Abstract
Induction of a sustained and broad antibody (Ab) response is a major goal in developing a protective HIV-1 vaccine. DNA priming alone shows reduced levels of immunogenicity; however, when combined with protein boosting is an attractive vaccination strategy for induction of humoral responses. Using the VC10014 DNA and protein-based vaccine consisting of HIV-1 envelope (Env) gp160 plasmids and trimeric gp140 proteins derived from an HIV-1 clade B infected subject who developed broadly neutralizing serum Abs, and which has been previously demonstrated to induce Tier 2 heterologous neutralizing Abs in rhesus macaques, we evaluated whether MPLA and IL-33 when administered during the DNA priming phase enhances the humoral response in mice. The addition of IL-33 during the gp160 DNA priming phase resulted in high titer gp120-specific plasma IgG after the first immunization. The IL-33 treated mice had higher plasma IgG Ab avidity, breadth, and durability after DNA and protein co-immunization with alum adjuvant as compared to MPLA and alum only treated mice. IL-33 was also associated with a significant IgM Env-specific response and expansion of peritoneal and splenic B-1b B cells. These results indicate that DNA priming in the presence of exogenous IL-33 qualitatively alters the HIV-1 Env-specific humoral response, improving the kinetics and breadth of potentially protective Ab.
Keywords: HIV-1, envelope, vaccine, antibody, IgM, IL-33
Introduction
HIV-1 infection remains a serious health issue worldwide with 36.9 million people living with HIV-1 and 1.8 million estimated new infections in 2017 [1]. Although with increased access to anti-retroviral therapy, particularly in sub-Saharan Africa, the incidence of new infections world-wide is decreasing, it is becoming evident that there are numerous recalcitrant regions and high-risk groups in which rates of new infections are stable or even increasing [2] underscoring the need for an effective preventive HIV-1 vaccine. Induction of a sustained broadly neutralizing antibody response to HIV-1 is a primary goal of HIV-1 vaccine development, however, despite the development HIV broadly neutralizing antibodies in a subset of HIV-infected patients, their induction through immunization has been difficult to achieve. Elegant technological advancements have been made in obtaining and characterizing broadly neutralizing antibodies (bNAbs) isolated from HIV-infected subjects [3] and this work has provided rich insight into the key sites of vulnerability on HIV-1 Envelope (Env), the molecular commonalities of these Abs, and their evolution during the infectious process. In turn this has led to the design and pre-clinical evaluation of immunogens that attempt to recapitulate the evolutionary process of bNAb development [4, 5]. Such rational immunogen design will likely need to be coupled with proper conditioning to ensure robust B cell engagement gives rise to persistent long-lived humoral immunity to confer durable protection.
Priming conditions, mainly determined by adjuvant and platform (e.g. protein, DNA, viral vector) can substantially influence quality of the B cell response, including breadth and durability. DNA is an attractive priming strategy when combined with protein or viral vector boosting; however, DNA priming alone typically fails to induce substantial Ab, limiting its potential as a stand-alone immunogen [6, 7]. The microenvironment in which B cell priming occurs, defined in large part by the local composition of cytokines and growth factors, is likely to influence the quality of the B cell memory and antibody response. This microenvironment can be conditioned through the delivery of exogenous factors [8].
One such factor is IL-33, which remains under-explored in the context of B cell responses, and several lines of observation suggest it could be advantageous in enhancing the HIV-1 Env-specific response. IL-33 is an alarmin, expressed by numerous cell types, particularly initiated during host defenses and or tissue damage [9, 10]. IL-33 has pleiotropic cytokine activities including mediating diverse pro-inflammatory responses [11], activation and recruitment of antigen-presenting cells [12], and enhancing adaptive immunity [13]. A few studies have demonstrated its adjuvant-like ability to enhance antibody and T cell responses [14, 15]. IL-33, an IL-1 family member mediates its biological effects via ST2 complexed with the IL-1R accessory protein (IL1RAcP) notably expressed on Th2 CD4+ T cells, ILC2, basophils, and mast cells [16–19]. IL-33 has been shown to enhance B-1 B cell proliferation, and IL-5 and IgM secretion [20]. IL-33 exposure of B-1b B cells also increases production of IL-13, a potent Th2 cytokine [21]. IL-33 is a potent inducer mucosal IgM+ IL-10-producing regulatory B cells [22] in mice, and correlates with increased plasma total IgM and auto-reactive antibodies in rheumatoid arthritis patients [23].
Numerous features of IgM memory suggest that with adequate engagement it could be a valuable contributor to an effective B cell response to HIV. These features include their rapid response, unique and polyreactive immunoglobulin (Ig) repertoire with intrinsic Env reactivity, neutralizing activity, expansive mucosal distribution, strong complement activation, and enhanced antigen presentation abilities [24–26]. Additionally, IgM has been shown to contribute to neutralization of other viruses including dengue, influenza, VZV, and smallpox [27–30]. The ability of IgM memory to differentiate upon antigen-stimulation into IgG and IgA memory and antibody secreting cell (ASC) populations [31] suggest they may contribute to qualitatively distinct Env-specific Ab responses.
Various studies in animal models and humans have shown the ability of the adjuvant, MPLA (3-O-desacyl-4′-monophosphoryl lipid A), a TLR4 agonist, and non-toxic derivative of LPS (lipopolysaccharide), to enhance both antibody and cellular immune response to vaccination [32]. MPLA upregulates innate immune responses including activating antigen-presenting cells and inducing pro-inflammatory cytokines such as TNF-α, IL-1β and IL-12 which in turn enhances features of the adaptive immune response, inducing T helper cells and B cell responses [33–35]. After immunization with MPLA as an adjuvant, higher antibody persistence, enhanced immunological memory and a superior anamnestic response has been observed [36, 37]. Priming in the absence of inflammation (antigen only) and boosting with MPLA [36, 38] results in the induction and long-term persistence of antigen-specific B-1 B cell and IgM Ab responses in mice [38].
In this study, we used the VC10014 DNA and protein-based HIV-1 vaccine which consists of gp160 plasmids and trimeric gp140 proteins derived from an HIV-1 clade B infected subject who developed broadly neutralizing Abs [39, 40], and which has been previously shown to induce Tier 2 heterologous neutralizing Abs in rabbits [41] and rhesus macaques [42]. Using this immunogen platform, we evaluated the impact of IL-33 and MPLA provided during the DNA priming phase to impact the kinetics and quality of the antibody and B cell response.
MATERIALS AND METHODS:
Mice
Young (6 to 8 weeks of age) C57BL/6J female mice were obtained from The Jackson Laboratories. All animal experiments were approved by the University of Rochester University Committee on Animal Resources and performed in compliance with guidelines defined by National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
HIV envelope DNA and protein VC10014 immunogen
Six HIV envelope DNA (F8, G6a, E5a, C6a, H10 and G10a clade B gp160 DNA) and 2 trimeric proteins (F8 and C6a clade B gp140 protein) from VC10014 were a kind gift from Haigwood Laboratory (OHSU, OR). The trimeric gp140 proteins were prepared as previously described, briefly the gp140 DNA was derived from the gp160 env sequence by site-directed mutagenesis (Stratagene, La Jolla, CA) to insert the previously described mutations [5, 43] in the primary and secondary protease cleavage sites respectively: REKR → RSKS and KAKRR → KAISS. A large-scale endotoxin-free plasmid preparation (Qiagen, Valencia, CA) was used for stable expression in 293F cells for protein production as described previously [44]. Epitope exposure and antigenicity of gp140 trimeric protein immunogens was assessed by ELISA, biolayer interferometry (BLI), and surface plasmon resonance for binding of multiple bNmAbs as described previously [41]. The HIV envelope DNA obtained from Haigwood laboratories were first used to transform E. coli DH5α and developed into permanent stocks. Plasmid purification for DNA immunizations was performed using endotoxin free mega prep kits (Qiagen# 12381, Germantown, MD) according to manufacturer’s protocol.
Immunizations
C57BL/6J mice were injected intramuscularly (i.m.) and intraperitoneally (i.p.) either with MPLA (20 μg) (InvivoGen # vac-mpls, San Diego, CA) or recombinant mature IL-33 protein (17.9 kDa) (2.5 μg) (Peprotech, Rocky Hill, NJ, #210–33) at one week prior to and at the time of priming (week 0 and week 3) with VC10014 envelope DNA plasmids (F8, G6a, E5a, C6a, H10 and G10a Clade B gp160 DNA). Following priming phase, mice were co-immunized with DNA plasmids and gp140 proteins (C6a and F8 gp140) at week 7 and week 11. A total of 30 μg of DNA (5 μg of each DNA plasmid) were given intramuscularly along with 25 μg of recombinant gp140 trimeric protein (12.5 μg each recombinant protein) were delivered intramuscularly by needle injection with alum (aluminium hydroxide, Alhydrogel adjuvant, Invivogen vac-alu-250) as the adjuvant. Non-immunized mice did not receive any injections. Blood was collected by submandibular bleed into EDTA containing tubes; plasma was separated and stored at −80 °C until the assays were performed. C57BL/6J mice were injected intramuscularly (i.m.) and intraperitoneally (i.p.) with IL-33 (2.5 μg) at one week prior to and at the time of priming (week 0 and week 3) with 100 μg of ovalbumin (Invivogen # vac-pova, San Diego, CA) and alum.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs), peritoneal cells (PerC),and splenocytes were collected from mice and were stained for 1 h with anti-IgG-FITC (Biolegend-406001, San Diego, CA), anti-CD95-PerCPefluor710 (Ebioscience-46–0951-82), anti-CD21-Pacific Blue (Biolegend-123414,San Diego, CA), anti-CD14-DyLight405LS (Novus Biologicals, Centennial, CO), anti-CD11b-BV570 (Biolegend-101233, San Diego, CA), anti-CD4-Qdot605 (Invitrogen-Q10092,), anti-CD23-BV786 (BD Horizon-563988, San Jose, CA), anti-CD19-BV711 (Biolegend-115555, San Diego, CA), anti-CD23-BV650 (BD-Horizon-563545, San Jose, CA), anti-GL7-AlexaFluor647 (Biolegend-144606, San Diego, CA), anti-CD45R-AlexaFluor700 (Biolegend-103232), anti-IgDAPC-Cy7 (Biolegend-405716), anti-CD43-PE (Biolegend-143206, San Diego, CA), anti-IgM-PE-Dazzle594 (Biolegend-406530, San Diego, CA), anti-CD5-PE-Cy5 (Biolegend-100610, San Diego, CA), and anti-CD1d-PE-Vio77 (Miltenyi Biotec-130–105-157, San Diego, CA). Cells were washed twice and stained with live/dead fixable yellow (Invitrogen) for 20 m. One-to-five million total events per sample were recorded on an LSRII instrument (BD Biosciences) and analysis was performed using FlowJo software (Treestar, Inc, Ashland, OR).
ELISA
The binding antibody response to multiple HIV-1 Env proteins was measured by enzyme-linked immunosorbent assay (ELISA). To measure HIV-1 Env-specific or OVA-specific IgG or IgM, 96 well flat-bottom immunoplates (Thermo Scientific) were coated overnight either with Clade A 92RW020 gp120 (Immune Technology Corp. # IT-001–001p), Clade B gp41 (Prospec # hiv-112-a), or proteins obtained from the NIH AIDS reagent repository: Clade B MN gp120 (#12570), Clade C 96ZM651 gp120 (#10080), Clade C CN54 gp120 (#7749) , Clade B gp140 SF162 (#12026), RSC 3 Clade B gp120 (#12042), Clade B F8 gp140, or Clade B C6a gp140 produced in the Haigwood laboratory, or ovalbumin protein. Proteins were coated at a concentration of 0.5 μg/ml in PBS, blocked with 3% BSA in PBS for 1 h, then washed with 0.05% Tween 20 in PBS. Samples were diluted to 1:500 and 1:2,500 in PBS containing 0.05% Tween 20 and added in duplicate to plate and incubated for 1h. Plates were washed and binding was detected using anti-mouse IgG-HRP (Jackson ImmunoResearch #115–035-003, West Grove, PA), anti-mouse IgM-HRP (Jackson ImmunoResearch #115–035-075, West Grove, PA), anti-mouse IgG1-HRP (Southern Biotech #1071–05, Birmingham, AL), or anti-mouse IgG2b-HRP (Southern Biotech #1091–05, Birmingham, AL) at a dilution of 1:2000 and developed by KPL SureBlue TMB Substrate. OD values of test plasma sample were divided by assay plate-specific PBST only control wells to obtain relative units (RU), and subsequent area under the curve values across the dilutions determined, unless otherwise noted.
For avidity ELISA, assay was performed similar to previously described [45–47], briefly plates were coated with CN54 gp120 (Acro Biosystems, # GP4-V15227–100ug Newark), coated plates were blocked with 3% BSA and washed thrice with PBS. Plasma samples were diluted to 1:100 and added to plates and incubated for 1 h, washed, and then 8M urea was added for 30 min. Plates were washed and anti-mouse IgG-HRP or anti-mouse IgM-HRP were added and developed by KPL SureBlue (Sera care, Milford, MA) TMB Substrate.
Statistical Analysis
Data were analyzed using GraphPad Prism (version 5.0d, GraphPad Software Inc, La Jolla, CA) to calculate area under the curve values and statistical significance. A minimum group size of n=5 mice was used for experiments. For two group comparisons, a two-tailed unpaired Mann-Whitney test was used.
Results
IL-33 influences the kinetics and isotype of the antibody response.
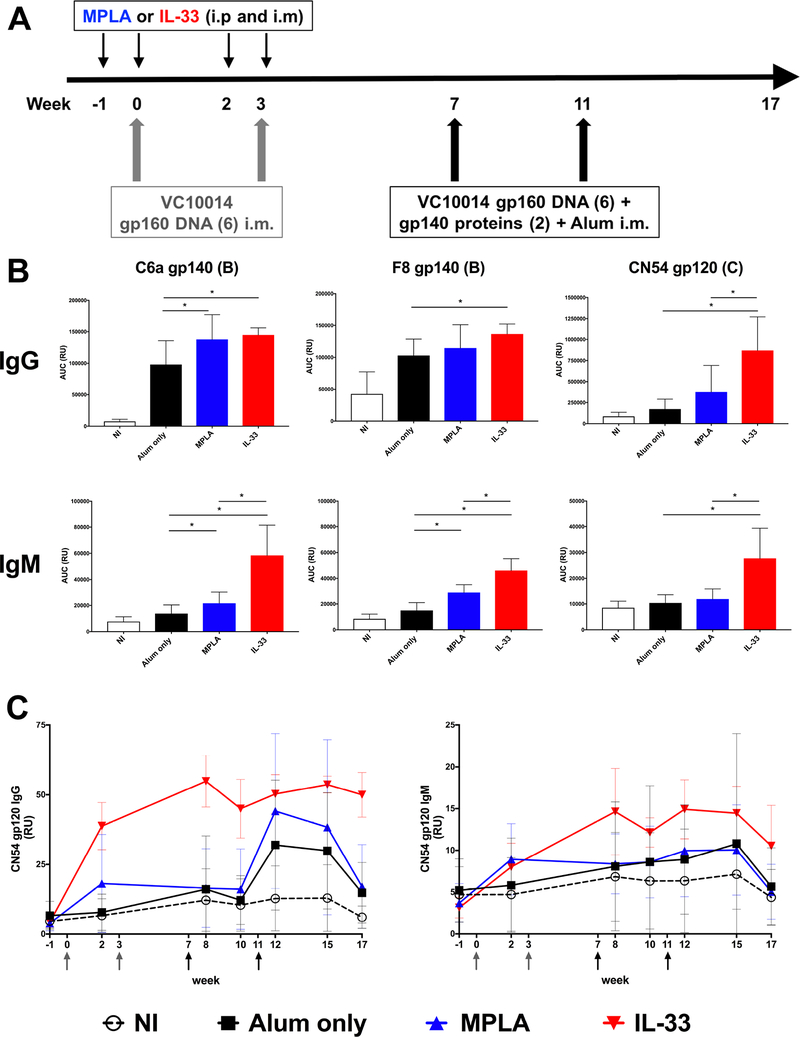
Using the clade B VC10014 HIV-1 Env-based immunogens, C57BL/6 mice were primed with VC10014 gp160 plasmid DNA at wk 0 and wk 3. To specifically influence the conditions in which priming occurs, mice were treated with exogenous conditioning agents, either IL-33 or MPLA, one week prior to and at time of plasmid DNA immunizations. Following this priming phase, mice were boosted with co-immunizations of VC10014 gp160 DNA plasmids and gp140 proteins in alum at wk 7 and wk 11 (Fig. 1A). Examining the plasma binding antibody response at one week following the final immunization (wk 12), treatment with IL-33 or MPLA enhanced the Env-specific IgG response compared to immunization with alum only, with IL-33 treatment resulting in significantly increased (p<0.05) autologous (C6a and F8) gp140 and heterologous clade C CN54 gp120 Env-specific IgG compared to alum only, and the CN54 gp120 IgG response was also significantly greater (p<0.05) compared to MPLA treatment (Fig. 1B), suggesting IL-33 is increasing the breadth of the Env-specific IgG response. IL-33 treatment resulted in ~2–3-fold greater Env-specific IgM that was significant (p<0.05) compared to both alum only and MPLA treated mice.
Figure 1. IL-33 improves the Env-specific IgG and IgM response.
A. Immunization schedule. Mice received MPLA (20 μg) or IL-33 (2.5 μg) i.p. and i.m. one week prior to and at time of i.m. priming immunizations with VC10014 DNA plasmids and were then boosted i.m. with co-immunization of VC10014 DNA plasmids and proteins in alum. B. Plasma Env-specific IgG and IgM determined by ELISA at week 12. C. Longitudinal plasma (1:500) CN54 gp120-specific IgG and IgM. Symbols represents group mean ± SD. NI: n=16, Alum only: n=16, MPLA: n=11, IL-33: n=5. * indicates significant difference (p<0.05) as determined by two-tailed Mann-Whitney test. Data are representative of three independent experiments.
Examining the longitudinal plasma heterologous CN54 gp120 Ab response; as soon as week 2, which is just after the first VC10014 DNA immunization, significant levels (p<0.05) of Env-specific IgM and IgG are evident in the IL-33 group, and to a lesser degree in the MPLA group compared to the alum only group (Fig. 1C). The CN54 IgG titers in all the immunized groups further increased after the DNA and protein co-immunizations. The CN54 IgM titers were consistently highest in the IL-33 group following week 2. Together, these results indicate IL-33 influences the kinetics of the plasma Ab response and promotes the development of Env-specific IgM.
IL-33 enhances the quality of the antibody response.
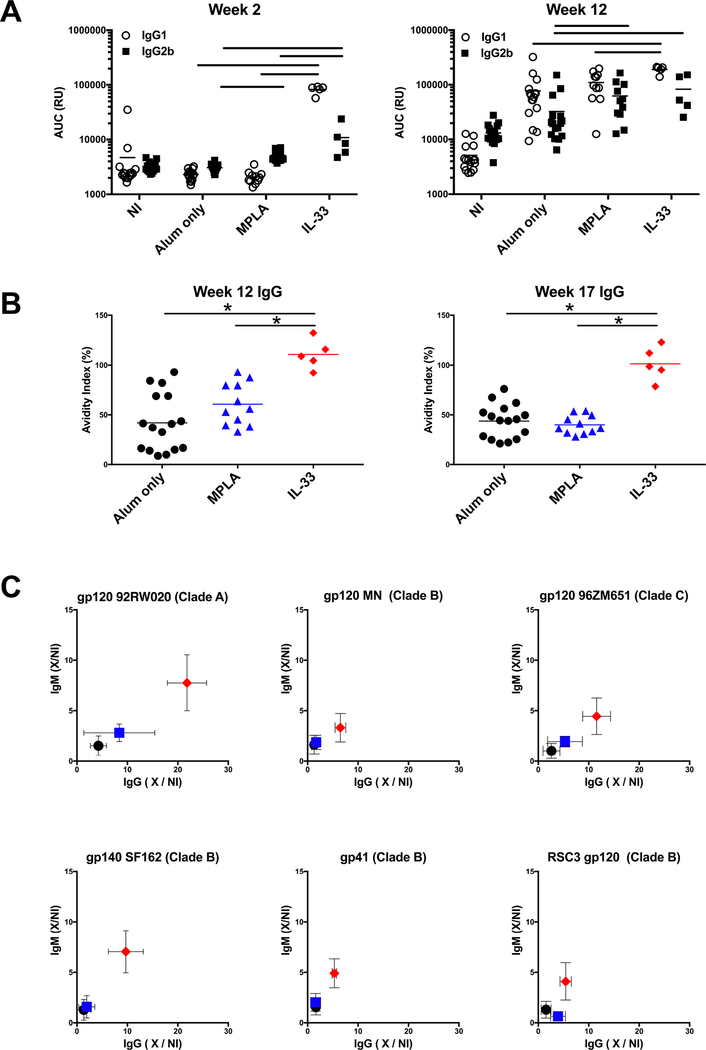
The influence of the priming in the presence of IL-33 or MPLA on the HIV Env-specific IgG subclass utilization was evaluated. While the early gp120 response at wk 2 is dominated by IgG2b in MPLA treated mice, IL-33 treatment results in an IgG1 dominated response (~10:1 IgG1:IgG2b ratio) (Fig. 2A). After the last immunization (wk 12), IgG1 dominated the gp120-specific response in all the immunized groups, with the IL-33 group having significantly higher IgG1 compared to all groups, and significantly higher IgG2b compared to alum only.
Figure 2. IL-33 impacts the quality of the Env-specific antibody response.
A. CN54 gp120-specific plasma IgG1 and IgG2b was determined by ELISA. Symbols represent individual mice. Lines indicate significant difference (p<0.05) between groups. B. Avidity index of CN54 gp120-specific plasma IgG determine by ELISA in the absence or presence of 8M urea treatment. Symbols represent individual mice. * indicates significant difference (p<0.05) between groups as determined by two-tailed Mann-Whitney test. C. Plasma (wk 12) was evaluated for IgM and IgG binding to indicated Env by ELISA. Symbols represent group mean ± SD fold increase in titer compared to the non-immunized group (X/NI). NI: n=16, Alum only: n=16, MPLA: n=11, IL-33: n=5. Data are representative of three independent experiments.
Next, we assessed the influence of IL-33 and MPLA on the strength of binding of the HIV Env-specific IgG response by measuring the amount of binding that is maintained following treatment with a chaotropic agent (avidity index). The presence of IL-33 during the priming phase resulted in significantly enhanced avidity compared to the alum only and MPLA groups, with nearly all IgG binding activity maintained following 8M Urea treatment for both the peak plasma Ab response (wk 12) and the durable (wk 17) plasma Ab (Fig. 2B).
To assess the breadth of the plasma Ab response the IgM and IgG binding Ab at wk 12 that was induced to various Env proteins was measured. The IL-33 group demonstrated high titer IgM and IgG binding against the various clade A, B, and C gp120 proteins, including Resurfaced Core 3 (RSC3) gp120, which lacks the V1-V3 variable loops and is indicative of Abs targeting the more conserved core region which contains the CD4 binding site [48]. Increased titers to gp41 were also evident in the IL-33 group (Fig. 2C). Overall these results indicate that IL-33 treatment during priming results in greater Env-specific IgG1, avidity, and breadth.
The influence of IL-33 on the early phase of the B cell response.
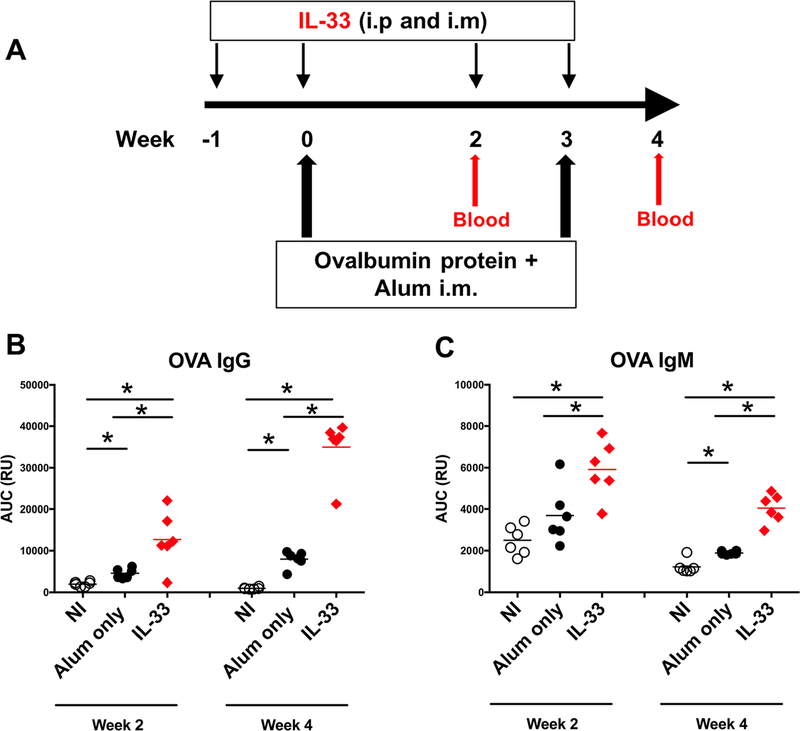
Observing the pronounced influence of IL-33 when present during the priming phase, we sought to better resolve the cellular impacts of IL-33 during the early phase of the response. For this we used ovalbumin protein (OVA), which would enable the assessment of impact of IL-33 beyond HIV-1 and DNA immunogens. For this, again IL-33 was administered one week before and at time of immunization with OVA in alum, and mice were euthanized at wk 4, the peak of the priming phase (Fig. 3A). A single experiment utilizing a group size of 6 mice, maintaining individual mice as replicates was performed to evaluate the serological OVA-specific Ab response and the B cell dynamics. The IL-33 group had a significantly higher IgG plasma Ab response (p<0.05) than the alum only group after the first OVA immunization that further increased after the second OVA immunization (Fig. 3B). A significantly higher IgM plasma Ab response after each immunization was apparent in the IL-33 group (Fig. 3C), which is consistent with the results using VC10014 (Fig. 1). The IgM plasma Ab response did not increase following the second immunization, which may suggest class-switching.
Figure 3. IL-33 increases OVA-specific IgG and IgM.
A. Immunization schedule. Mice (n=6 per group) received IL-33 (2.5 μg) i.p. and i.m. one week prior to and at time of i.m. immunization with ovalbumin protein (100 μg) in alum. Plasma OVA-specific IgG (B) and IgM (C) determined by ELISA. Symbols represent individual mice. * indicates significant difference (p<0.05) between groups as determined by two-tailed Mann-Whitney test. This experiment was conducted once.
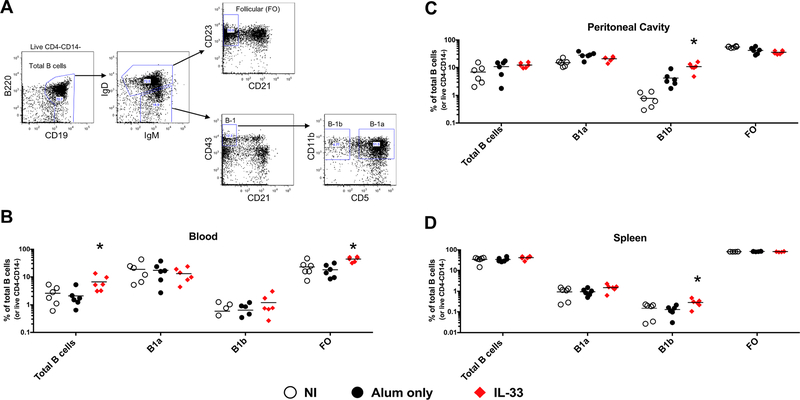
To determine if IL-33 impacts the dynamics of B cells flow cytometry phenotyping (Fig. 4A) was performed at wk 4, one week after the final IL-33 treatment. The IL-33 group had a significant 3-fold higher frequency of total CD19+ peripheral blood B cells compared to the alum only group (2.1+/−1.6% vs 6.7+/−4.1%, p<0.05) (Fig. 4B). The frequency of B-1a (CD19+IgDlowIgMhiCD21−CD43+CD11b+CD5+) and B-1b (CD19+IgDlowIgMhiCD21−CD43+CD11b+CD5−) peripheral blood B cells did not differ significantly between groups, however, the IL-33 group had a 2-fold higher frequency of follicular (FO: CD19+IgD+CD21−CD23+) peripheral blood B cells (18.0+/−9.2% vs 43.6+/−8.4%, p<0.005) compared to the alum only group. In the peritoneal cavity, the IL-33 group had a ~10-fold and 2-fold higher frequency of B-1b cells (10.8+/−3.8%) compared to non-immunized (0.8+/−0.5%, p<0.005) and alum only (4.2+/−2.6%, p<0.005) groups, respectively (Fig. 4C). A similar ~2-fold increase in B-1b cells in the IL-33 group (0.30+/−0.1%) was observed in the spleen, compared to the non-immunized (0.15+/−0.09%, p<0.05) and alum only (0.13+/−0.06%, p<0.05) groups, respectively (Fig. 4D). Together, these results suggest that peripheral FO, and peritoneal and splenic B-1b B cells are responding to IL-33 treatment.
Figure 4. IL-33 increases B1-b B cells.
A. Gating strategy. Mice (n=6 per group) were euthanized at wk 4 following IL-33 treatment and immunizations and B cell subsets defined by flow cytometry from the peripheral blood (B), peritoneal cavity (C), and spleen (D). Frequency of total B cells are presented as % of live CD4-CD14- cells. Symbols represent individual mice. * indicates significant difference (p<0.05) as compared to alum only group as determined by two-tailed Mann-Whitney test. This experiment was conducted once.
Discussion
Treatment with IL-33 during the initial priming immunization with gp160 DNA plasmids resulted in a significant induction of plasma Env-specific IgG within two weeks that was not evident in the alum only group, and only minimal in the MPL treated group (Fig. 1C). This rapid IgG response was dominated by IgG1, but also included substantial IgG2b Env-specific antibody (Fig. 2A). Intramuscular immunization with DNA plasmids alone is poorly immunogenic, however, a few studies have described increased Ab responses when combined with an adjuvant such as GM-CSF or QS-21 [49–52] yet these were primarily only evident following repeated DNA plasmid immunizations. IL-33 may be acting in multi-faceted manner to increase DNA immunogenicity, such through increasing Env expression, enhancing CD4+ T cell help, as IL-33 itself has been indicated in promoting Th1, Th2, and Th9 differentiation [18, 53, 54]. Further, the direct impact of IL-33 on B cells may also be contributing. As the clinical development of plasmid DNA-based immunogens continue, combining IL-33 with other delivery modalities which increase the humoral response such as electroporation and intradermal injection should be evaluated. IL-33 has been previously demonstrated to increase the plasma and mucosal IgG response when delivered intranasally in combination with influenza HA protein in mice [14], and interestingly it has been reported that alum delivered i.p. induces the release of endogenous IL-33 and in-part mediates the adjuvant effect of alum [55]. This study exclusively delivered IL-33 via i.p., however delivery via traditional i.m. route should be evaluated to adequately determine the translational feasibility of IL-33 as an adjuvant. Similarly, it remains to be determined if pre-treatment with IL-33 in advance of immunization is a requirement for its optimal adjuvant-like activity.
The autologous clade B IgG response to F8 and C6a gp140 at the peak timepoint (wk 12) was modestly increased compared to the alum only and MPLA treated groups, however, the superiority of the IL-33 treatment in promoting a heterologous IgG response to the clade C CN54 gp120 and the other representative Envs was most apparent (Fig 1 and Fig 2). Additionally, the predominant IgG1 bias and increased IgG avidity resulting from IL-33 treatment further indicate that IL-33 during the priming phase is qualitatively altering the Env-specific IgG response in a manner that remains evident even following boosting immunizations. The consistent induction of Env-specific IgM was dominant feature of the IL-33 treatment, frequently at least two-fold greater than that observed with MPLA or alum only (Fig 1B and Fig 2C) further substantiate the qualitative uniqueness associated with IL-33 treatment, and may be an integral mechanistic component to the development of the enhanced kinetics and breadth of the IgG response.
Although the cellular source of the Env-specific plasma IgM was not defined in this study, the increase in B-1b B cells observed following IL-33 treatment (Fig 4) suggests they should be considered as a potential contributor to the Env-specific humoral response. B-1 B cells are commonly associated their preferential anatomic distribution in the peritoneal and pleural cavities and omentum, providing a first line of defense against pathogens through the constitutive production of natural IgM [56, 57] and are the primary antibody producers along with marginal zone B cells in response to T cell independent antigens [58, 59]. Although bacterial capsular polysaccharides are the most well described B-1 antigens, B-1 responses to viruses particularly influenza have been suggested [30, 60, 61]. Among B-1 b cells, B-1a are thought to be the primary source of steady state natural IgM and B-1b are associated with a greater degree of antigen-specific responsiveness, including memory and recall responses [62–64]. Although IgM production dominates B-1b Ab secretion, they can also give rise to IgG and IgA expressing plasma cells at mucosal sites and in the bone marrow [65–67]. Follow-up studies to determine if pre-treatment with IL-33 prior to immunization, enhances B cell localization and/or responsiveness to the immunization are warranted. Additionally, experiments to define the precise cellular source of the Env-specific Ab that develops with IL-33 treatment and the modulation or potential contribution of CD4+ T cell help that might be occurring should be pursued and may resolve a unique developmental pathway for HIV-1 specific B cell responses.
Our results in mice substantiate the previous findings in rabbits [41] and rhesus macaques [42] that the VC10014 HIV-1 Env-based immunogen platform can induce a broad cross-clade Ab response. The trimeric gp140 proteins used in this study are not of the recently developed native-like trimer formats, and generating and testing the VC10014 HIV-1 Envs as native-like trimers [68, 69] may further increase their ability to induce broad and potent Ab responses. Our results do indicate that the addition of IL-33 to the current VC10014 immunization regimen can further increase the magnitude, avidity, and breadth of the IgG response and also promote the expansion of B-1b cells and a robust Env-specific IgM response which are not apparent in the absence of IL-33 treatment. These findings provide justification for evaluating the ability of IL-33 in combination with VC10014 to induce protective immunity to HIV-1 infection.
Acknowledgements
We are very grateful the technical assistance provided by Danielle DeLooze Noah Chernosky, Claire Ruben and Haein Son, and the assistance of the University of Rochester Flow Cytometry Core Facility.
Funding: This work was supported by the National Institutes of Health (5R01AI117787 to JJK), the University of Rochester Center for AIDS Research P30AI078498 (NIH/NIAID), and institutional funding provided by the University of Rochester.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- [1].UNAIDS/WHO. 2017.
- [2].Kharsany AB, Karim QA. HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J. 2016;10:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, et al. A Blueprint for HIV Vaccine Discovery. Cell Host Microbe. 2012;12:396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Malherbe DC, Doria-Rose NA, Misher L, Beckett T, Puryear WB, Schuman JT, et al. Sequential immunization with a subtype B HIV-1 envelope quasispecies partially mimics the in vivo development of neutralizing antibodies. J Virol. 2011;85:5262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. [DOI] [PubMed] [Google Scholar]
- [7].Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin Infect Dis. 2011;53:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, et al. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122:2711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Villarreal DO, Wise MC, Walters JN, Reuschel EL, Choi MJ, Obeng-Adjei N, et al. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res. 2014;74:1789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Villarreal DO, Weiner DB. Interleukin 33: a switch-hitting cytokine. Curr Opin Immunol. 2014;28:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–65. [DOI] [PubMed] [Google Scholar]
- [13].Villarreal DO, Siefert RJ, Weiner DB. Alarmin IL-33 elicits potent TB-specific cell-mediated responses. Hum Vaccin Immunother. 2015;11:1954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kayamuro H, Yoshioka Y, Abe Y, Arita S, Katayama K, Nomura T, et al. Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus. J Virol. 2010;84:12703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Villarreal DO, Svoronos N, Wise MC, Shedlock DJ, Morrow MP, Conejo-Garcia JR, et al. Molecular adjuvant IL-33 enhances the potency of a DNA vaccine in a lethal challenge model. Vaccine. 2015;33:4313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998;95:6930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schneider E, Petit-Bertron AF, Bricard R, Levasseur M, Ramadan A, Girard JP, et al. IL-33 activates unprimed murine basophils directly in vitro and induces their in vivo expansion indirectly by promoting hematopoietic growth factor production. J Immunol. 2009;183:3591–7. [DOI] [PubMed] [Google Scholar]
- [18].Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–30. [DOI] [PubMed] [Google Scholar]
- [19].Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–4. [DOI] [PubMed] [Google Scholar]
- [20].Komai-Koma M, Gilchrist DS, McKenzie AN, Goodyear CS, Xu D, Liew FY. IL-33 activates B1 cells and exacerbates contact sensitivity. J Immunol. 2011;186:2584–91. [DOI] [PubMed] [Google Scholar]
- [21].Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. [DOI] [PubMed] [Google Scholar]
- [22].Sattler S, Ling GS, Xu D, Hussaarts L, Romaine A, Zhao H, et al. IL-10-producing regulatory B cells induced by IL-33 (Breg(IL-33)) effectively attenuate mucosal inflammatory responses in the gut. J Autoimmun. 2014;50:107–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mu R, Huang HQ, Li YH, Li C, Ye H, Li ZG. Elevated serum interleukin 33 is associated with autoantibody production in patients with rheumatoid arthritis. J Rheumatol. 2010;37:2006–13. [DOI] [PubMed] [Google Scholar]
- [24].Pujanauski LM, Janoff EN, McCarter MD, Pelanda R, Torres RM. Mouse marginal zone B cells harbor specificities similar to human broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A. 2013;110:1422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Peachman KK, Wieczorek L, Matyas GR, Polonis VR, Alving CR, Rao M. The importance of antibody isotype in HIV-1 virus capture assay and in TZM-bl neutralization. Viral immunology. 2010;23:627–32. [DOI] [PubMed] [Google Scholar]
- [26].Gong S, Tomusange K, Kulkarni V, Adeniji OS, Lakhashe SK, Hariraju D, et al. Anti-HIV IgM protects against mucosal SHIV transmission. AIDS. 2018;32:F5–F13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Puschnik A, Lau L, Cromwell EA, Balmaseda A, Zompi S, Harris E. Correlation between dengue-specific neutralizing antibodies and serum avidity in primary and secondary dengue virus 3 natural infections in humans. PLoS neglected tropical diseases. 2013;7:e2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moyron-Quiroz JE, McCausland MM, Kageyama R, Sette A, Crotty S. The smallpox vaccine induces an early neutralizing IgM response. Vaccine. 2009;28:140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Baumgarth N How specific is too specific? B-cell responses to viral infections reveal the importance of breadth over depth. Immunological reviews. 2013;255:82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. The Journal of experimental medicine. 2008;205:3053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. The Journal of experimental medicine. 2012;209:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin Immunol. 2018. [DOI] [PubMed] [Google Scholar]
- [33].Martin M, Michalek SM, Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun. 2003;71:2498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ismaili J, Rennesson J, Aksoy E, Vekemans J, Vincart B, Amraoui Z, et al. Monophosphoryl lipid A activates both human dendritic cells and T cells. J Immunol. 2002;168:926–32. [DOI] [PubMed] [Google Scholar]
- [35].De Becker G, Moulin V, Pajak B, Bruck C, Francotte M, Thiriart C, et al. The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int Immunol. 2000;12:807–15. [DOI] [PubMed] [Google Scholar]
- [36].Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24:5937–49. [DOI] [PubMed] [Google Scholar]
- [37].O’Ryan M, Vidal R, del Canto F, Salazar JC, Montero D. Vaccines for viral and bacterial pathogens causing acute gastroenteritis: Part I: Overview, vaccines for enteric viruses and Vibrio cholerae. Hum Vaccin Immunother. 2015;11:584–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang Y, Ghosn EE, Cole LE, Obukhanych TV, Sadate-Ngatchou P, Vogel SN, et al. Antigen-specific memory in B-1a and its relationship to natural immunity. Proc Natl Acad Sci U S A. 2012;109:5388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sather DN, Carbonetti S, Malherbe DC, Pissani F, Stuart AB, Hessell AJ, et al. Emergence of broadly neutralizing antibodies and viral coevolution in two subjects during the early stages of infection with human immunodeficiency virus type 1. J Virol. 2014;88:12968–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chukwuma VU, Kose N, Sather DN, Sapparapu G, Falk R, King H, et al. Increased breadth of HIV-1 neutralization achieved by diverse antibody clones each with limited neutralization breadth. PLoS One. 2018;13:e0209437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Malherbe DC, Pissani F, Sather DN, Guo B, Pandey S, Sutton WF, et al. Envelope variants circulating as initial neutralization breadth developed in two HIV-infected subjects stimulate multiclade neutralizing antibodies in rabbits. J Virol. 2014;88:12949–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hessell AJ, Malherbe DC, Pissani F, McBurney S, Krebs SJ, Gomes M, et al. Achieving Potent Autologous Neutralizing Antibody Responses against Tier 2 HIV-1 Viruses by Strategic Selection of Envelope Immunogens. J Immunol. 2016;196:3064–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Srivastava IK, VanDorsten K, Vojtech L, Barnett SW, Stamatatos L. Changes in the immunogenic properties of soluble gp140 human immunodeficiency virus envelope constructs upon partial deletion of the second hypervariable region. J Virol. 2003;77:2310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sellhorn G, Caldwell Z, Mineart C, Stamatatos L. Improving the expression of recombinant soluble HIV Envelope glycoproteins using pseudo-stable transient transfection. Vaccine. 2009;28:430–6. [DOI] [PubMed] [Google Scholar]
- [45].Shepherd SJ, McAllister G, Kean J, Wallace LA, Templeton KE, Goldberg DJ, et al. Development of an avidity assay for detection of recent HIV infections. J Virol Methods. 2015;217:42–9. [DOI] [PubMed] [Google Scholar]
- [46].Dimitrov JD, Lacroix-Desmazes S, Kaveri SV. Important parameters for evaluation of antibody avidity by immunosorbent assay. Anal Biochem. 2011;418:149–51. [DOI] [PubMed] [Google Scholar]
- [47].Nogales A, Piepenbrink MS, Wang J, Ortega S, Basu M, Fucile CF, et al. A Highly Potent and Broadly Neutralizing H1 Influenza-Specific Human Monoclonal Antibody. Sci Rep. 2018;8:4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Okuda K, Wada Y, Shimada M. Recent Developments in Preclinical DNA Vaccination. Vaccines (Basel). 2014;2:89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kim JJ, Ayyavoo V, Bagarazzi ML, Chattergoon MA, Dang K, Wang B, et al. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol. 1997;158:816–26. [PubMed] [Google Scholar]
- [51].Sasaki S, Sumino K, Hamajima K, Fukushima J, Ishii N, Kawamoto S, et al. Induction of systemic and mucosal immune responses to human immunodeficiency virus type 1 by a DNA vaccine formulated with QS-21 saponin adjuvant via intramuscular and intranasal routes. J Virol. 1998;72:4931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cristillo AD, Wang S, Caskey MS, Unangst T, Hocker L, He L, et al. Preclinical evaluation of cellular immune responses elicited by a polyvalent DNA prime/protein boost HIV-1 vaccine. Virology. 2006;346:151–68. [DOI] [PubMed] [Google Scholar]
- [53].Duan L, Chen J, Zhang H, Yang H, Zhu P, Xiong A, et al. Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3(+) regulatory T-cell responses in mice. Mol Med. 2012;18:753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Arima H, Nishikori M, Otsuka Y, Kishimoto W, Izumi K, Yasuda K, et al. B cells with aberrant activation of Notch1 signaling promote Treg and Th2 cell-dominant T-cell responses via IL-33. Blood Adv. 2018;2:2282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rose WA, 2nd, Okragly AJ, Patel CN, Benschop RJ. IL-33 released by alum is responsible for early cytokine production and has adjuvant properties. Sci Rep. 2015;5:13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Baumgarth N The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. [DOI] [PubMed] [Google Scholar]
- [57].Kreslavsky T, Wong JB, Fischer M, Skok JA, Busslinger M. Control of B-1a cell development by instructive BCR signaling. Curr Opin Immunol. 2018;51:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunological reviews. 2000;175:70–9. [PubMed] [Google Scholar]
- [59].Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–29. [DOI] [PubMed] [Google Scholar]
- [60].Carbonari M, Caprini E, Tedesco T, Mazzetta F, Tocco V, Casato M, et al. Hepatitis C virus drives the unconstrained monoclonal expansion of VH1–69-expressing memory B cells in type II cryoglobulinemia: a model of infection-driven lymphomagenesis. J Immunol. 2005;174:6532–9. [DOI] [PubMed] [Google Scholar]
- [61].Viau M, Veas F, Zouali M. Direct impact of inactivated HIV-1 virions on B lymphocyte subsets. Mol Immunol. 2007;44:2124–34. [DOI] [PubMed] [Google Scholar]
- [62].Yammani RD, Haas KM. Primate B-1 cells generate antigen-specific B cell responses to T cell-independent type 2 antigens. J Immunol. 2013;190:3100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–90. [DOI] [PubMed] [Google Scholar]
- [64].Defrance T, Taillardet M, Genestier L. T cell-independent B cell memory. Curr Opin Immunol. 2011;23:330–6. [DOI] [PubMed] [Google Scholar]
- [65].Kim SH, Kim TH, Han DU. The main peritoneal source of precursors of the murine intestinal IgA plasma cell. Anticancer Res. 1996;16:2821–4. [PubMed] [Google Scholar]
- [66].Haas KM. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J Immunol. 2011;187:5183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Taillardet M, Haffar G, Mondiere P, Asensio MJ, Gheit H, Burdin N, et al. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood. 2009;114:4432–40. [DOI] [PubMed] [Google Scholar]
- [68].Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9:e1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sharma SK, de Val N, Bale S, Guenaga J, Tran K, Feng Y, et al. Cleavage-independent HIV-1 Env trimers engineered as soluble native spike mimetics for vaccine design. Cell Rep. 2015;11:539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]