Abstract
The objective of this study was to evaluate the utility of a complement-dependent C3d assay to risk-stratify donor-specific antibodies (DSA) in a multicenter cohort of kidney recipients presenting with new-onset clinical dysfunction. One hundred and six subjects with evidence of DSA at a mean period of 5.3 ± 5.0 years posttransplant underwent testing using C3d reagents. C3d positivity was strongly associated with both the peak and sum IgG DSA MFI, with 98.3% (n=57/58) of strongly reactive sera (peak MFI >10,000) eliciting a positive signal. Patients with C3d+ DSA had a higher creatinine (p=0.03), more significant graft fibrosis (p=0.035), and a faster rate of graft loss post-test compared to those with C3d- DSA (p=0.05). Sub-analysis of patients with low-moderate level DSA confirmed the inferior outcome associated with C3d positivity. Despite the prognostic value of C3d as a standalone test, the assay did not provide independent risk prediction after incorporation of graft fibrosis in a multivariate model (p=0.94). Overall, C3d offered limited discriminatory value for strong DSA with peak IgG MFI >10,000 and in patients where histologic data is available, but its utilization may be considered in those with low-moderate level DSA and where an allograft biopsy is not accessible.
Introduction
The initial DeKAF (long-term deterioration of kidney allograft function) study found antibody-mediated rejection (AMR) defined by the presence of donor-specific antibodies (DSA) and/or intragraft C4d positivity to be the dominant cause of late kidney allograft failure (1). Accordingly, detection of anti-HLA antibodies on the Luminex single antigen bead (SAB) platform has become a critical component in the diagnosis of AMR. In recent years, modifications of the conventional SAB assay (C4d, C1q, C3d) to further discriminate the complement-activating potential of antibodies opened another dimension to the risk assessment of DSA (2–10). Although initial studies found complement fixation to be predictive of outcome (3, 4, 6, 8, 9, 11), other reports did not confirm this association (7, 12–15). Furthermore, recent technical experiments challenge the additional value provided by these modified assays after taking into account of antibodies’ MFI strength (16, 17), which is recognized as a major determinant of complement activity (18). Currently, the group of patients who would benefit from complement-dependent evaluation is not well-defined.
Introduced after C1q, the present assay detects complement split product C3d positioned downstream in the classical pathway, which may represent a better measure of physiologic complement activity and perhaps more indicative of complement-mediated injury in the graft. In this study, our objectives were to: 1) determine the utility of C3d to predict graft loss in a multicenter cohort of kidney recipients presenting with late clinical dysfunction; 2) assess the prognostic value of C3d after accounting for antibody strength as measured in MFI values.
Materials and Methods
Patient Population
DeKAF is a multicenter consortium of 7 US-Canadian centers aimed at correlating specific clinico-pathologic entities with allograft dysfunction (19). Patients in the prospective arm (n=1948) were enrolled at the time of kidney transplantation – they were required to have a stable baseline renal function (average of 3 serum creatinine values) at 3 months posttransplant. Patients in the cross-sectional cohort (n=559) received a transplant prior to October 1, 2005 and had a serum creatinine level ≤ 2 mg/dL prior to study entry. All subjects were followed longitudinally for development of graft dysfunction, defined as a ≥ 25% increase in serum creatinine or new-onset proteinuria, which then triggered an allograft biopsy and concurrent serum testing for DSA by a central laboratory (UCLA Immunogenetics) (19). Biopsies were read both locally and by a blinded central pathologist (Mayo, Rochester).
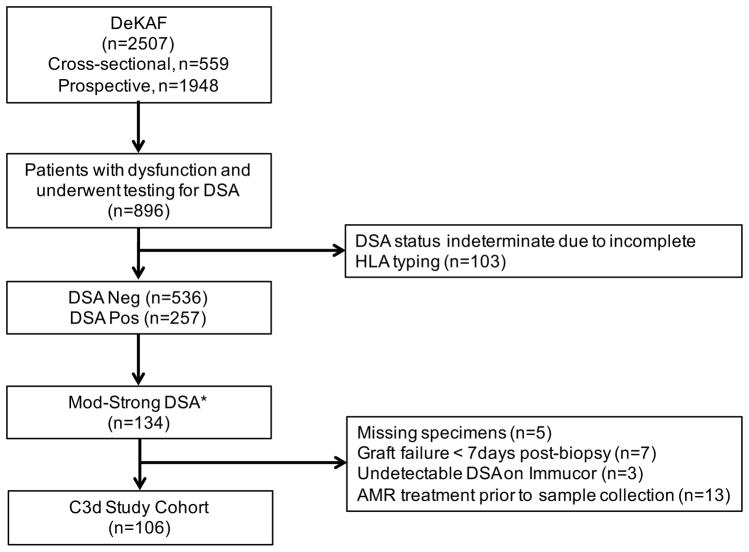
Figure 1 depicts the selection of patients analyzed in this study. Among patients that experienced dysfunction and testing positive for DSA (n=257), those who initially screened positive with sum MFI ≥5,000 using LABScreen single antigen beads (One Lambda, Canoga Park, CA) underwent parallel antibody testing using Immucor LSA-IgG and C3d reagents (Immucor Transplant Diagnostics, Inc., Stamford, CT) (n=134). Twenty-eight patients were excluded due to: missing samples (n=5), undetectable DSA on Immucor LSA-IgG platform (n=3), graft failure <7 days from time of dysfunction (n=7), and patient exposure to antibody-depleting therapy prior to serum collection for DSA testing (n=13), leaving 106 patients for analysis. This study was approved by the Institutional Review Board at UCLA (IRB 11-000456).
Figure 1.
Flow diagram of the study cohort. *Patients with moderate-strong antibodies screened positive for DSA with sum MFI ≥5,000 using One Lambda LABScreen single antigen beads.
Anti-HLA Antibody Testing
Donor-recipient HLA typing was performed at each of the participating DeKAF centers’ HLA laboratories using their existing protocols. Given the era of transplants, typing for HLA-Cw, -DQA, -DPA/B were generally unavailable. Assignment of donor-reactivity in HLA-Cw and -DQA loci was based on strong linkage disequilibrium; DSA to DP antigens could not be determined. Overall, 4.7% (5/106) of the study recipients had evidence of anti-DP antibodies.
Patient sera stored at the time of kidney biopsy were retested using Immucor LSA Class I and II Single Antigens (Lot 04234B, Immucor Transplant Diagnostics, Inc., Stamford, CT) by an experienced technologist (George Benzuela) at the UCLA Immunogenetics Laboratory. Antibody specificities with MFI ≥500 were defined as positive. The same serum samples were re-analyzed using the Immucor LSA C3d assay (Lot 04234B) according to manufacturer’s instructions. Antibody reactivities were adjusted for background by subtracting the MFI value of beads in patient wells from those derived from the negative control well. A threshold of MFI ≥500 was used to define C3d positivity. Whenever multiple donor-specific reactivities were present in the same serum, peak DSA referred to the antibody specificity exhibiting the highest MFI value relative to the others present. Relationships between antibody strength and C3d positivity were derived using Immucor MFI values.
Biopsy Data
Representative biopsy sections were submitted to a central pathologist blinded to patient outcome for further review, using the Banff 97 classification. Scoring of peritubular capillary infiltrates was performed as per the Banff 2005 classification. Overall, 93/106 (87.7%) of patients had both central and local biopsy data available, 11/106 (10.4%) with only local results, and 2/106 (1.9%) with missing data. In this study, moderate-severe interstitial fibrosis/tubular atrophy (IFTA) was defined as ci+ct ≥2. Presence of microvascular inflammation was defined as g+ptc >0. C4d interpretation by the central pathologist was available in 62% (66/106) of biopsy specimens while 33% (35/106) only had C4d analysis by the local pathologist; C4d results were unavailable in 5 patients.
Statistical Analysis
Graft survival rates were defined as time from biopsy to return to dialysis. Survival functions, censoring for death, were calculated using the Kaplan-Meier method and compared with the Wilcoxon test. Categorical variables were compared using Fisher’s exact test; data for race were compared using the chi-square test. Continuous variables were expressed as mean±SD and analyzed using Student’s t-test. Predictors of graft loss were evaluated using univariate and multivariate Cox proportional hazards models. Variables with p-values <0.1 were selected to construct the multivariate model. All comparisons were two-sided, and p-values <0.05 were considered to be statistically significant. STATA version 13, R version 3.4.3 and GraphPad version 7.0d were used for statistical analysis.
Results
Baseline Patient and Anti-HLA Antibody Characteristics
The study cohort was primarily Caucasian (65.1%), with a significant proportion comprised of African-Americans (23.6%). The majority of patients (71.7%) belonged to the cross-sectional cohort. The average time to graft dysfunction was 5.3 ± 5.0 years. Table 1 depicts the baseline clinico-pathologic and antibody characteristics between C3d+ and C3d− groups. While no significant demographic differences were observed between the two groups, C3d+ patients had a higher serum creatinine (2.88 ± 1.71 vs. 2.08 ± 0.89 mg/dL, p=0.03), a larger burden of graft fibrosis (44.3% vs. 20.0%, p=0.035), and a trend toward greater proteinuria (1735 ± 1830 vs. 923 ± 1110 mg/g, p=0.10) compared with the C3d− group at dysfunction.
Table 1.
Baseline demographic, clinico-pathologic data, and DSA characteristics of study patients by C3d status.
| All (N=106) | C3d− (N=25) | C3d+ (N=81) | P-value | |
|---|---|---|---|---|
|
| ||||
| Patient Characteristics | ||||
|
| ||||
| Gender (% male) | 54.7% | 52% | 55.6% | 0.82 |
|
| ||||
| Age at transplant (mean ± std) | 39.6 ± 15.5 | 43.6 ± 13.9 | 38.3 ± 15.8 | 0.14 |
|
| ||||
| Age at enrollment (mean ± std) | 44.4 ± 14.9 | 46.9 ± 14.2 | 43.7 ± 15.1 | 0.35 |
|
| ||||
| Race: | ||||
| White (%) | 65.1% | 80% | 60.5% | 0.19 |
| Black (%) | 23.6% | 12% | 27.2% | |
| Other (%) | 11.3% | 8% | 12.3% | |
|
| ||||
| Time to biopsy (yrs) | 5.3 ± 5.0 | 3.92 ± 3.42 | 5.66 ± 5.38 | 0.13 |
|
| ||||
| No. HLA A/B/DRB1 mismatches | 3.86 ± 1.46 | 3.59 ± 1.53 | 3.95 ± 1.43 | 0.32 |
|
| ||||
| Creatinine at biopsy (mg/dL) | 2.71 ± 1.60 | 2.08 ± 0.89 | 2.88 ± 1.71 | 0.03 |
|
| ||||
| Creatinine at biopsy (μmol/L) | 239.2 ± 141.6 | 183.8 ± 78.7 | 254.9 ± 151.6 | 0.03 |
|
| ||||
| Protein/Cr ratio (mg/g) | 1515.0 ± 1696.0 | 923.0 ± 1110.1 | 1735.3 ± 1830.0 | 0.10 |
|
| ||||
| Protein/Cr ratio (mg/mmol) | 171.2 ± 191.6 | 104.3 ± 125.4 | 196.1 ± 206.8 | 0.10 |
|
| ||||
| DSA Characteristics (%) | ||||
|
| ||||
| Class I only | 5.7% | 4% | 6.2% | |
|
| ||||
| Class II only | 69.8% | 80% | 66.7% | |
|
| ||||
| Class I and II | 24.5% | 16% | 27.1% | |
|
| ||||
| DSA to HLA-A | 9.6% | 5.3% | 10.5% | |
|
| ||||
| DSA to HLA-B | 10.0% | 7.9% | 10.5% | |
|
| ||||
| DSA to HLA-C | 0.9% | 0% | 1.1% | |
|
| ||||
| DSA to HLA-DR | 38.4% | 34.2% | 39.2% | |
|
| ||||
| DSA to HLA-DQ | 41.1% | 52.6% | 38.7% | |
|
| ||||
| Number of DSA (mean ± std) | 2.1 ± 1.4 | 1.5 ± 0.7 | 2.2 ± 1.5 | 0.02 |
|
| ||||
| Biopsy Banff Scores (mean ± std) | ||||
|
| ||||
| g | 0.63 ± 0.74 | 0.72 ± 0.84 | 0.59 ± 0.71 | 0.46 |
|
| ||||
| ptc | 1.27 ± 0.93 | 1.26 ± 0.93 | 1.27 ± 0.94 | 0.98 |
|
| ||||
| t | 0.95 ± 1.17 | 0.96 ± 1.17 | 0.95 ± 1.18 | 0.97 |
|
| ||||
| i | 1.02 ± 1.11 | 0.96 ± 1.14 | 1.04 ± 1.11 | 0.76 |
|
| ||||
| v | 0.10 ± 0.36 | 0.04 ± 0.20 | 0.11 ± 0.39 | 0.37 |
|
| ||||
| cg | 0.69 ± 1.06 | 0.44 ± 0.82 | 0.77 ± 1.12 | 0.17 |
|
| ||||
| ci | 1.39 ± 1.00 | 1.08 ± 0.70 | 1.49 ± 1.06 | 0.07 |
|
| ||||
| ct | 1.56 ± 0.86 | 1.20 ± 0.65 | 1.67 ± 0.89 | 0.02 |
|
| ||||
| Microvascular inflammation (%)a | 78.9% | 76.0% | 79.7% | 0.78 |
|
| ||||
| Moderate-severe IFTA (%)b | 37.7% | 20.0% | 44.3% | 0.035 |
|
| ||||
| C4d+ (%) | 62.4% | 29.2% | 72.7% | 0.0002 |
g+ptc >0;
ci+ct ≥2.
The average number of DSA identified at the time of kidney biopsy was 2.1, with skewing toward class II (69.8% class II only, 24.5% class I and II). DSA to HLA-DQ was the most frequently observed locus (41.1%), followed by antibodies against HLA-DR (38.4%). The locus distribution of DSA was comparable between C3d+ and C3d− groups. C3d+ patients on average had a higher number of DSA compared to the C3d− group (2.2 ± 1.5 vs. 1.5 ± 0.7, p=0.02).
Relationship between SAB IgG MFI and C3d Status
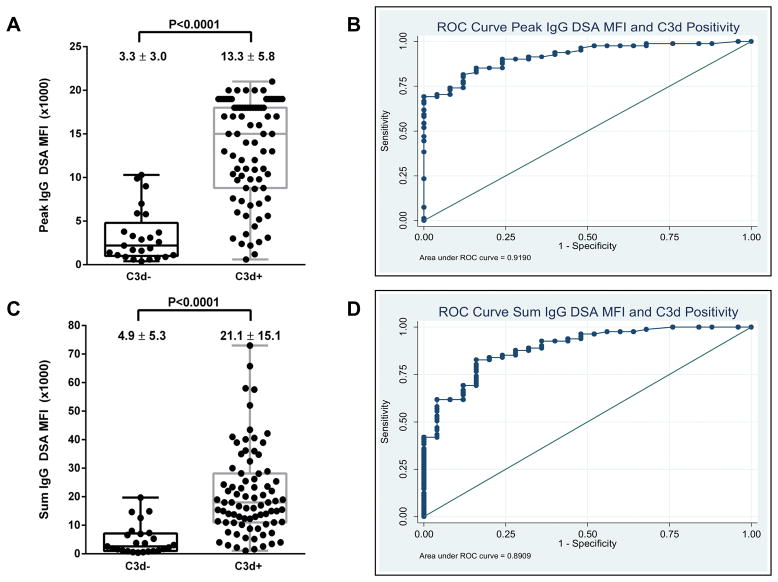
81/106 (76.4%) kidney patients had circulating C3d+ DSA at the time of dysfunction. In keeping with previous studies (15, 16), there was a strong relationship between antibodies’ strength in MFI and their ability to fix complement. The peak IgG DSA MFI was significantly higher in C3d+ DSA compared to those that were negative (13.3 ± 5.8 vs. 3.3 ± 3.0 x1000, p<0.0001) (Figure 2A). Area under the receiver operating curve between the peak DSA MFI and C3d status was 0.919 (Figure 2B). A similar correlation was observed by analyzing the sum DSA MFI in relation to C3d status (Figure 2C and 2D). Interestingly, almost all patients (98.3%, n=57/58) with peak DSA MFI >10,000 elicited C3d positivity; thus, C3d offered little discriminatory value for strong antibodies defined using this threshold.
Figure 2.
(A) Relationship of peak IgG DSA MFI and the C3d status of antibodies. (B) ROC curve of peak IgG DSA MFI and C3d status. (C) Relationship of sum IgG DSA MFI and C3d positivity. (D) ROC curve of sum IgG DSA MFI and C3d status.
Relationship between C3d Positivity and Intragraft C4d Staining
The correlation between C3d positivity and intragraft C4d was analyzed in 101 biopsy specimens with available C4d staining. As expected, the majority of C3d+ DSA (73%, n=56/77) were correlated with peritubular C4d deposition in the tissue (Figure 3A). Surprisingly, a proportion (29%) of patients with C3d− DSA also had evidence of C4d detection in the allograft (Figure 3A, circle). We hypothesized that this discordance may be related to differences in the method of complement-activation evaluation: whereas the C3d test interrogates the individual capacity of each HLA antibody specificity to trigger the complement cascade in vitro, C4d formation in tissue depends on the cumulative amount of antigen-antibody interaction on graft surface. Further analysis of the number of DSA in the C3d− group revealed a strong trend toward multiple DSA being detected among C3d−C4d+ patients compared to the C3d−C4d− group (71% vs. 24%, p=0.06) (Figure 3B).
Figure 3.
Relationship between circulating DSA’s C3d status and C4d detection in tissue biopsy. (A) The majority of C3d+ DSA correlated with intragraft C4d staining. (B) A small subset of C4d+ biopsies were associated with C3d− DSA. Among patients with C3d− DSA, the majority of C3d−C4d+ biopsies were associated with presence of multiple DSA compared with C3d−C4d− biopsies.
Performance of the C3d Test and its Association with Graft Survival
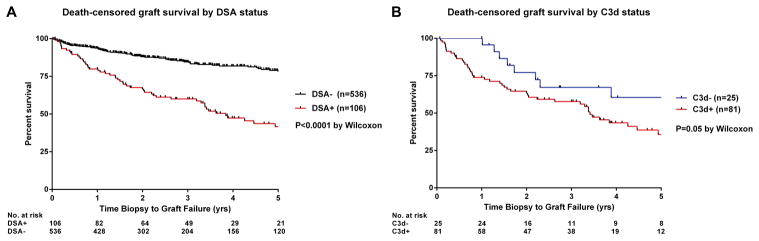
Compared with the historical control of DSA− patients in DeKAF (n=536, baseline clinico-pathologic data in Supplementary Table 1), recipients in this study had a significantly worse posttransplant graft survival rate of 42% at 5 years postbiopsy versus 78% in DSA− recipients (Figure 4A). The C3d test further separated the graft outcome of DSA+ patients, with those screening positive for C3d exhibiting inferior graft survival (Figure 4B). The rate of graft loss among C3d+ patients was fastest early after biopsy; in contrast, allograft failure in C3d− patients all occurred at least one year after clinical dysfunction. Comparison of DSA characteristics between patients with graft failure versus those with a functioning allograft at 5 years postbiopsy showed no significant differences with respect to DSA class, number, peak and sum IgG DSA MFI, and C3d peak and sum MFI (Supplementary Table 2).
Figure 4.
(A) Death-censored graft survival of patients in this study cohort compared to the historical control of DSA− patients in DeKAF. (B) Graft survival by C3d status.
The diagnostic performance of C3d and intragraft C4d in predicting graft loss is shown in Table 2. Both tests yielded reasonable sensitivity and negative predictive value, particularly when predicting outcome within the first year after biopsy. C3d offered superior sensitivity compared to C4d at all time points analyzed, although a decrease in assay performance was observed when predicting graft survival distant to the test. Complement activation as evaluated by both tests showed poor specificity and positive predictive value for graft survival.
Table 2.
Performance of the C3d assay and intragraft C4d detection in predicting graft loss at 1, 2, and 3 years post allograft dysfunction. GL, graft loss.
| Assay | Test Performance | GL 1st year | GL 2nd year | GL 3rd year |
|---|---|---|---|---|
| C3d test | Sensitivity | 100% | 85% | 83% |
| Specificity | 30% | 28% | 27% | |
| Positive predictive value | 26% | 36% | 41% | |
| Negative predictive value | 100% | 80% | 72% | |
| Intragraft C4d | Sensitivity | 83% | 70% | 67% |
| Specificity | 42% | 41% | 40% | |
| Positive predictive value | 24% | 33% | 38% | |
| Negative predictive value | 92% | 76% | 68% |
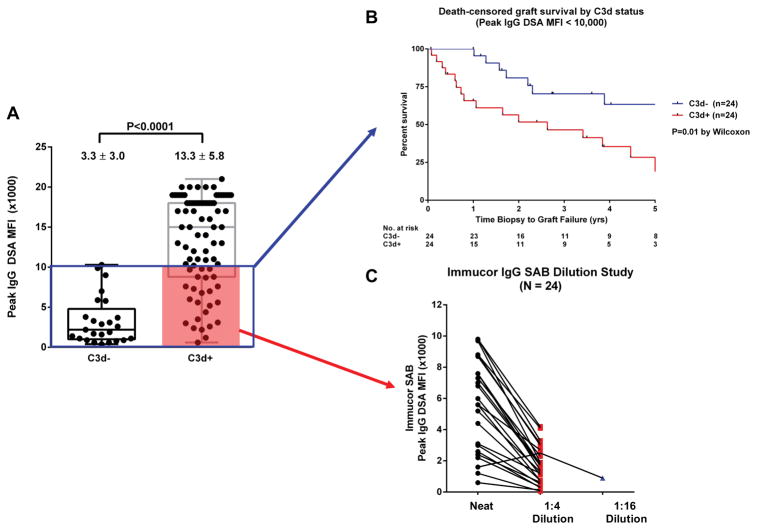
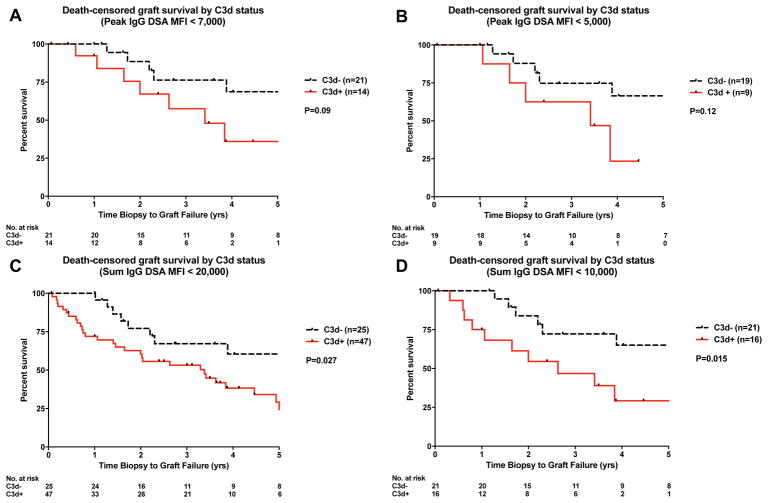
Given the strong dependency of complement activation on antibody strength, we next analyzed a subset of recipients with peak DSA MFI <10,000 (box in Figure 5A). Despite exclusion of patients with the strongest antibody reactivities (MFI >10,000), the inferior graft outcome associated with C3d positivity remained unchanged (Figure 5B). Tambur et al recently described the common occurrence of complement interference (prozone) on the solid phase platform (20). To ensure prozone was not a confounder in the interpretation of graft survival data, we performed serial dilution of sera from C3+ patients involved in the sub-analysis (shaded box in Figure 5A). At 1:4 dilution all sera exhibited the expected decrease in MFI values, with the exception of one specimen that showed the decrease at 1:16 dilution (Figure 5C). Thus, we concluded that complement interference did not have a significant effect in the graft survival comparison. We also analyzed the graft survival of recipient subsets using different peak IgG MFI (<7,000 and <5,000) and sum MFI (<20,000 and <10,000) cut-offs. The inferior outcome associated with C3d positivity was consistent under these different conditions (Figure 6).
Figure 5.
(A) Patients with peak DSA MFI <10,000 (blue box) were further evaluated for their graft survival according to C3d status. (B) Patients with C3d+ DSA had inferior graft survival compared with the C3d− group, even after exclusion of patients with DSA MFI >10,000. (C) Dilution of C3d+ sera (shaded) showed that prozone/complement interference was not a significant confounder in the graft survival data.
Figure 6.
Death-censored graft survival of recipient subsets by C3d status using different MFI cut-offs. (A) Peak MFI <7,000; (B) Peak MFI <5,000; (C) Sum MFI <20,000; (D) Sum MFI <10,000.
Univariate and Multivariate Models
In a univariate Cox proportional hazards model, significant predictors for death-censored graft loss at 5 years postbiopsy included: race, creatinine and proteinuria at time of biopsy, biopsy IFTA, and C3d positivity (Table 3). We next sought to determine whether C3d could provide independent prognostic value beyond standard clinico-pathologic data. In a multivariate Cox model, only biopsy IFTA emerged as a significant predictor of graft loss (HR 1.84; 95% CI 1.04–3.26, p=0.035). A crosstabulation between C3d and IFTA showed that the two variables were correlated (p=0.04), but in terms of risk prediction the value of C3d was usurped after integration with histology.
Table 3.
Univariate and multivariate models of risk factors for death-censored graft survival at 5 years post dysfunction. Variables with p-values <0.10 in the univariate analysis were selected to construct the multivariate model.
|
|
||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
|
| ||||
| Variable | Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value |
| Baseline clinical characteristics | ||||
| Gender | ||||
| Female | 1.00 (reference) | |||
| Male | 1.12 (0.64–1.9) | 0.693 | ||
| Race | 1.35 (0.84–2.16) | 0.216 | ||
| Caucasian | 1.00 (reference) | |||
| Black | 1.47 (0.78–2.79) | 0.235 | ||
| Other | 2.52 (1.19–5.35) | 0.016 | ||
| Age at transplant (per 1-yr increment) | 0.99 (0.97–1.00) | 0.238 | ||
| Type of cohort | ||||
| Prospective | 1.00 (reference) | |||
| Cross-sectional | 1.58 (0.81–3.1) | 0.178 | ||
| Number of ABDR mismatches | 1.16 (0.95–1.4) | 0.145 | ||
| Biochemical data at time of biopsy | ||||
| Creatinine (per 1 umol/L increment) | 1.003 (1.002–1.004) | <0.0001 | 1.003 (1.000–1.005) | 0.054 |
| Proteinuria (per 1 mg/mmol increment) | 1.002 (1.000–1.003) | 0.0300 | 1.001 (1.000–1.003) | 0.126 |
| Histologic data | ||||
| Microvascular inflammation (g+ptc >0) | 0.61 (0.33–1.1) | 0.109 | ||
| Biopsy C4d positivity | 1.44 (0.78–2.7) | 0.242 | ||
| Moderate-Severe IFTA (ci+ct ≥2) | 1.98 (1.3–3.0) | 0.001 | 1.84 (1.04–3.26) | 0.035 |
| Antibody Characteristics | ||||
| DSA Class | ||||
| Class I | 1.00 (reference) | |||
| Class II | 1.88 (0.45–7.82) | 0.380 | ||
| Class I and II | 2.01 (0.47–9.35) | 0.330 | ||
| Number of DSA (per 1 unit increment) | 0.85 (0.5–1.5) | 0.570 | ||
| Peak IgG DSA MFI (per 1000 increment) | 1.03 (0.99–1.1) | 0.212 | ||
| Sum IgG DSA MFI (per 1000 increment) | 1.01 (0.99–1.0) | 0.417 | ||
| C3d positivity | 2.01 (0.94–4.3) | 0.070 | 1.04 (0.37–2.94) | 0.942 |
Discussion
In this multicenter observational cohort study, the major findings are that patients with circulating C3d+ DSA had a worse renal function and more established graft fibrosis at the time of dysfunction. These patients developed a rapid rate of graft loss within the first year after dysfunction, leading to an inferior long-term graft survival compared with patients who produced non-complement-fixing DSA. Notwithstanding the prognostic value of C3d as a standalone test, in a multivariate model the amount of fibrosis was the dominant predictor of graft loss, indicating a limited role of the assay when biopsy data is already available.
Previous studies examining the utility of complement-dependent assays in kidney transplant patients have yielded mixed results (4, 6, 9, 12–15, 21). In a large population-based study, Loupy et al found C1q binding DSA within the first year posttransplant to be associated with a higher degree of graft injury on histopathology and a significantly higher risk of graft failure on follow-up (4). More recently, both Sicard and Comoli et al found a C3d assay to be more predictive of graft loss compared to C1q (6, 8). In the cross-sectional screening of a stable cohort of kidney recipients however, Eskandary et al found complement assays (C1q, C3d, C4d) failed to add additional diagnostic value over standard IgG MFI in the prediction for AMR, although graft outcome was not directly examined in this study (7). In contrast to Loupy’s cohort, patients with C3d+ DSA in this study did not exhibit more active micro-circulatory inflammation (g+ptc) on biopsy but rather, showed a higher degree of chronic graft fibrosis (ci+ct). This finding likely reflects differences in the timing of allograft biopsy, with biopsies performed in Loupy’s cohort all occurring within the first year posttransplant, while the average time to biopsy in this study was 5.3 ± 5.0 years posttransplant. Several studies show that early and late antibody-mediated injury represent different time points along the spectrum of AMR and carry distinct phenotypes (22–24). In our cohort enriched for late graft dysfunction, it is not surprising that C3d+ DSA were associated with a higher burden of chronic injury, but this is further evidence that even in the setting of a carefully monitored study protocol, a substantial degree of chronicity may already be present by the time clinical dysfunction is evident.
The association of complement-activating antibodies with inferior outcome should be interpreted in the context of antibody strength as assessed by conventional SAB platforms. In line with most complement-dependent studies, our data revealed a strong correlation between the SAB IgG MFI of antibodies and their C3d status (AUC 0.919). In support of this finding, Diebolder et al’s experimental data and images from cryo-electron-tomography show that efficient complement activation requires a sufficient density of antibody binding to be achieved on the cell surface, thereby initiating formation of a stable hexameric-IgG arrangement to bind C1q, the first component of the classical complement cascade (18). While we did not directly profile IgG subclasses, our data indicate that the occurrence of isolated weak/non-complement-fixing (IgG2/IgG4) strong antibodies is relatively rare in the clinical setting, as the majority (98.3%) of DSA with peak MFI >10,000 in our study yielded in vitro C3d positivity. Taken together, these findings suggest that the C3d test provides little discriminatory value, nor is it cost-effective, in the risk-stratification of strong antibodies where the conventional MFI strength already exceeds the predicted threshold for a positive signal on the assay. Even in the rare event that isolated strong non-complement-fixing DSA are identified, complement-independent mechanisms of graft injury are well-described (25), and these antibodies should not be ascribed as clinically irrelevant until more outcome data become available.
In the next part of analysis we focused on the ability of C3d to discriminate outcome in subsets of DSA positive patients with low-moderate range MFI. Despite exclusion of patients with the strongest MFI values, C3d positivity showed a persistent correlation with inferior graft survival. Furthermore, dilution of C3d+ sera showed that complement interference was not a confounder in this sub-analysis. In a recent comparative study, antibody specificities detected using kits from two SAB vendors showed a tendency toward higher MFI values being observed on the One Lambda (OL) platform compared to Immucor (26). This difference may in part relate to the extra serum dilution step associated with the Immucor protocol but not in OL’s, and perhaps the reason our dilution study identified only a low prevalence of complement interference in patient sera, which was already mitigated through Immucor’s protocol. This protocol difference has important implications in terms of test interpretation and application. First, although we performed parallel IgG SAB and C3d testing using Immucor reagents, many centers use OL SAB as their primary antibody detection method. Without serum pretreatment to ameliorate complement interference, apparent “low-moderate” MFI DSA detected on the OL SAB platform may actually represent strong antibodies if prozone is at play – in these cases further complement-dependent testing may not provide additional risk prediction. Second, given significant assay differences between OL and Immucor SAB across the MFI continuum, relationships defined between C3d and MFI strength in this study should be viewed as assay-specific until confirmatory data are available on the OL platform.
It is intriguing that while a positive C3d signal was found to be closely associated with IgG MFI, the assay was able to further discriminate the pathogenicity of a proportion of low-moderate strength antibodies, where factors other than antibody strength produced a positive signal on the test. In previous experimental work, synergy between polyspecific antibodies binding in close proximity to distinct epitopes on the same HLA molecule was a major determinant of in vivo complement activation (27, 28). Whether a similar mechanism occurs in vitro on antigen-coated beads remains to be elucidated. In a related finding, 29% of patients of C3d− DSA had detectable intragraft C4d. While issues with assay sensitivity and/or alternative complement pathway activation in the graft could account for this discordance, we found a higher proportion of multiple DSA being detected among C3d−C4d+ patients compared with the C3d−C4d− group (71% vs. 24%, p=0.06). This raises the possibility that in vitro evaluation of individual antibody specificity’s ability to activate complement may not accurately reflect the combined deleterious effect of multiple anti-donor antibodies acting synergistically on cell surface.
Examination of C3d’s test performance showed that it offered improved sensitivity and negative predictive value compared to biopsy C4d for predicting graft loss, while both tests showed poor specificity and positive predictive value. Whereas C3d positivity correlated with a rapid rate of graft loss early post-test, both C3d+ and C3d− patients demonstrated a comparable rate of graft failure over the subsequent years. This finding may be explained by: (1) the dynamic waxing-weaning nature of antibody titer can alter the C3d classification of DSA at serial time points as shown by Comoli et al (8); (2) differential deleterious impact of complement-fixing and non-complement fixing antibodies on graft injury (29); and (3) unknown treatment differences between the two groups.
Although we found C3d to be prognostic of graft failure as a standalone test along the continuum of MFI values, we did not confirm the independent predictive value of C3d as demonstrated in a previous publication (6). In two recent studies that evaluated C3d in kidney recipients, one did not directly examine graft failure as the primary outcome, and the other did not include histology in the risk prediction model (7, 8). While C3d positivity and graft fibrosis were found to be correlated in our analysis, in a combined model graft fibrosis was a better predictor of graft loss, lending further support to the value of histology both in the diagnosis of AMR and prognosis of outcome (7, 15). Despite this limitation, the C3d test may be considered in cases where a kidney biopsy is not permitted, or in patients where longitudinal risk stratification is desired after an incident biopsy. In these scenarios, both our data and others (15) showed that a sensitivity and specific threshold for predicting in vitro complement activity is achievable; thus, the assay is most efficient in the risk discrimination of low-moderate strength antibodies as identified on the Immucor platform. Within this context, it is also important for clinicians and HLA laboratories to discuss how the test result would change clinical management, considering the additional turn-around-time and cost of the test, and the general lack of effective treatment for AMR (30, 31). One rationale for testing may be to inform the selection of patients to undergo treatment with complement inhibitors. Given the current cost-prohibitive nature of these therapeutics however, the C3d test may be best reserved for patients enrolled in clinical trials until access to these medications becomes more prevalent.
Results of this study should be interpreted in view of certain limitations. First, as with all retrospective studies, our analysis is subject to unknown confounders between the two groups. One example is differences in the selection criteria for patients to receive AMR treatment and the drug regimens used by different centers, which were not captured in this study. Despite this limitation, there was no convincing evidence in literature during the study period that demonstrated the superiority of any AMR treatment protocol relative to others. Second, on first sight it may appear that the study underrepresented patients with low-level DSA as all subjects were required to screen positive for DSA with sum MFI >5,000 on the OL SAB platform before testing using Immucor products. Due to differences in assay protocol and sensitivity, a large proportion of our study patients in fact had MFI values <5,000 on the Immucor platform (Figure 2); moreover, 3 patients that screened positive using OL SAB had to be excluded from analysis as they showed no detectable DSA on Immucor SAB. Third, we acknowledge that C3d is only one of several available assays (C4d, C1q, IgG subclass) that can interrogate the complement activity of antibodies. Without performing these tests in parallel our results are not directly applicable to information derived from other assays. Similarly, given key differences between antibody detection platforms, conclusions regarding C3d and its utility should be limited to laboratories that use Immucor reagents. Fourth, given the general unavailability of HLA typing for DPA/B loci, potential DSA against DP antigens could not be determined, although we note that only a minority (5/106, 4.7%) of patients tested positive for anti-DP antibodies. Finally, as our dataset lacks patient-specific clinical data (i.e. non-adherence) that have been shown by others (15) to be prognostically important, the additional benefit of C3d relative to certain clinical predictors remains undefined.
In summary, C3d positivity in kidney recipients presenting with late dysfunction was associated with a worse renal outcome and faster progression to graft loss compared to patients with C3d− DSA. Despite the prognostic value of C3d as a standalone test, the assay did not provide independent risk prediction after integration of graft fibrosis into the model. For laboratories that run the Immucor assay, the C3d test adds marginal risk-stratification for strong antibodies with peak IgG MFI >10,000 and in patients where histology is available, but its utilization may be considered in those with low-moderate level DSA and where an allograft biopsy is not accessible.
Supplementary Material
Acknowledgments
This work was supported by funding from the UBC Clinician Investigator Program (JHL) and NIH RO1 AIO42819 (EFR). The authors express gratitude for all the staff and personnel involved in the initial recruitment, data collection, specimen storage, care and follow-up of patients enrolled in the DeKAF study. We would like to thank Immucor for providing the LSA and C3d reagents, and Ivan Balazs and Diana Dalfo for their meticulous coordination of reagent shipments and technical expertise. We also thank Sean Barbour for his statistical input. Finally, we are indebted to George Benzuela at UCLA for performing the assays with great care and efficiency.
Abbreviations
- AMR
antibody-mediated rejection
- DeKAF
long-term deterioration of kidney allograft function
- DSA
donor-specific antibodies
- IFTA
interstitial fibrosis and tubular atrophy
- MFI
mean fluorescence intensity
- OL
One Lambda Inc
- SAB
single antigen bead
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Immucor provided reagents used in this study but had no input regarding the study design, data analysis, interpretations and conclusions of this study. EFR receives grant funding from Immucor (20102702-B and 20145481) that are unrelated to the present C3d study.
References
- 1.Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90(1):68–74. doi: 10.1097/TP.0b013e3181e065de. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Sequeira F, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Hum Immunol. 2011;72(10):849–858. doi: 10.1016/j.humimm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Chin C, Chen G, Sequeria F, Berry G, Siehr S, Bernstein D, et al. Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. J Heart Lung Transplant. 2011;30(2):158–163. doi: 10.1016/j.healun.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369(13):1215–1226. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 5.Smith JD, Hamour IM, Banner NR, Rose ML. C4d fixing, luminex binding antibodies - a new tool for prediction of graft failure after heart transplantation. Am J Transplant. 2007;7(12):2809–2815. doi: 10.1111/j.1600-6143.2007.01991.x. [DOI] [PubMed] [Google Scholar]
- 6.Sicard A, Ducreux S, Rabeyrin M, Couzi L, McGregor B, Badet L, et al. Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J Am Soc Nephrol. 2015;26(2):457–467. doi: 10.1681/ASN.2013101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eskandary F, Bond G, Kozakowski N, Regele H, Marinova L, Wahrmann M, et al. Diagnostic Contribution of Donor-Specific Antibody Characteristics to Uncover Late Silent Antibody-Mediated Rejection-Results of a Cross-Sectional Screening Study. Transplantation. 2017;101(3):631–641. doi: 10.1097/TP.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 8.Comoli P, Cioni M, Tagliamacco A, Quartuccio G, Innocente A, Fontana I, et al. Acquisition of C3d-Binding Activity by De Novo Donor-Specific HLA Antibodies Correlates With Graft Loss in Nonsensitized Pediatric Kidney Recipients. Am J Transplant. 2016;16(7):2106–2116. doi: 10.1111/ajt.13700. [DOI] [PubMed] [Google Scholar]
- 9.Yabu JM, Higgins JP, Chen G, Sequeira F, Busque S, Tyan DB. C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation. 2011;91(3):342–347. doi: 10.1097/TP.0b013e318203fd26. [DOI] [PubMed] [Google Scholar]
- 10.Lan JH, Tinckam K. Clinical Utility of Complement Dependent Assays in Kidney Transplantation. Transplantation. 2018;102(1S Suppl 1):S14–S22. doi: 10.1097/TP.0000000000001819. [DOI] [PubMed] [Google Scholar]
- 11.Zeevi A, Lunz J, Feingold B, Shullo M, Bermudez C, Teuteberg J, et al. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32(1):98–105. doi: 10.1016/j.healun.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otten HG, Verhaar MC, Borst HP, Hené RJ, van Zuilen AD. Pretransplant donor-specific HLA class-I and -II antibodies are associated with an increased risk for kidney graft failure. Am J Transplant. 2012;12(6):1618–1623. doi: 10.1111/j.1600-6143.2011.03985.x. [DOI] [PubMed] [Google Scholar]
- 13.Crespo M, Torio A, Mas V, Redondo D, Pérez-Sáez MJ, Mir M, et al. Clinical relevance of pretransplant anti-HLA donor-specific antibodies: does C1q-fixation matter? Transpl Immunol. 2013;29(1–4):28–33. doi: 10.1016/j.trim.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Ginevri F, Nocera A, Comoli P, Innocente A, Cioni M, Parodi A, et al. Posttransplant de novo donor-specific hla antibodies identify pediatric kidney recipients at risk for late antibody-mediated rejection. Am J Transplant. 2012;12(12):3355–3362. doi: 10.1111/j.1600-6143.2012.04251.x. [DOI] [PubMed] [Google Scholar]
- 15.Wiebe C, Gareau AJ, Pochinco D, Gibson IW, Ho J, Birk PE, et al. Evaluation of C1q Status and Titer of De Novo Donor-Specific Antibodies as Predictors of Allograft Survival. Am J Transplant. 2016 doi: 10.1111/ajt.14015. [DOI] [PubMed] [Google Scholar]
- 16.Schaub S, Hönger G, Koller MT, Liwski R, Amico P. Determinants of C1q binding in the single antigen bead assay. Transplantation. 2014;98(4):387–393. doi: 10.1097/TP.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 17.Yell M, Muth BL, Kaufman DB, Djamali A, Ellis TM. C1q Binding Activity of De Novo Donor-specific HLA Antibodies in Renal Transplant Recipients With and Without Antibody-mediated Rejection. Transplantation. 2015;99(6):1151–1155. doi: 10.1097/TP.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 18.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014;343(6176):1260–1263. doi: 10.1126/science.1248943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourishankar S, Leduc R, Connett J, Cecka JM, Cosio F, Fieberg A, et al. Pathological and clinical characterization of the ‘troubled transplant’: data from the DeKAF study. Am J Transplant. 2010;10(2):324–330. doi: 10.1111/j.1600-6143.2009.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tambur AR, Herrera ND, Haarberg KM, Cusick MF, Gordon RA, Leventhal JR, et al. Assessing Antibody Strength: Comparison of MFI, C1q, and Titer Information. Am J Transplant. 2015;15(9):2421–2430. doi: 10.1111/ajt.13295. [DOI] [PubMed] [Google Scholar]
- 21.Freitas MC, Rebellato LM, Ozawa M, Nguyen A, Sasaki N, Everly M, et al. The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation. 2013;95(9):1113–1119. doi: 10.1097/TP.0b013e3182888db6. [DOI] [PubMed] [Google Scholar]
- 22.Dörje C, Midtvedt K, Holdaas H, Naper C, Strøm EH, Øyen O, et al. Early versus late acute antibody-mediated rejection in renal transplant recipients. Transplantation. 2013;96(1):79–84. doi: 10.1097/TP.0b013e31829434d4. [DOI] [PubMed] [Google Scholar]
- 23.Papadimitriou JC, Drachenberg CB, Ramos E, Kukuruga D, Klassen DK, Ugarte R, et al. Antibody-mediated allograft rejection: morphologic spectrum and serologic correlations in surveillance and for cause biopsies. Transplantation. 2013;95(1):128–136. doi: 10.1097/TP.0b013e3182777f28. [DOI] [PubMed] [Google Scholar]
- 24.Sun Q, Liu ZH, Ji S, Chen J, Tang Z, Zeng C, et al. Late and early C4d-positive acute rejection: different clinico-histopathological subentities in renal transplantation. Kidney Int. 2006;70(2):377–383. doi: 10.1038/sj.ki.5001552. [DOI] [PubMed] [Google Scholar]
- 25.Valenzuela NM, McNamara JT, Reed EF. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant. 2014;19(1):33–40. doi: 10.1097/MOT.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clerkin KJ, See SB, Farr MA, Restaino SW, Serban G, Latif F, et al. Comparative Assessment of Anti-HLA Antibodies Using Two Commercially Available Luminex-Based Assays. Transplant Direct. 2017;3(11):e218. doi: 10.1097/TXD.0000000000000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuller TC, Fuller AA, Golden M, Rodey GE. HLA alloantibodies and the mechanism of the antiglobulin-augmented lymphocytotoxicity procedure. Hum Immunol. 1997;56(1–2):94–105. doi: 10.1016/s0198-8859(97)00174-2. [DOI] [PubMed] [Google Scholar]
- 28.Kushihata F, Watanabe J, Mulder A, Claas F, Scornik JC. Human leukocyte antigen antibodies and human complement activation: role of IgG subclass, specificity, and cytotoxic potential. Transplantation. 2004;78(7):995–1001. doi: 10.1097/01.tp.0000136966.63957.e2. [DOI] [PubMed] [Google Scholar]
- 29.Guidicelli G, Guerville F, Lepreux S, Wiebe C, Thaunat O, Dubois V, et al. Non-Complement-Binding De Novo Donor-Specific Anti-HLA Antibodies and Kidney Allograft Survival. J Am Soc Nephrol. 2016;27(2):615–625. doi: 10.1681/ASN.2014040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sautenet B, Blancho G, Büchler M, Morelon E, Toupance O, Barrou B, et al. One-year Results of the Effects of Rituximab on Acute Antibody-Mediated Rejection in Renal Transplantation: RITUX ERAH, a Multicenter Double-blind Randomized Placebo-controlled Trial. Transplantation. 2016;100(2):391–399. doi: 10.1097/TP.0000000000000958. [DOI] [PubMed] [Google Scholar]
- 31.Eskandary F, Regele H, Baumann L, Bond G, Kozakowski N, Wahrmann M, et al. A Randomized Trial of Bortezomib in Late Antibody-Mediated Kidney Transplant Rejection. J Am Soc Nephrol. 2018;29(2):591–605. doi: 10.1681/ASN.2017070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.