturer’s protocol (Invitrogen). RNA was extracted from microglia using the Single Cell RNA purification kit (Norgen Biotek Corp., Thorold, Canada). Samples were reverse transcribed, and real-time PCR conducted as previously described (22).
Percoll gradient isolation of enriched microglia.
Dissected frontal cortex was passed through a 70 μm cell strainer. Homogenates were centrifuged at 600xg for 6 min. Supernatants were removed and cell pellets were re-suspended in 70% isotonic Percoll (GE Healthcare). A discontinuous Percoll density gradient was layered as follows: 50%, 35%, and 0% isotonic Percoll. The gradient was centrifuged for 20 min at 2000xg and enriched microglia were collected from the interphase between the 70% and 50% Percoll layers (23, 24).
Immunohistology.
Mice were transcardially perfused with sterile PBS and 4% paraformaldehyde (PFA). Brains were post-fixed in 4% PFA for 24 h and incubated in 30% sucrose for an additional 24 h. Frozen brains were sectioned with Microm HM550 cryostat. Free-floating sections were washed, then blocked for 1 hour at room temperature. Sections were washed, then incubated with primary antibodies: rabbit anti-Iba-1 (Wako; 019–19741), mouse anti-CSF1 (R&D Systems; AF416), mouse anti-CD68 (Abcam; ab31630), goat anti-Iba-1 (Novus Biologicals; NB100–1028), or rabbit anti-P2Y12R (Anaspec; AS-55043A) overnight at 4°C. Sections were washed and incubated with conjugated secondary antibody overnight at 4°C.
Short-hairpin RNA and viral preparation.
CSF1 short-hairpin (sh)RNA sequence was designed targeting transcript (25). CSF1 shRNA (5’-TTTGTCTCATCTATTATGTCTTGTACCCTTCCTG TCAGGTACAAGACATAATAGATGAGAATTTTT-3’) or scrambled control (Integrated DNA Technologies, Coralville, IA) was ligated into the pEGFP-shRNA construct, designed to co- express enhanced green fluorescent protein (EGFP) under the CMV promoter and ligated shRNA driven by U6 promoter. Construct was packaged in adeno-associated virus-2 (AAV2) as previously described (6, 26).
Surgery and cortical infusion.
Mice were anesthetized with ketamine/xylazine (100/10 mg/kg). Bilateral viral infusions in the medial PFC (1 μl; 0.1 μl/minute) were performed with coordinates (from bregma), +2.0 mm anterior-posterior, ±0.2 mm medial-lateral, −2.8 mm dorsal-ventral (27). Incisions were closed with sutures and mice received intraperitoneal injection of carprofen (5 mg/kg) immediately after surgery and daily for the next two days.
Quantitative Immunofluorescence.
Confocal images were obtained on Olympus BX61WI microscope (Tokyo, Japan) and captured with Fluoview (FV1000) and Hamamatsu high- resolution digital camera (ORCA-ER; Hamamatsu City, Japan. Quantification of immunolabeling and dendritic spine density was performed as previously described (28, 29). Further details are provided in supplemental information.
Statistical analysis.
Data were subjected to statistical analyses with GraphPad Prism 6 (La Jolla, California). Significant main effects and interactions were determined using one- (genotype, treatment), or two- (genotype × treatment) way ANOVA. Differences between group means were evaluated with Fischer’s least significant differences (LSD) test.
Study Approval.
Animal use and procedures were in accordance with the National Institutes of Health guidelines and approved by the Yale University Animal Care and Use Committees.
Results:
Male and female mice develop anxiety- and depressive-like behaviors following CUS.
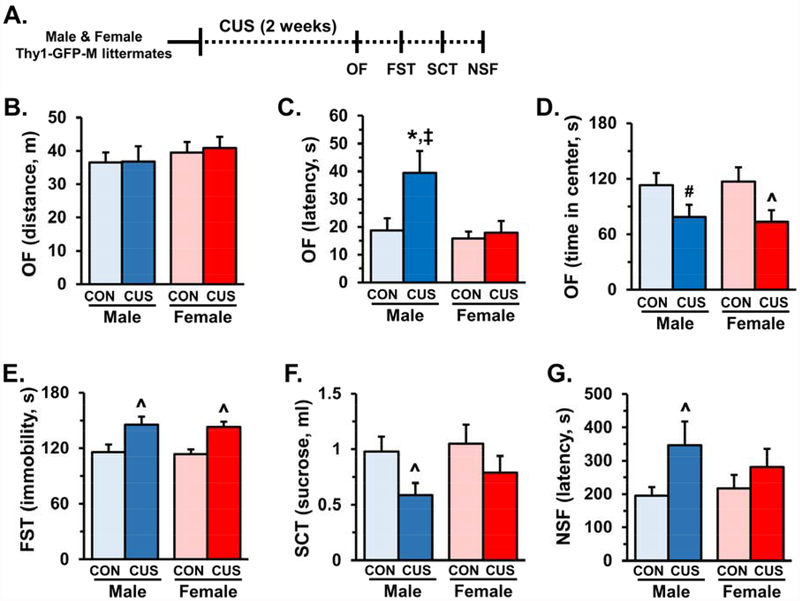
Male and female mice were exposed to 14 days of CUS and then assessed in the open field (OF), forced swim test (FST), sucrose consumption test (SCT), and novelty suppressed feeding (NSF) on subsequent days (Fig.1A). In the OF, male and female mice showed similar locomotor activity, however, males showed a significant increase in latency to enter the open field (F1,31=3.49, p<0.07, Fig.1B&C). Both male and female mice had decreased time spent in the open field following CUS (F1,31=8.21, p<0.007, Fig.1D). CUS significantly increased FST immobility in both male and female mice (F1,30=16.04, p<0.0004, Fig.1E). There was an overall decrease in sucrose consumption (F1,31=5.05, p<0.03), but post-hoc analyses showed a significant reduction only in male mice compared to controls (Fig.1F). Similarly, there was a general increase in latency to feed in the NSF for both males and females following CUS (F1,30=4.45, p<0.04), but only male mice were significantly different than controls (Fig.1G). Thus, anxiety- and depressive-like behaviors were observed in both male and female mice after CUS, with male mice demonstrating greater susceptibility to CUS.
Figure 1. Male and female mice develop anxiety- and depressive-like behaviors following chronic unpredictable stress.
Male and female Thy1-GFP-M littermates were exposed to 14 days of chronic unpredictable stress (CUS) and then assessed in the open field (OF), forced swim test (FST), sucrose consumption test (SCT), and novelty-suppressed feeding (NSF) on subsequent days (n = 8–10/ group). A) Schematic showing experimental approach and timeline. On the final day of CUS activity in the open field (OF) was assessed and total distance (B), latency to enter the open field (C), and time spent in the center of the open field (D) are shown. The following day immobility in the forced swim test (FST) was measured, immobility in minutes 2–6 is shown (E). On the subsequent day the sucrose consumption test (SCT) was performed, and total sucrose consumer is shown (F). Last, the novelty-suppressed feeding test was administered, latency to feed is shown (G). Bars represent the mean ± S.E.M. Means significantly different than respective control group based on ANOVA are denoted (main effect: ^, p < 0.05 or #, p = 0.08), (interaction: *, p < 0.05). Means significantly different than respective female experimental group based on ANOVA are noted by (‡, p < 0.05).
CUS altered PFC expression of genes that regulate microglia function.
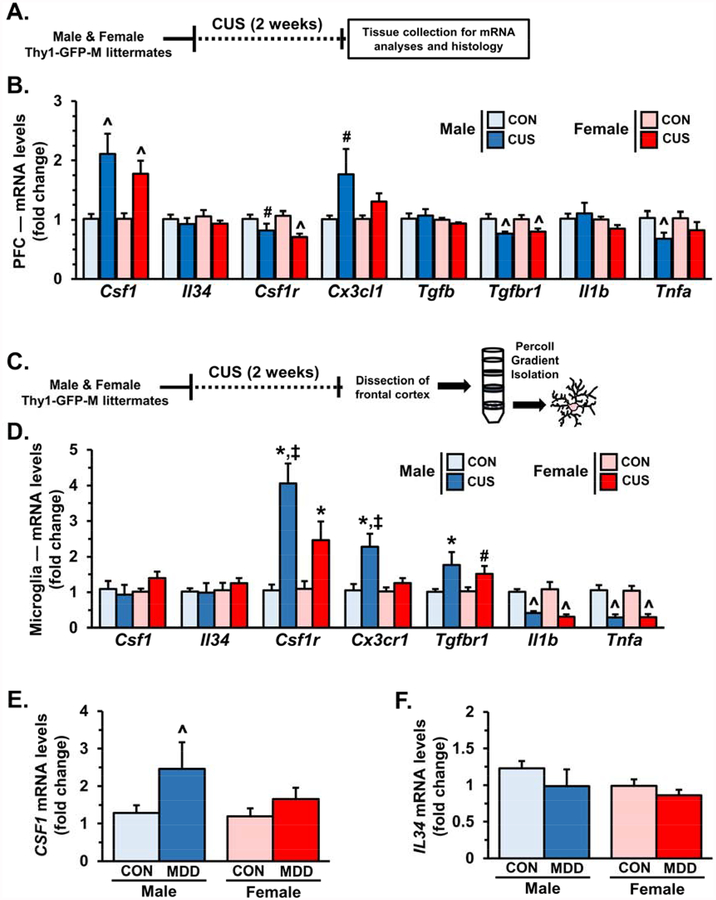
Coincident neuronal atrophy and ‘microglia activation’ in the PFC suggest that neuron- microglia interactions may be dysregulated (4). To test this hypothesis, male and female mice were exposed to 14 days of CUS and 2 h after the final stressor PFC was dissected for mRNA analyses (Fig.2A). CUS exposure caused robust up-regulation of csf1 expression (F1,19=19.75, p<0.0003) with a modest increase in cx3cl1 expression (F1,20=6.91, p<0.02). Several markers, including csf1r (F1,19=12.12, p<0.003), tgfbr1 (F1,19=13.28, p<0.002), and tnfa (F1,19=5.09, p<0.04) were significantly reduced following CUS (Fig.2B). Other regulatory factors, such as il34, tgfb, and il1b were not altered by CUS exposure (Fig.2B).
Figure 2. Chronic unpredictable stress altered gene expression of factors that mediate neuron-microglia interactions in the prefrontal cortex (PFC).
A) Male and female Thy1- GFP-M littermates were exposed to 14 days of chronic unpredictable stress (CUS) and 2 hours after the final stressor PFC was dissected for mRNA analyses. B) Relative fold change of mRNA levels in the PFC of male and female mice are shown for: csf1, il34, csf1r, cx3cl1, tgfb, tgfbr1, il1b, and tnfa (n =5–7/ group). C) In separate cohorts, male and female wild-type littermates of Thy1-GFP-M mice were exposed to 14 days of chronic unpredictable stress. Following the final stressor frontal cortex was dissected and microglia were enriched through Percoll gradient isolation. D) Gene expression of csf1, csf1r, cx3cr1, tgfbr, il1b, and tnfa in enriched microglia is shown (n = 4–5/ group). Isolated mRNA from post mortem dorsolateral prefrontal cortex was obtained from controls and major depressive disorder patients. Relative fold change of E) CSF1 and F) IL34 mRNA levels are shown (n = 8–14/ group). Bars represent the mean ± S.E.M.. Means significantly different than respective control group based on ANOVA are denoted (main effect: ^, p < 0.05 or #, p = 0.08), (interaction: *, p < 0.05). Means significantly different than respective female experimental group based on ANOVA are noted by (‡, p < 0.05).
To further examine CUS-induced neuroimmune alterations, male and female mice were exposed to CUS and 2 hours after the final stressor frontal cortex was dissected and enriched microglia were obtained via Percoll gradient isolation (23, 24) (Fig.2C). Gene expression of several neuroimmune markers were examined, including csf1, il34, csf1r, tgfbr, cx3cr1, il1b, and tnfa (Fig.2D). In enriched microglia from the frontal cortex of male and female mice, csf1r (F1,15=31.84, p<0.0001), tgfbr (F1,15=22.02, p<0.0003), and cx3cr1 (F1,15=13.02, p<0.003) were up-regulated after CUS (Fig.2D). Of note, enriched microglia had increased expression of immunomodulatory receptors in a sex-dependent manner with male mice showing greater fold change of csf1r (F1,15=4.49, p<0.05), tgfbr (F1,15=3.08, p=0.10), and cx3cr1 (F1,15=5.93, p<0.03) compared to female mice. The expression of pro-inflammatory cytokines il1b (F1,16=33.59, p<0.0001) and tnfa (F1,16=41.44, p<0.0001) were robustly decreased in both male and female mice following CUS. These findings indicate that CUS exposure significantly altered gene expression of immunomodulatory receptors and cytokines in a sex-dependent manner (Fig.2D).
To examine if CSF1 was modulated in clinical samples, mRNA analyses were performed on dorsolateral PFC samples obtained from individuals diagnosed with Major Depressive Disorder (MDD) and assessed in a prior publication (6). Consistent with the rodent model, CSF1 mRNA was up-regulated in post mortem dorsolateral PFC of MDD samples (F1,48=4.36, p<0.04, Fig.2E) with men showing significant up-regulation of CSF1 compared to controls (p<0.051, Fig.2E). Notably, these samples did not show significant changes in IL34 mRNA levels (Fig.2F).
Divergent sex-dependent microglia activation is associated with reduced dendritic spine density in the medial prefrontal cortex (PFC) after CUS
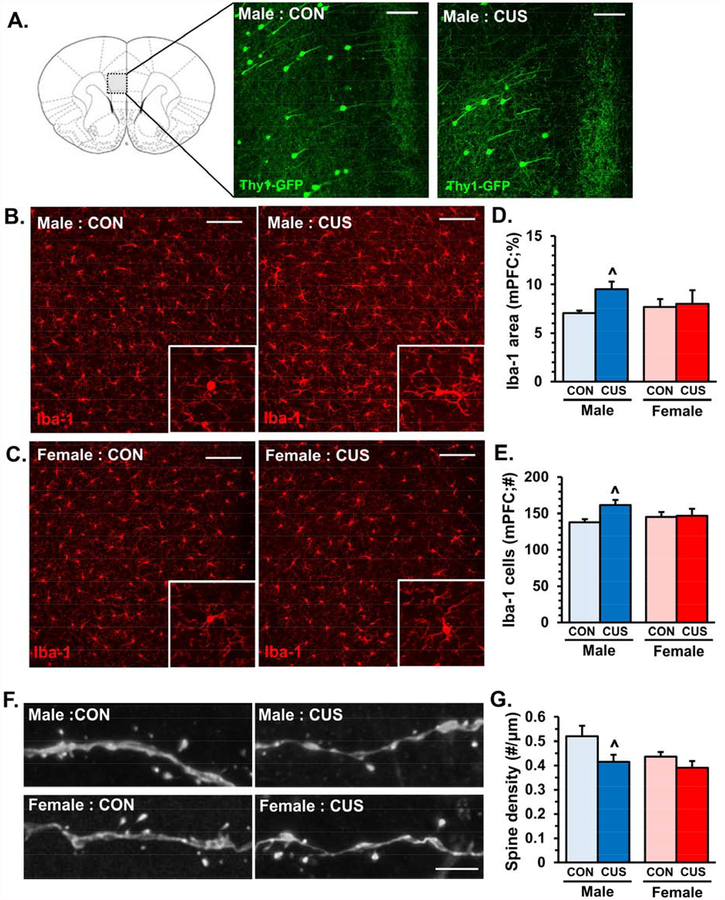
Several studies demonstrate that microglia undergo morphological changes in the medial PFC following stress exposure (9, 10), which may represent alterations in gene transcription as well as function (30). To examine the coincidence of microglia alterations and neuronal atrophy we used Thy1-GFP-M mice, which have a subset of PFC pyramidal neurons that express GFP enabling dendritic and synaptic analyses (Fig.3A). Male and female Thy1-GFP-M mice exposed to 14 days of CUS were perfused 2−4 hours later for immunohistology. Representative images of Iba-1 (microglia marker) immunofluorescence in control or CUS male (Fig.3B) and female (Fig.3C) mice are shown. Proportional area analyses of Iba-1 showed that CUS caused modest elevations in Iba-1 immunolabeling in medial PFC (F1,15=2.58, p=0.12, Fig.3D), with male mice showing elevations in Iba-1 proportional area (p<0.059, Fig.3D). The number of Iba-1+ microglia in the medial PFC was modestly increased following CUS (F1,15=3.26, p<0.09), particularly in male mice (p<0.03, Fig.3E). Dendritic spine analyses were performed on the same samples processed above. CUS significantly reduced dendritic spine density in the medial PFC (F1,12=5.10, p<0.04) and post-hoc analyses revealed that synaptic deficits were more pronounced in male mice (p<0.03, Fig.3F&G). Taken together, these findings show that CUS caused sex-dependent morphological alterations in microglia that were associated with synaptic deficits in layer I of the medial PFC.
Figure 3. Divergent sex-dependent microglia activation is associated with reduced dendritic spine density in the medial prefrontal cortex (PFC) after chronic unpredictable stress.
Male and female Thy1-GFP-M mice were exposed to 14 days of chronic unpredictable stress (CUS), 2–4 hours after the final stressor mice were perfused and brains collected for immunohistology. Representative images of Iba-1 immunofluorescence in the medial PFC of control (CON) or CUS male (A) and female (B) mice. White scale bar represents 100 μm. Enlarged images of representative microglia are shown. C) Quantification of Iba-1 proportional (%) area in medial PFC. D) Number of Iba-1 microglia per field in the medial PFC (n = 4–6/ group). In the same samples apical dendrites were identified in layer I of medial PFC and dendritic spine density was analyzed. E) Representative confocal images of layer I apical dendrites in the medial PFC of control (CON) and CUS male and female Thy1-GFP-M mice. White scale bar represents 5 m. F) Quantification of average dendritic spine density in the medial PFC of control (CON) and CUS male and female Thy1-GFP-M mice (n = 5–6/ group). Bars represent the mean ± S.E.M. Means significantly different than respective control group based on ANOVA are denoted (main effect: ^, p < 0.05).
CUS increased microglia-mediated neuronal remodeling in layer I of the medial PFC
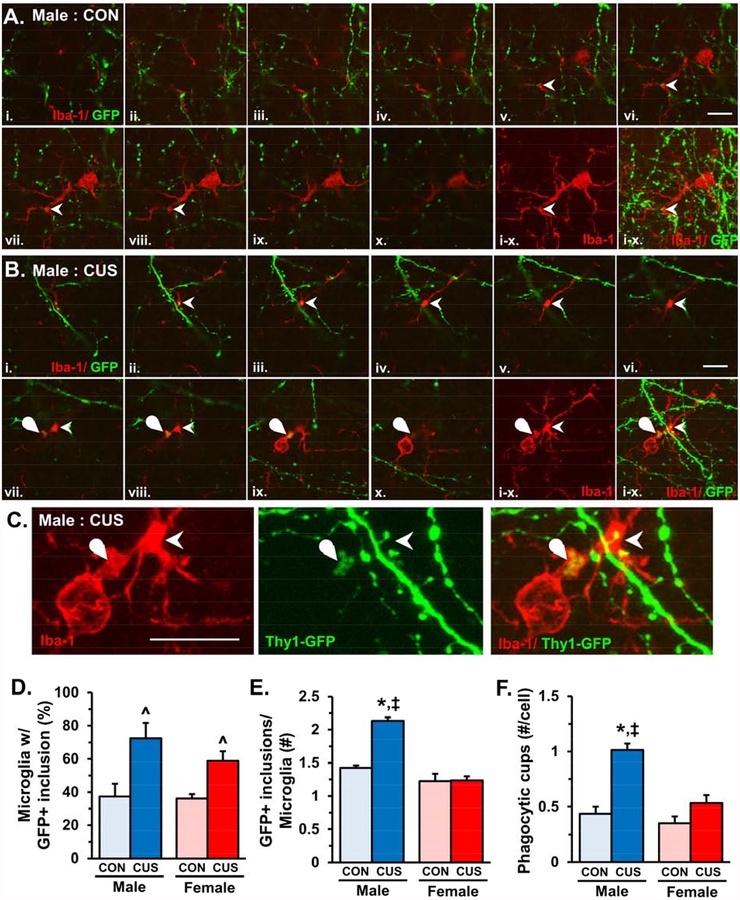
To determine if microglia contribute to synaptic deficits in the medial PFC, as reported in the hippocampus (38), neuron-microglia interactions were examined in the same samples of male and female Thy1-GFP-M mice used for histological analyses of dendritic spine density (Fig.3). Confocal images of individual microglia were taken in the medial PFC and stacks were analyzed for neuron-microglia interactions. Representative images show that microglia in both control and CUS mice had processes closely apposed GFP+ neuronal elements (denoted by arrowheads, Fig.4A&B). Further analyses showed that a portion of microglia in layer I of the medial PFC showed GFP+ inclusions (Fig.4B, rounded arrowhead). The proportion of microglia with GFP+ inclusions was increased by CUS exposure in both male and female mice in layer I of the medial PFC (F1,15=11.10, p<0.004, Fig.4D). Male mice had significantly more GFP+ inclusions per microglia compared to controls and females exposed to CUS (F1,15=33.07, p<0.0001, Fig.4E), which coincided with an increased frequency of microglial phagocytic cups (F1,16=10.00, p<0.006, Fig.4F). Within microglia, GFP+ inclusions co-localized with the lysosomal marker CD68, indicating that these neuronal elements are phagocytosed in layer I of the medial PFC (Fig.S1). In addition, all microglia (Iba-1+) in the medial PFC co-localized with the resident microglia-specific marker P2Y12 receptor (Fig.S2). Furthermore, microglia in medial PFC layer V or in somatosensory cortex layer I did not show increased engulfment of neuronal elements (Fig.S3), indicating that resident microglia mediate neuronal remodeling in a laminar- and region-specific manner.
Figure 4. Chronic unpredictable stress increased microglia-mediated neuronal remodeling in layer I of the medial PFC.
Male and female Thy1-GFP-M mice were exposed to 14 days of chronic unpredictable stress (CUS), 2–4 hours after the final stressor mice were perfused and brains collected for immunohistology. Representative sequence of confocal images (i.-x.) obtained from layer I of the medial PFC in control (A) and CUS (B) male Thy1-GFP-M mice. Arrowhead shows microglia process in close proximity to dendrite or dendritic spine and curved arrow notes GFP+ neuronal elements engulfed in microglia process. White scale bar represents 10 m. C) Enlarged and merged confocal stacks are shown from panel B. White scale bar represents 10 m. D) Proportion of microglia in layer I of medial PFC with GFP+ inclusions (n = 18–22 microglia/ sample). E) Average number of GFP+ inclusions in microglia with neuronal elements (n = 2–20 microglia/ sample). Bars represent the mean ± S.E.M. (n = 4–6/ group). Means that are significantly different than respective control group based on ANOVA are denoted (main effect: ^, p < 0.05 or #, p = 0.08), (interaction: *, p < 0.05). Means significantly different than respective female experimental group based on ANOVA are noted by (‡, p <0.05).
CSF1 knockdown in the medial PFC normalized neuroimmune responses following CUS
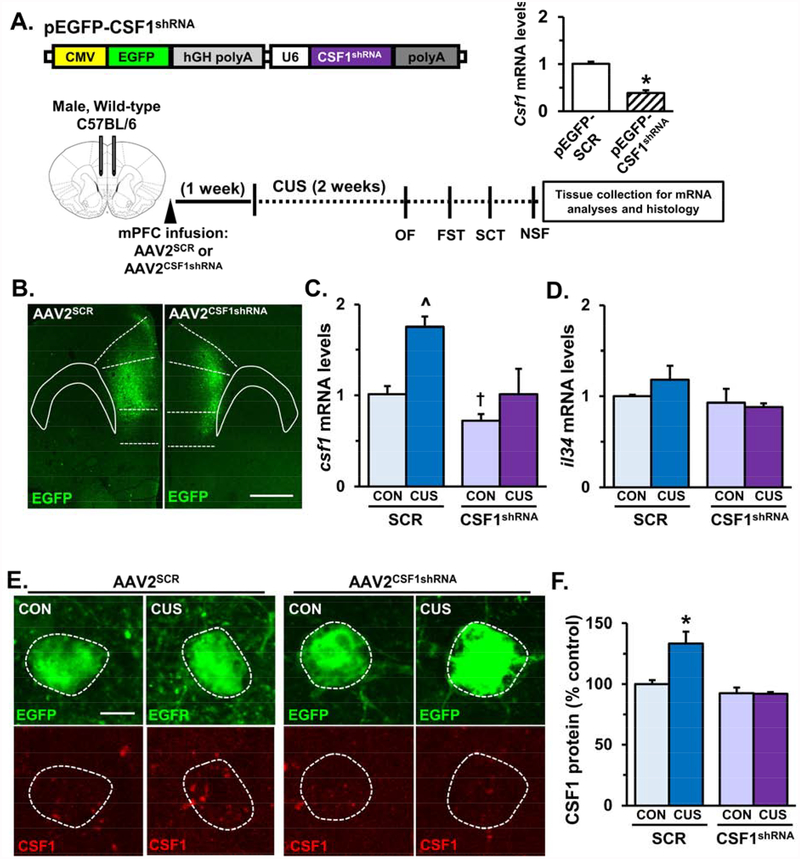
Experiments using viral-mediated CSF1 knockdown were performed to test the hypothesis that CSF1 signaling in the medial PFC promotes microglia-mediated neuronal remodeling in male mice. Constructs were designed to co-express a short-hairpin RNA (shRNA) targeting CSF1 and an EGFP reporter (Fig.5A). The pEGFP-CSF1shRNA construct significantly reduced CSF1 mRNA levels compared to scrambled control (pEGFP-SCR) in transfected N2a cells (Fig.5A, p<0.0001). Importantly, pEGFP-CSF1shRNA did not significantly change cx3cl1, il34, or tgfb mRNA levels in transfected N2a cells (Fig.S4A). Following viral packaging, wild- type C57BL/6 male mice received bilateral infusion of AAV2SCR or AAV2CSF1shRNA in the medial PFC and after recovery, mice were subjected to CUS (Fig.5A). After behavioral testing, one subset of mice was perfused and brains were collected for immunohistology. Representative images of AAV2SCR and AAV2CSF1shRNA virus infection (EGFP reporter) in the medial PFC are shown (Fig.5B). In a separate subset of mice, the medial PFC was dissected and mRNA analyses were performed. As expected CUS significantly increased csf1 mRNA levels in mice that received AAV2SCR (F =10.18, p<0.008). Moreover, mice that received AAV2CSF1shRNA showed reductions of csf1 mRNA levels in the medial PFC (F1,12=10.20, p<0.008). Post-hoc analyses showed that AAV2CSF1shRNA/CON mice had decreased csf1 mRNA levels compared to AAV2SCR/CON mice (p = 0.07) and csf1 mRNA levels were attenuated in AAV2CSF1shRNA/CUS mice compared to AAV2SCR/CUS mice (Fig.5C). Similar to previous results il34 mRNA levels were not significantly changed following CUS and AAV2CSF1shRNA did not influence il34 expression (Fig.5D). AAV2CSF1shRNA did alter some neuroimmune factors as tgfb (F1,12=8.755, p<0.01), il1b (F1,12=15.04, p<0.002), and tnfa (F1,12=15.23, p<0.002) were significantly reduced in AAV2CSF1shRNA/CON mice (Fig.S4A&B), suggesting that reduced CSF1 may elicit adaptations in neuron-microglia signaling factors. Additional immunohistology showed that CUS induced elevation of CSF1 protein in AAV2SCR-infected neurons was blocked in AAV2CSF1shRNA- infected neurons (F1,11=7.41, p<0.02, Fig.5E&F).
Figure 5. AAV2CSF1shRNA attenuated -stress-induced up-regulation of neuronal CSF1 in the medial PFC.
A) Schematic of plasmid design for pEGFP-CSF1shRNA. Relative mRNA levels of csf1 in N2a cells transfected with pEGFP-SCR or pEGFP-CSF1shRNA are shown. Experimental timeline is shown for in vivo CSF1 knockdown studies. Male wild-type mice received bilateral infusion of AAV2SCR or AAV2CSF1shRNA and after recovery, mice were subjected to 14 days of CUS. B) Representative images of AAV2SCR and AAV2CSF1shRNA viral infection in the medial PFC. White scale bar represents 500 m. Following CUS and behavioral testing medial PFC was collected from a subset of mice for mRNA analyses. Relative mRNA levels of (C) csf1 and (D) il34 are shown (n = 4). A subset of control and CUS male mice infused with AAV2SCR or AAV2CSF1shRNA were perfused and brains were collected for CSF1 immunohistology. E) Representative images of EGFP+ neurons with CSF1 co-labeling are shown for each experimental group. White scale bar represents 10 m. F) Quantification of average relative fluorescent intensity for CSF1 immunolabeling is represented as percent AAV2SCR: CON (n = 4). Bars represent the mean ± S.E.M. Means that are significantly different than respective control group based on ANOVA are denoted (main effect: ^, p < 0.05 or †, p < 0.07), (interaction: *, p < 0.05).
Knockdown of neuron-derived CSF1 blocked microglia-mediated neuronal remodeling in the medial PFC following CUS.
To examine the role of neuron-derived CSF1 in stress-associated changes in microglia function, male mice were infused with AAV2SCR or AAV2CSF1shRNA in the medial PFC and then exposed to CUS. After behavioral analyses mice were perfused and brains were collected for immunohistology. CUS-induced increase in Iba-1+ proportional area in microglia morphology were attenuated in mice that received AAV2CSF1shRNA (F1,11=12.36, p<0.005, Fig.S5A–C). The modest increase in the number of Iba-1+ microglia (F1,11=2.84, p=0.12) after CUS was also blocked in mice that received AAV2CSF1shRNA (F1,11=6.463, p<0.03, Fig.S4D). These findings indicate that knockdown of neuronal CSF1 with AAV2CSF1shRNA prevented CUS-induced alterations in morphology and number of Iba-1+ microglia.
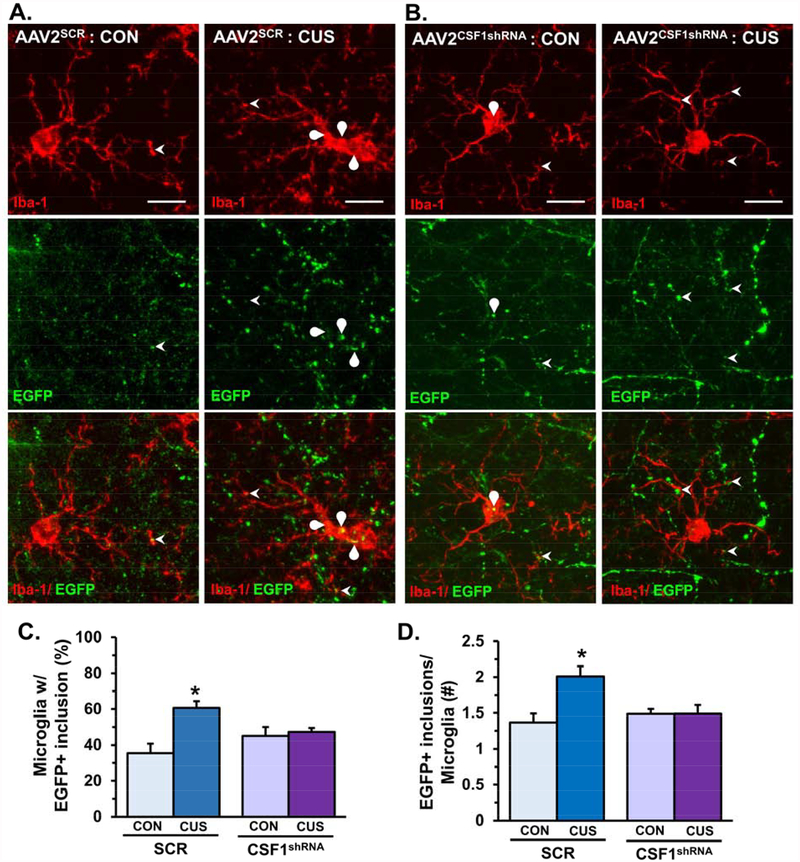
The EGFP reporter of AAV2SCR and AAV2CSF1shRNA enabled further analyses of microglia interactions with pyramidal neuron apical dendrites in layer I of the medial PFC (Fig.6A&B). Consistent with prior results (Fig.4) CUS significantly increased the proportion of microglia in layer I of the medial PFC with EGFP+ inclusions, and these microglia responses were attenuated in mice that received AAV2CSF1shRNA (F1,11=7.55, p<0.02, Fig.6C). Moreover, CUS increased the average number of EGFP+ inclusions in microglia and AAV2CSF1shRNA reduced the number of EGFP+ inclusions per microglia after CUS (F1,11=7.18, p<0.02, Fig.6D). These results demonstrate that neuron-derived CSF1 promotes microglia-mediated phagocytosis of dendritic elements in the medial PFC after CUS.
Figure 6. Knockdown of neuron-derived CSF1 blocked microglia-mediated dendritic remodeling in the medial PFC following chronic unpredictable stress.
Male wild-type mice received bilateral infusion of AAV2SCR or AAV2CSF1shRNA and after recovery, mice were subjected to 14 days of CUS. Mice were perfused and brains collected for immunohistology 2–4 hours after NSF. Representative confocal images obtained from layer I of the medial PFC (proximal to virus infection) in control or CUS male mice infused with (A) AAV2SCR or (B) AAV2CSF1shRNA. Arrowhead shows microglia process interacting with dendrite or dendritic spine and curved arrow notes EGFP+ neuronal elements engulfed in microglia process. White scale bar represents 10 m. C) Proportion of microglia in layer I of medial PFC with EGFP+ inclusions (n = 12–15 microglia/ sample). E) Average number of EGFP+ inclusions in microglia with neuronal elements (n = 3–10 microglia/ sample). Bars represent the mean ± S.E.M. (n = 4/ group). Means significantly different than respective control group based on ANOVA are denoted (interaction: *, p < 0.05).
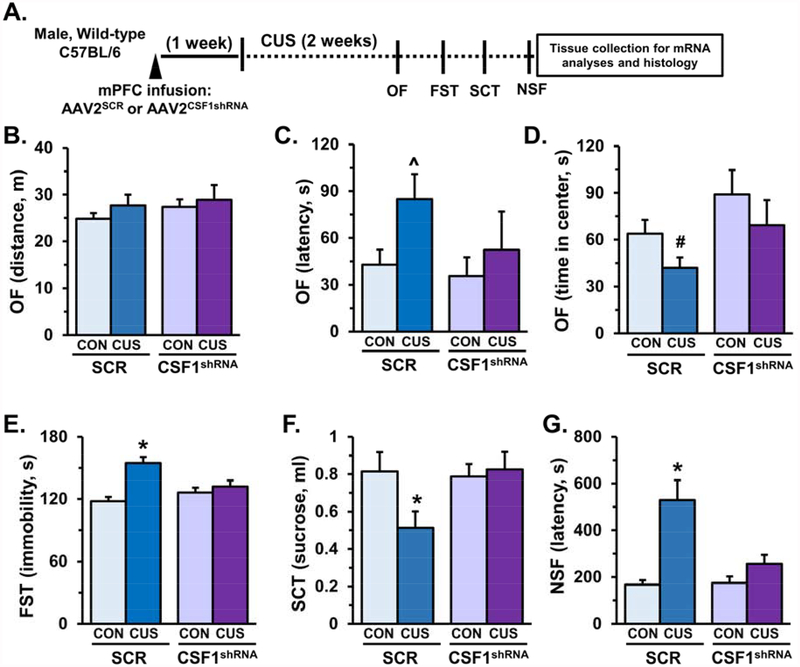
Knockdown of neuron-derived CSF1 prevented CUS-induced anxiety- and depressive-like behaviors.
Behavioral effects of neuronal CSF1 knockdown were examined in male mice exposed to 14 days of CUS (Fig.7A). In the OF, no significant differences in total distance traveled were observed (Fig.7B), however, in mice that received AAV2SCR CUS increased the latency to enter the center (F1,27=3.05, p=0.09, Fig.7C) and reduced time spent in center (F1,27=2.64, p=0.11, Fig.7D). In both metrics mice that received AAV2CSF1shRNA had blunted responses following CUS and showed a relative increase in time spent in center (F1,27=4.25, p<0.05, Fig.7D). In the FST, CUS increased immobility in mice that received AAV2SCR and these effects were prevented in mice that received AAV2CSF1shRNA (F1,26=8.42, p<0.008, Fig.7E). Similarly, in the SCT, CUS-induced reductions in sucrose consumption were blocked in mice that received AAV2CSF1shRNA (F1,27=3.66, p=0.07, Fig.7F). In the NSF assay, AAV2SCR mice exposed to CUS had increased latency to feed, while mice that received AAV2CSFshRNA had similar latency to feed as controls (F1,27=7.43, p<0.01, Fig.7G).
Figure 7. AAV2CSF1shRNA prevented stress-induced anxiety- and depressive-like behaviors.
Male wild-type mice received bilateral infusion of AAV2SCR orAAV2CSF1shRNA and after recovery, mice were subjected to 14 days of chronic unpredictable stress (CUS). On subsequent days behavior was assessed in the open field (OF), forced swim test (FST), sucrose consumption test (SCT), and novelty-suppressed feeding (NSF) on subsequent days. A) Schematic showing experimental approach and timeline. On the final day of CUS activity in the open field (OF) was assessed and total distance (B), latency to enter the open field (C), and time spent in the center of the open field (D) are shown. The following day immobility in the forced swim test (FST) was measured, immobility in minutes 2–6 is shown (E). On the subsequent day the sucrose consumption test (SCT) was performed, and total sucrose consumer is shown (F). Last, the novelty-suppressed feeding test was administered, latency to feed is shown (G). Bars represent the mean ± S.E.M. Means that are significantly different than respective control group based on ANOVA are denoted (main effect: ^, p < 0.05 or #, p = 0.08), (interaction: *, p < 0.05).
Discussion
Clinical and preclinical research demonstrates that the pathophysiology of MDD includes neuronal atrophy and synaptic deficits in the medial PFC (3–5). While intrinsic molecular mechanisms that contribute to neuronal atrophy have been studied (31, 32), the roles of other cellular mediators in stress-induced synaptic deficits have not been extensively studied. Recent findings indicate that microglia support homeostatic neuronal function and modulate synaptic plasticity (11, 12, 33). Moreover, repeated stress exposure causes morphological and functional alterations in microglia (4, 9, 10), implicating microglia in the neurobiology of depressive-like behaviors. Here we show that CUS caused anxiety- and depressive-like behaviors in male and female mice, with changes more pronounced in male mice. These behavioral effects were associated with markers of dysregulated microglia function, including altered expression of neuron-derived factors that modulate microglia activation as well as morphological changes in microglia in the medial PFC.
One of the prominent neuron-derived factors increased in the medial PFC following CUS was colony stimulating factor-1 (CSF1). In addition, consistent with the murine CUS model, CSF1 mRNA levels were elevated in postmortem dorsolateral PFC obtained from depressed individuals. CSF1 is a key neuron-derived signal that modulates microglia in physiological and pathological conditions (15). Indeed, studies show that dystrophic neuronal responses in models of kainic acid-induced excitotoxicity or sensory nerve injury increased CSF1 expression, leading to microglia activation (18, 19). Consistent with these findings, CUS-induced CSF1 expression in the medial PFC corresponded with increased expression of immunomodulatory receptors, including CSF1 receptor, in enriched microglia. It is notable that different patterns of gene expression were observed between whole homogenates and enriched microglia in the frontal cortex. In particular, CSF1R and TGFβR expression was decreased in the whole PFC, but expression of these receptors was increased in enriched microglia. These opposing expression patterns may be linked to the differential role of CSF1R and TGFβR in neurons as compared to microglia. For instance, CSF1R and TGFβR signaling in neurons mediates neurodevelopment and promotes neuroplasticity (34, 35), while CSF1R signaling in microglia is essential for their viability (15) and TGFβR signaling promotes the unique molecular and functional profile of microglia (17, 36). These divergent roles in neurons and microglia may underlie differential expression of CSF1R and TGFβR following stress. In addition, mRNA levels of IL-1β and TNFα were significantly decreased in enriched microglia, and not whole PFC, as these cytokines are enriched in immune cells. Other studies using longer bouts of CUS increased IL-1β and/or TNFα levels in the hippocampus, which contributed to development of depressive-like behaviors (20, 37). These contrasting results may reflect dynamic neuroimmune effects dependent on stress duration, which is consistent with other findings that show brain region-specific microglia responses that elicit divergent neurobiological effects (38–40). Together, the findings indicate that CUS-induced anxiety- and depressive-like behaviors are linked to neuron-derived factors that promote functional and molecular alterations in microglia.
The functional role of microglia has expanded as seminal reports demonstrate that microglia sculpt neurocircuitry during neurodevelopment in an activity-dependent manner (41, 42) and eliminate dystrophic synapses in disease models (43, 44). Using Thy1-GFP-M mice we show that microglia processes in unstressed mice are in close proximity to dendrites and axons in layer I of the medial PFC, and subsets of microglia (~40%) contained GFP+ inclusions. This is consistent with other studies showing microglia regularly sample synaptic and dendritic elements in the cortex (43). Further analyses revealed that CUS significantly increased the proportion of microglia in layer I of the medial PFC (~75%) that contained GFP+ inclusions. CUS also increased the frequency of microglial phagocytic cups in layer I of the medial PFC, which is pertinent because these structures are observed in other models of microglia-mediated neuronal remodeling (41, 45, 46). Further immunohistology showed that GFP+ inclusions were co- localized with CD68+ lysosomal markers within microglia, indicating that these neuronal elements had been internalized via phagocytosis. These functional changes in microglia coincided with decreased dendritic spine density on apical dendrites of pyramidal neurons in the medial PFC. Moreover, microglia (Iba-1+) in the medial PFC of control and CUS mice co- localized with resident microglia-specific marker, P2Y12 receptor. These results indicate that stress-induced neuronal remodeling can be attributed to resident microglia, rather than trafficking peripheral monocytes/macrophages. This is consistent with recent studies that show trafficking peripheral monocytes/macrophages utilize neurovascular signaling to promote development of social defeat-induced anxiety-like behavior (47–49). Notably, the proportion of microglia with GFP+ inclusions in layer V of medial PFC or layer I of somatosensory cortex did not change following CUS. Altogether, these findings demonstrate that resident microglia mediate neuronal remodeling in a laminar- and region-specific manner, which likely contributes to selective synaptic deficits in layer I of the medial PFC following repeated stress (14, 50). It is unclear how microglia target specific neuronal elements, and it is possible that GFP+ inclusions in microglia may comprise axonal and dendritic components (51). Further studies will need to utilize higher resolution microscopy to assess spatial relationships between microglia and neuronal elements and examine the specificity of microglia phagocytosis in the medial PFC.
An important finding in these studies was that microglia showed differential sex- dependent responses to CUS, which corresponded with susceptibility to anxiety- and depressive- like behaviors. Microglia from male mice had higher CSF1 receptor expression that corresponded with increased phagocytosis of GFP+ neuronal elements and significant reductions in dendritic spine density following CUS. Since CSF1 was increased in both males and females, intrinsic molecular pathways (i.e., CSF1R) may underlie divergent microglia responses after CUS. Our findings are consistent with preclinical studies that show females are resilient to stress-induced neuronal impairments and behavioral or cognitive deficits (52–54), which may be linked to blunted microglia responses following stress (55). These findings contrast with clinical reports that show higher incidence of MDD in women than men (56, 57). Several factors may contribute to the increased risk of MDD in females, including drastic fluctuations in sex hormones (e.g., during adolescence and pregnancy) (58, 59), as well as differential neuroendocrine and immune reactivity to environmental and social stressors (60). In all, our findings suggest that females demonstrate partial resiliency in this stress paradigm, and more pronounced changes in behavior and neuroimmune dysfunction may be unmasked with longer CUS exposure. Further studies into sex-dependent differences in microglia, including paradigms that model fluctuations in sex hormones, and subsequent response to stressors may provide insight into susceptibility for mental health disorders.
Prior studies indicate that stress-induced neuroendocrine and neurotransmitter fluctuations mediate morphological and functional changes in microglia following stress exposure (24, 61, 62). Here we provide initial evidence that local neuron-derived signals promote functional alterations in microglia, which, in turn shape neuronal and behavioral responses to stress. Of note, knockdown of neuronal CSF1 attenuated microglia-mediated dendritic remodeling in layer I of the medial PFC and prevented development of anxiety- and depressive- like behaviors following CUS. Previous studies implicated microglia in the neurobiology of stress as minocycline blocked stress-induced microglia activation and neuronal FosB in the medial PFC (63). Furthermore, recent work revealed that microglia phagocytosed neuronal elements in CA1 of the hippocampus, which corresponded with synaptic plasticity deficits and anhedonia after CUS (51). Taken together, these findings indicate that microglia actively shape neuronal responses to stress, leading to dendritic remodeling and development of depressive-like behaviors.
In summary our findings provide further evidence that brain-resident microglia are critical mediators of neuroplasticity and reveal a novel cellular pathway that contributes to the pathophysiology of stress-induced depressive-like behaviors. Moreover, chronic stress exposure elicited divergent neuroimmune responses in males and females, demonstrating sex-dependent differences in neuron-microglia interactions. Further microglia contributed to neuronal remodeling in layer I, but not layer V, of the medial PFC indicating that neuron-microglia interactions are compartmentalized within specific brain regions. While microglia responses are typically directed to restore neuronal homeostasis (64, 65), chronic stress exposure causes microglia-mediated neuronal remodeling that contributes to synaptic deficits and anxiety- and depressive-like behaviors. These findings indicate that interventions aimed at normalizing neuron-microglia interactions, such as CSF1 signaling, may provide therapeutic benefits in affective disorders.
Supplementary Material
Acknowledgements:
This research was supported by NIMH Grants MH045481 and MH093897 to R.S.D, the State of CT, as well as a NARSAD Young Investigator Grant and University of Cincinnati Neurobiology Research Center Pilot Grant to E.S.W. We would like to thank Dr. Ralph DiLeone and Colin Bond for providing plasmids and assistance in molecular techniques. We would also like to thank the generous donation of human postmortem samples for these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors report no biomedical financial interests or potential conflicts of interest.
References:
- 1.Kessler RC (2012): The costs of depression. The Psychiatric clinics of North America. 35:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. (2013): The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA : the journal of the American Medical Association. 310:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016): Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nature medicine. 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wohleb ES, Franklin T, Iwata M, Duman RS (2016): Integrating neuroimmune systems in the neurobiology of depression. Nature reviews Neuroscience. 17:497–511. [DOI] [PubMed] [Google Scholar]
- 5.Christoffel DJ, Golden SA, Russo SJ (2011): Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 22:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ota KT, Liu RJ, Voleti B, Maldonado-Aviles JG, Duric V, Iwata M, et al. (2014): REDD1 is essential for stress-induced synaptic loss and depressive behavior.Nature medicine. 20:531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. (2010): mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. (2012): Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nature medicine. 18:1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yirmiya R, Rimmerman N, Reshef R (2015): Depression as a microglial disease. Trends in neurosciences. 38:637–658. [DOI] [PubMed] [Google Scholar]
- 10.Walker FR, Nilsson M, Jones K (2013): Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 14:1262–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Dissing-Olesen L, MacVicar BA, Stevens B (2015): Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 36:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A (2011): The role of microglia in the healthy brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 31:16064–16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnsten AF (2009): Stress signalling pathways that impair prefrontal cortex structure and function. Nature reviews Neuroscience. 10:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu RJ, Aghajanian GK (2008): Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proceedings of the National Academy of Sciences of the United States of America. 105:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, et al. (2014): Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 82:380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morganti JM, Nash KR, Grimmig BA, Ranjit S, Small B, Bickford PC, et al. (2012): The soluble isoform of CX3CL1 is necessary for neuroprotection in a mouse model of Parkinson’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 32:14592–14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. (2014): Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nature neuroscience. 17:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo J, Elwood F, Britschgi M, Villeda S, Zhang H, Ding Z, et al. (2013): Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J Exp Med. 210:157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, et al. (2016): Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nature neuroscience. 19:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, et al. (2015): Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biological psychiatry. [DOI] [PubMed] [Google Scholar]
- 21.Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS (2012): Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biological psychiatry. 72:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, et al. (2016): GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. The Journal of clinical investigation. 126:2482–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP (2012): Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice Psychoneuroendocrinology. 37:1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. (2011): β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 31:6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A (2004): Rational siRNA design for RNA interference. Nature biotechnology. 22:326–330. [DOI] [PubMed] [Google Scholar]
- 26.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ (2003): Local gene knockdown in the brain using viral-mediated RNA interference. Nature medicine. 9:1539–1544. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Franklin KBJ (2001): The mouse brain: in stereotaxic coordinates. 2nd edition. [Google Scholar]
- 28.Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, et al. (2016): GLYX-13 Produces Rapid Antidepressant Responses with Key Synaptic and Behavioral Effects Distinct from Ketamine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne S (2008): Automated Three-Dimensional Detection and Shape Classification of Dendritic Spines from Fluorescence Microscopy Images. PLoS ONE. 3:e1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker FR, Beynon SB, Jones KA, Zhao Z, Kongsui R, Cairns M, et al. (2014): Dynamic structural remodelling of microglia in health and disease: a review of the models, the signals and the mechanisms. Brain, behavior, and immunity. 37:1–14. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan V, Nestler EJ (2010): Linking molecules to mood: new insight into the biology of depression. The American journal of psychiatry. 167:1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnan V, Nestler EJ (2008): The molecular neurobiology of depression. Nature.455:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong S, Dissing-Olesen L, Stevens B (2016): New insights on the role of microglia in synaptic pruning in health and disease. Current opinion in neurobiology. 36:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chitu V, Gokhan S, Nandi S, Mehler MF, Stanley ER (2016): Emerging Roles for CSF-1 Receptor and its Ligands in the Nervous System. Trends in neurosciences. 39:378–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koeglsperger T, Li S, Brenneis C, Saulnier JL, Mayo L, Carrier Y, et al. (2013): Impaired glutamate recycling and GluN2B-mediated neuronal calcium overload in mice lacking TGF-beta1 in the CNS. Glia. 61:985–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, et al. (2014): Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 159:1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koo JW, Duman RS (2008): IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proceedings of the National Academy of Sciences of the United States of America.105:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vecchiarelli HA, Gandhi CP, Gray JM, Morena M, Hassan KI, Hill MN (2016): Divergent responses of inflammatory mediators within the amygdala and medial prefrontal cortex to acute psychological stress. Brain, behavior, and immunity. 51:70–91. [DOI] [PubMed] [Google Scholar]
- 39.Delpech JC, Madore C, Nadjar A, Joffre C, Wohleb ES, Laye S (2015): Microglia in neuronal plasticity: Influence of stress. Neuropharmacology. 96:19–28. [DOI] [PubMed] [Google Scholar]
- 40.Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, et al. (2016):Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nature neuroscience. 19:504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tremblay ME, Lowery RL, Majewska AK (2010): Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8:e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. (2012): Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 74:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J (2009): Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 29:3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. (2016): Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 352:712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, et al. (2010): Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 7:483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frost JL, Schafer DP (2016): Microglia: Architects of the Developing Nervous System. Trends Cell Biol. 26:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF (2014): Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 34:2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, et al. (2017): Microglial recruitment of IL-1beta-producing monocytes to brain endothelium causes stress-induced anxiety. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wohleb ES, McKim DB, Sheridan JF, Godbout JP (2015): Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 8:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duman CH, Duman RS (2015): Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett. 601:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milior G, Lecours C, Samson L, Bisht K, Poggini S, Pagani F, et al. (2015): Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain, behavior, and immunity. [DOI] [PubMed] [Google Scholar]
- 52.Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, et al. (2014): Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Molecular psychiatry. 19:588–598. [DOI] [PubMed] [Google Scholar]
- 53.Garrett JE, Wellman CL (2009): Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 162:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahmoud R, Wainwright SR, Chaiton JA, Lieblich SE, Galea LA (2016): Ovarian hormones, but not fluoxetine, impart resilience within a chronic unpredictable stress model in middle-aged female rats. Neuropharmacology. 107:278–293. [DOI] [PubMed] [Google Scholar]
- 55.Bollinger JL, Bergeon Burns CM, Wellman CL (2016): Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain, behavior, and immunity. 52:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker G, Fletcher K, Paterson A, Anderson J, Hong M (2014): Gender differences in depression severity and symptoms across depressive sub-types. Journal of affective disorders. 167:351–357. [DOI] [PubMed] [Google Scholar]
- 57.Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, et al. (2005): Gender differences in depression: findings from the STAR*D study. Journal of affective disorders. 87:141–150. [DOI] [PubMed] [Google Scholar]
- 58.Kessler RC (2003): Epidemiology of women and depression. Journal of affective disorders. 74:5–13. [DOI] [PubMed] [Google Scholar]
- 59.Steiner M, Dunn E, Born L (2003): Hormones and mood: from menarche to menopause and beyond. Journal of affective disorders. 74:67–83. [DOI] [PubMed] [Google Scholar]
- 60.Nemeth CL, Harrell CS, Beck KD, Neigh GN (2013): Not all depression is created equal: sex interacts with disease to precipitate depression. Biol Sex Differ. 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nair A, Bonneau RH (2006): Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 171:72–85. [DOI] [PubMed] [Google Scholar]
- 62.Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, et al. (2010): Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain, behavior, and immunity. [DOI] [PubMed] [Google Scholar]
- 63.Hinwood M, Morandini J, Day TA, Walker FR (2012): Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex. 22:1442–1454. [DOI] [PubMed] [Google Scholar]
- 64.Davies LC, Jenkins SJ, Allen JE, Taylor PR (2013): Tissue-resident macrophages. Nat Immunol. 14:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wohleb ES (2016): Neuron-Microglia Interactions in Mental Health Disorders: “For Better, and For Worse”. Front Immunol. 7:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.