Abstract
Peripheral inflammation is associated with poor response to antidepressant treatments. However, whether sex differentially affects this association remains unknown. Participants of Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care (EMBARC) with baseline plasma samples were included in this study (n=220; male n=75, female n=145). Depression severity [Hamilton Rating Scale for Depression 17-item (HAMD-17)] was measured at baseline and weeks- 1, 2, 3, 4, 6, and 8. Plasma c-reactive protein (CRP) was measured with commercially-available ELISA kits at baseline, week-1, and week-8. Sex difference in prediction of baseline-to-week-8 HAMD-17 change by baseline CRP was tested with sex-by-baseline-CRP-by-time interaction in mixed model analysis. Additionally, changes in CRP from baseline-to-week-8 CRP and its association with HAMD-17 changes over that period were also evaluated. Covariates included body mass index, site, smoking status, and age. There was a significant sex difference in association of baseline-to-week-8 HAMD-17 reduction with baseline CRP (p=0.033). Higher baseline CRP was associated with lower baseline-to-week-8 HAMD-17 reduction in females (p<0.0001) but not in males (p=0.632). Additionally, CRP was significantly reduced (p=0.041, effect size=0.254) from baseline-to-week-8, but there were no sex differences in this reduction (p=0.249). Baseline-to-week-8 changes in HAMD-17 and CRP were not significantly associated either overall (p=0.348) or based on sex (p=0.370). In a large study of depressed outpatients, we replicated previous findings that elevated baseline CRP levels are associated with worse antidepressant treatment outcomes. However, this effect was limited only to females. These findings emphasize the importance of studying sex differences in biological mechanisms linking inflammation and depression.
Keywords: Inflammation, depression, sex differences, c-reactive protein, antidepressant response, major depressive disorder
Introduction
C-reactive protein (CRP) is an easily available and inexpensive biomarker of inflammation that can prognosticate clinical course of major depressive disorder (MDD) (Uher et al., 2014, Miller et al., 2017, Jha et al., 2017). Elevated levels of CRP are associated with greater severity of depressive symptoms (Howren et al., 2009), higher risk of hospitalization (Wium-Andersen and Nielsen, 2013) and mortality (Wium-Andersen et al., 2014), and poorer response to commonly used antidepressant treatments (Haroon et al., 2018). Emerging evidence suggests that CRP in blood is a good surrogate for inflammation in the central nervous system, as levels of CRP in plasma and cerebrospinal fluid (CSF) are highly correlated (coefficient= 0.855) (Felger et al., 2018). Additionally, elevated CRP (>3 mg/L) in plasma is also associated with higher levels of several inflammatory cytokines and their soluble receptors in CSF (Felger et al., 2018). However, the strength of association between CRP and symptoms of MDD remains modest (Howren et al., 2009). This may be related in part to sex differences in pathophysiology of depression (Labonté et al., 2017), especially as it relates to immune dysfunction (Jha et al., 2018). In a recent report, elevated inflammatory biomarkers in CSF were associated with anhedonia in females but not in males (Felger et al., 2018). Other cross-sectional studies of sex differences in association of CRP with depression severity have reported conflicting results. While some reports have found greater depressive symptom severity with higher CRP levels in females only (Köhler-Forsberg et al., 2017), others have either reported stronger association of CRP with depressive symptoms in males as compared to females (Tayefi et al., 2017, Vetter et al., 2013, Liu et al., 2014) or no association of CRP with depressive symptoms in either sex (de Menezes et al., 2017). Hence, longitudinal studies that test association of CRP with depressive symptoms at multiple time points are necessary to better understand the sex differences in its association with depressive symptom severity.
Arguably, the clinical utility of CRP may be most evident in predicting response to antidepressant treatments (Miller et al., 2017). In two recent reports, higher levels of CRP were associated with worse outcomes with escitalopram, a selective serotonin reuptake inhibitor (SSRI) antidepressant (Jha et al., 2017, Uher et al., 2014). As SSRIs are the most commonly used antidepressant treatment (Olfson and Marcus, 2009), early identification of SSRI non-response may enable early use of treatment resistant depression (TRD)-specific treatments (such as repetitive transcranial magnetic stimulation, electroconvulsive therapy, or ketamine/esketamine) and reduce the morbidity and mortality of failed treatment trials. However, studies predicting poor response to SSRIs with elevated CRP levels have been limited so far by lack of both a placebo comparator and a consideration of sex differences (Jha et al., 2017, Uher et al., 2014).
This report aims to understand the sex differences in association of CRP with depressive symptoms and treatment outcomes in patients with MDD. The primary aim of this report is to test for sex differences in the prediction of acute-phase treatment outcomes based on baseline CRP. Exploratory aims include sex differences in changes in CRP with treatment and association of changes in CRP with those of depressive symptom severity. The specific questions asked in this report were:
Does association of baseline CRP with acute-phase antidepressant outcomes differ on the basis of sex?
Does change in CRP with antidepressant treatment differ on the basis of sex?
Does association of change in CRP with change in depressive symptom severity differ on the basis of sex?
We used a sample of convenience from the Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care (EMBARC) study that randomized outpatients with MDD to sertraline or placebo for eight weeks. To our knowledge, sex differences in association of CRP with acute-phase treatment outcomes has not been evaluated previously.
Material and Methods
Study overview
This report utilized data from participants of the EMBARC study who were randomized to sertraline versus placebo in a double-blind fashion for eight weeks and provided plasma samples prior to treatment initiation (n=220). As previously described, the EMBARC study enrolled 309 participants with MDD and 40 healthy controls in a two-stage study to identify biosignatures of antidepressant response. The first 10 participants with MDD were randomized to citalopram and three subjects were randomized though ineligible. Thus, the modified intent-to-treat sample of stage 1 of EMBARC study comprised of 296 participants who were randomized to either sertraline or placebo.
Participants were enrolled at four sites. The study design was reviewed and approved by the Institutional Review Board at each site and was registered on clinicaltrials.gov (NCT01407094) prior to enrollment of any participant. The study was conducted in accordance with the latest version of the Declaration of Helsinki. All participants signed informed consent prior to completing any study-related procedures. The inclusion and exclusion criteria have been described earlier by Trivedi et al. (2016) and are listed in detail at https://clinicaltrials.gov/ct2/show/NCT01407094. Briefly, participants were 18-65 years of age, met criteria for current episode of MDD on Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (W WJB and Gibbon, 2007), scored ≥14 on Quick Inventory of Depressive Symptomatology score (QIDS-SR) at both screening and randomization visits, did not meet criteria for any failed antidepressant trial in the current episode based on Massachusetts General Hospital Antidepressant Treatment Response Questionnaire (MGH-ATRQ), and agreed to and were eligible for all biomarker procedures (electroencephalography, psychological testing, magnetic resonance imaging, and blood draws). Participants were excluded if they did not tolerate sertraline or bupropion in the past, were pregnant/breastfeeding/planning to become pregnant, were medically or psychiatrically unstable, met criteria for psychotic/bipolar disorder in lifetime or substance abuse in past two months or substance dependence in past six months, or were on prohibited concomitant medications (antipsychotic, anticonvulsant, mood stabilizers, central nervous system stimulants, daily use of benzodiazepines or hypnotics, or antidepressants).
Assessments
Changes in depression severity as measured by the 17-item Hamilton Rating Scale for Depression (HAMD-17) was the primary outcome of EMBARC (Trivedi et al., 2016, Petkova et al., 2017). The individual items of HAMD-17 have three or five choices that are scored from 0-2 or 0-4, which are then summed to indicate depression severity of none (<6), mild (6-13), moderate (14-18), severe (19-23) and very severe (≥24) (Hamilton, 1960). The scale has high inter-rater reliability [r = 0.94; (Trajković et al., 2011)] and good construct validity [Cronbach’s α of 0.83 (Rush et al., 2003)]. Trained clinicians completed HAMD-17 at baseline and weeks 1, 2, 3, 4, 6, and 8 during stage 1 of EMBARC.
Measurement of CRP
Blood samples were obtained in potassium EDTA tubes for isolation of plasma by trained study members at each of the four sites. Plasma was extracted within four hours of blood draw by centrifugation at 1200 relative centrifugation force for 15 minutes at room temperature and aliquoted for storage at −80°C. Samples were shipped overnight on dry ice to the Biologic Core of National Institute of Mental Health Repository and Genomics Resource (NIMH RGR) for long-term storage. For this report, plasma samples were obtained from the NIMH RGR, transported to UT Southwestern Medical Center on dry ice, and subaliquoted by the Sanger Sequencing Core. Levels of CRP were estimated by the Metabolic Phenotyping Core using enzyme-linked immunosorbent assay (ELISA, EMD Millipore, Catalog #CYT298) according to the kit manufacturer’s instructions. Briefly, the standards, quality controls and plasma samples (100μl, 1:4 dilution) were incubated in the micro-titration 96-well plate coated with polyclonal anti-human CRP antibody. After a thorough wash, polyclonal anti-human CRP antibody labelled with horseradish peroxidase (HRP) was added to the wells and incubated with the immobilized antibody-CRP complex. Following another wash step, the HRP-conjugated antibody was allowed to react with the substrate and tetramethylbenzidine (100μl). The reaction was stopped by acidic solution (100μl), and absorbance (optical density) was measured at 450nm. The absorbance is proportional to the concentration of CRP. The background intensity was subtracted using appropriate controls (i.e., a “blank” sample), and CRP concentration (mg/L) was interpolated from a four-parameter logistic 4-PL standard curve generated with Graphpad Prism software, version 6.0. Using the 4-PL curve-fit measurements for each standard, coefficients of variation were estimated. The inter- and intra- assay coefficients of variation were in the range of 4.6% to 6.0%.
2.4. Statistical analysis plan
T-tests and chi-square tests, as appropriate, were used to compare baseline clinical and sociodemographic features between males and females, as well as those who were included in this report (n=220) and those who were excluded due to no available baseline plasma sample (n=76). Natural log transformation of CRP (log CRP) was conducted due to a skewed distribution. Separate repeated measure mixed model analyses with time-by-treatment arm and sex-by-time interactions were used to test if change in depression severity (HAMD-17) differed on the basis of treatment arm or sex, respectively. Covariates for all mixed model analyses included body mass index (BMI; categorized as normal or underweight, overweight, obese I, and obese II+), site, age, and smoking status. To test the primary hypothesis, a repeated measures mixed model analysis was conducted with HAMD-17 as the dependent variable and a three-way interaction of sex-by-baseline log CRP-by-time as the primary independent variable of interest, with all two-way interactions and main effects involving sex, baseline log CRP, and time (excluding baseline as it was included as a covariate). A significant three-way interaction suggests that rate of change of HAMD-17 based on baseline log CRP differed in males vs. females and were interpreted with analyses that were stratified by sex. An exploratory mixed model analyses with treatment arm-by-baseline log CRP-by-time interaction tested for differential effect of treatment arms. Repeated measures mixed model analysis with log CRP as the dependent variable and time (baseline, week-1, and week-8), sex, and sex-by-time interaction as independent variables was used to assess if changes in log CRP differed on the basis of sex. An exploratory mixed analysis with log CRP as the dependent variable and a treatment arm-by-time interaction was used to test if change in log CRP differed on the basis of treatment arm. Finally, to assess if changes in log CRP were differentially associated with changes in depression severity in males and females, linear regression analysis with baseline-to-week-8 change in HAMD-17 as dependent variable and sex-by-baseline-to-week-8 change in log CRP as the key independent variable of interest was used. Exploratory analyses tested for any treatment-arm differences in the association of baseline-to-week-8 changes in HAMD-17 and log CRP. All statistical analyses were conducted with SAS 9.3. Threshold of statistical significance was set at p<0.05.
Results
Participants of EMBARC study (n=220) included in this report were predominantly white, non-Hispanic and married/partnered. Those who were excluded from these analyses due to not having a plasma sample available (n=76) were younger in age, had lower BMI and self-reported depression severity, and were more likely to be employed full-time (Supplementary Table 1). Females were similar to males on most baseline clinical and sociodemographic variables except for significantly higher levels of CRP in females vs. males (Table 1). The two treatment arms (sertraline and placebo) did not significantly differ in HAMD-17 change (treatment arm-by-time interaction: F=1.05, df=6, 986, p=0.389) (Supplementary Table 2). The rates of remission (stage 1 exit HAMD-17 score ≤7) were 39.6% (40/101) and 37.1% (39/105) with sertraline and placebo respectively (missing n=14 due to no post-baseline visits). Similarly, the rates of response [score less than “much improved” on the Clinical Global Improvement scale at the end of stage 1 as defined a priori by (Trivedi et al., 2016)] with sertraline and placebo were 46.5% (47/101) and 40.0%(42/105) respectively (missing n=14 due to no post-baseline visits). There was no difference in HAMD-17 change based on sex either (sex-by-time interaction: F=0.33, df=6, 986, p=0.923) (Supplementary Table 3).
Table 1.
Clinical and sociodemographic features based on sex in EMBARC study participants who provided plasma at baseline (n=220)
| Males | Females | Comparison | ||||
|---|---|---|---|---|---|---|
| Number | 75 | 145 | ||||
| Categorical variables | N | % | N | % | χ2 (df) | p-value |
| Race | 2.14 (2) | 0.34 | ||||
| White | 54 | 72.0% | 91 | 62.7% | ||
| Black | 11 | 14.7% | 32 | 22.1% | ||
| Other | 10 | 13.3% | 22 | 15.2% | ||
| Hispanic ethnicity | 0.25 (1) | 0.615 | ||||
| No | 60 | 80.0% | 120 | 82.8% | ||
| Yes | 15 | 20.0% | 25 | 17.2% | ||
| Employment status at baseline | 4.20 (2) | 0.122 | ||||
| Full time | 22 | 29.3% | 40 | 28.0% | ||
| Part time | 23 | 30.7% | 28 | 19.6% | ||
| Unemployed | 30 | 40.0% | 75 | 52.4% | ||
| Marital Status | 7.30 (5) | 0.199 | ||||
| Married | 48 | 64.0% | 73 | 51.1% | ||
| Partnered | 16 | 21.3% | 34 | 23.8% | ||
| Single | 7 | 9.3% | 31 | 21.7% | ||
| Divorced | 3 | 4.0% | 3 | 2.1% | ||
| Separated | 1 | 1.3% | 1 | 0.7% | ||
| Widowed | 0 | 0.0% | 1 | 0.7% | ||
| Smoking status | 0.05 (1) | 0.818 | ||||
| No | 64 | 86.5% | 127 | 87.6% | ||
| Yes | 10 | 13.5% | 18 | 12.4% | ||
| Continuous variables | Mean | SD | Mean | SD | T value (df) | p-value |
| Age in years | 38.65 | 12.25 | 38.32 | 13.67 | 0.19 (1, 165) | 0.853 |
| Years of education | 15.09 | 2.35 | 15.07 | 2.40 | 0.08 (1, 153) | 0.940 |
| Episode duration in months | 59.31 | 99.21 | 37.23 | 67.78 | 3.64 (1)* | 0.057 |
| Age of onset of first major depressive episode | 17.20 | 5.75 | 15.65 | 5.89 | 1.88 (153) | 0.062 |
| QIDS-SR | 18.49 | 2.73 | 18.23 | 2.85 | 0.66 (1, 155) | 0.513 |
| HAMD-17 | 18.91 | 4.08 | 18.30 | 4.54 | 1.00 (1, 164) | 0.319 |
| Body mass index | 28.06 | 7.40 | 29.13 | 8.22 | −0.92 (1, 139) | 0.357 |
| C-reactive protein (CRP) | 1.31 | 1.57 | 3.22 | 5.16 | 11.74 (1)* | 0.0006 |
| Baseline log of c-reactive protein (log CRP) | −0.27 | 1.02 | 0.36 | 1.29 | −3.96 (183) | 0.0001 |
Non-parametric test results (Kruskal Wallis Test) as these variables were not normally distributed. EMBARC is Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care, SD is standard deviation, df is degrees of freedom, QIDS-SR is Quick Inventory of Depressive Symptomatology Self-Rated version, and HAMD-17 is Hamilton Rating Scale for Depression 17-item.
Does association of baseline CRP with acute-phase antidepressant outcomes differ on the basis of sex?
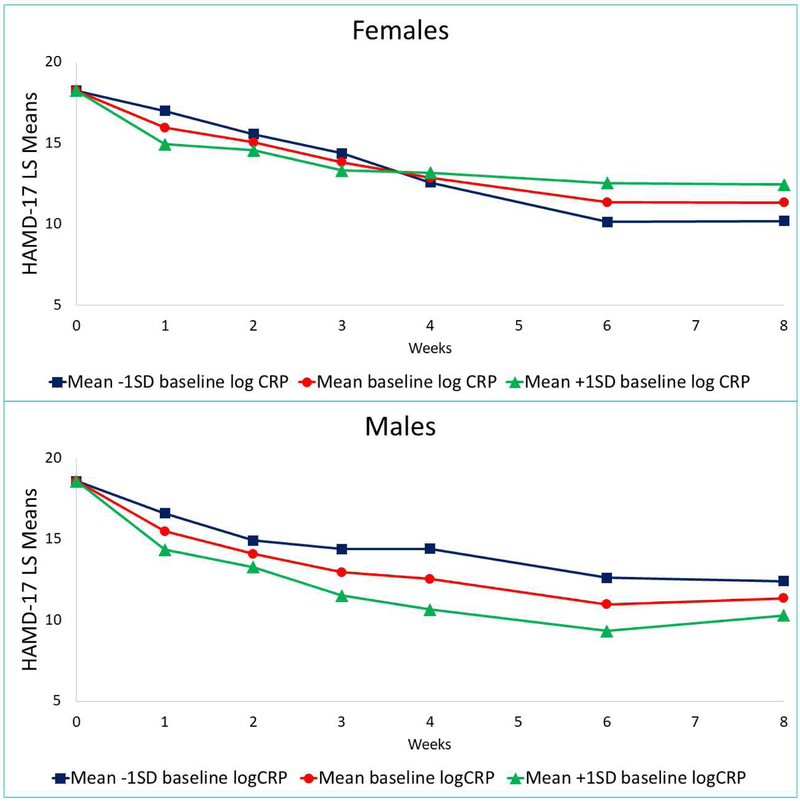
Yes. There was a significant sex-by-baseline log CRP-by-time interaction for HAMD-17 (F=2.44, df=5, 797, p=0.033) even after controlling for baseline depression severity, site, age, BMI, and smoking status (Supplementary Table 4). In subsequent analyses stratified by sex, there was a significant baseline log CRP-by-time interaction in females (p <0.0001) but not in males (p=0.632) (Table 2). As shown in Figure 1, females with lower log CRP at baseline had greater reduction in depressive symptom severity by week eight as compared to those with higher log CRP levels at baseline. The trend was reversed in males where higher log CRP levels at baseline were associated with greater reduction in depressive symptom severity as compared to those with lower baseline log CRP levels. Baseline log CRP levels did not differentially predict outcomes for sertraline vs. placebo (treatment arm-by-baseline log CRP-by-time interaction: F=0.37, df=6, 974, p=0.897) (Supplementary Table 5).
Table 2.
Association of baseline CRP with change in depression severity during acute-phase antidepressant treatment stratified by sex
| Males | Females | |||||
|---|---|---|---|---|---|---|
| F value | df | p value | F value | df | p value | |
| Baseline HAMD-17 | 34.50 | 1, 46 | <0.0001 | 17.22 | 1, 118 | <0.0001 |
| Baseline log CRP | 2.72 | 1, 48 | 0.101 | 0.03 | 1, 119 | 0.853 |
| Time | 12.98 | 5, 254 | <0.0001 | 26.76 | 5, 542 | <0.0001 |
| Site | 2.20 | 3, 47 | 0.101 | 4.98 | 3, 114 | 0.003 |
| Baseline body mass index | 0.92 | 3, 46 | 0.438 | 0.75 | 3, 117 | 0.522 |
| Age | 6.58 | 1, 46 | 0.014 | 4.85 | 1, 116 | 0.030 |
| Smoking status | 1.19 | 1, 46 | 0.281 | 0.25 | 1,118 | 0.621 |
| Baseline log CRP-by-time interaction | 0.69 | 5, 254 | 0.632 | 5.48 | 5, 543 | <0.0001 |
HAMD-17 is Hamilton Rating Scale for Depression 17-item, CRP is c-reactive protein, log CRP is log of c-reactive protein, df is degrees of freedom.
Figure 1. Sex differences in acute-phase outcomes based on baseline CRP in EMBARC study (n=220).
Changes in 17-item Hamilton Rating Scale for Depression (HAMD-17) in males and females on the basis of baseline c-reactive protein level (CRP). Least square (LS) means were obtained from mixed model analyses that utilized contrasts of mean ± 1 standard deviation (SD) in the Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care (EMBARC) study.
Does change in CRP with antidepressant treatment differ on the basis of sex?
No. While levels of CRP decreased significantly from baseline to week-8 (F=3.23, df=2, 259, p=0.041, effect size=0.254), there was no significant effect of sex on this reduction (sex-by-time interaction: F=1.40, df=2, 259, p=0.249) (Supplementary Table 6). The levels of CRP significantly differed on the basis of sex throughout the course of acute-phase antidepressant treatment (F=5.33, df=1, 190, p=0.022). In exploratory analyses of change in CRP based on treatment arm, there was neither a main effect of treatment arm (F=0.24, df=1,189, p=0.625) or a treatment arm-by-time interaction (F=2.07, df=2, 259, p=0.129) (Supplementary Table 7).
Does association of change in CRP with change in depressive symptom severity differ on the basis of sex?
No. Association of changes in log CRP and HAMD-17 from baseline to week-8 did not differ on the basis of sex (sex-by-baseline-to-week-8 change in log CRP p=0.370) (Table 3). The main effect of change in log CRP on change in HAMD-17 in this model was not statistically significant either (Table 3). In exploratory analyses, association of baseline-to-week-8 changes in log CRP and HAMD-17 did not differ on the basis of treatment arm either (treatment arm-by-log CRP interaction: F= 0.26, df=1, 108, p=0.792) (Supplementary Table 8).
Table 3.
Lack of differential association of baseline-to-week-8 changes in HAMD-17 and CRP based on sex
| T value | df | p value | |
|---|---|---|---|
| Baseline-to-week-8 change in log CRP | 0.94 | 1, 108 | 0.348 |
| Sex | −1.11 | 1, 108 | 0.268 |
| Sex-by-baseline-to-week-8 change in log CRP | −0.97 | 1, 108 | 0.370 |
| Site 1 * | 0.57 | 1, 108 | 0.571 |
| Site 2* | −1.53 | 1, 108 | 0.129 |
| Site 3* | −1.10 | 1, 108 | 0.275 |
| Age | −1.85 | 1, 108 | 0.067 |
| Normal weight@ | −0.10 | 1, 108 | 0.922 |
| Overweight@ | 0.23 | 1, 108 | 0.817 |
| Obese I@ | −0.31 | 1, 108 | 0.756 |
| Smoking status | 0.94 | 1, 108 | 0.350 |
Results of linear regression analyses with baseline-to-week-8 change in HAMD-17 as the dependent variable. CRP is c-reactive protein, the treatment arms included sertraline and placebo. HAMD-17 is Hamilton Rating Scale for Depression 17-item, CRP is c-reactive protein, log CRP is log of c-reactive protein, and df is degrees of freedom.
reference group is Site 4,
reference group is Obese II+
Discussion
This large study of depressed outpatients for the first time found that sex significantly moderated the association of baseline CRP and acute-phase antidepressant outcomes. Lower levels of baseline CRP were associated with greater reduction in depressive symptom severity in females. No similar association was seen in males. However, sex did not affect either the change in CRP or the association of changes in CRP and depression severity with antidepressant treatment. While CRP levels decreased with antidepressant treatment, the effect size of this decrease was small per Cohen’s convention (Cohen, 1992), and there was no difference between males and females in the baseline to week-8 reduction in CRP. Baseline to week-8 changes in CRP and depression severity were not significantly associated. In exploratory analyses, in the absence of a non-serotonergic antidepressant, baseline CRP did not significantly predict differential treatment outcomes with sertraline vs. placebo. Similarly, there were no significant differences between sertraline and placebo either on change in CRP or on association of changes in CRP and depression severity from baseline to week-8.
While no previous research has reported differential response based on sex and CRP levels, several findings of this report are consistent with previous ones. Elevated levels of CRP in females as compared to males is consistent with previous reports (Khera et al., 2005, Mehta et al., 2011). Increased adiposity may partly contribute to the differences in CRP levels as female outpatients with MDD report higher severity of increase in weight and appetite as compared to male outpatients with MDD (Marcus et al., 2008, Marcus et al., 2005). While BMI did not differ between males and females in our report, previous reports suggest that BMI is an imprecise measure of adiposity as visceral but not subcutaneous adiposity is associated with increased levels of CRP (Neeland et al., 2013). Findings of baseline CRP being a predictor of poor outcomes is consistent with those seen in CO-MED and GENDEP studies where patients treated with escitalopram, an SSRI similar to sertraline, had poorer response with elevated levels of CRP (Jha et al., 2017, Uher et al., 2014). Again to our knowledge, this is the first report to show that association of poorer treatment outcomes with elevated CRP levels is restricted only to females and not males. Notably, better treatment outcomes in females with lower CRP emerged only after 6 weeks of treatment. The potential biological reasons for such a time-dependent association are unclear. Response to antidepressant treatment is associated with changes in neural circuits (Castren and Hen, 2013). Elevated inflammation in females may interfere with these adaptive neural changes, thus preventing the maximal benefit with antidepressants which often requires 6 or more weeks of treatment (Trivedi et al., 2006). Reduction in CRP with antidepressant treatment is also consistent with previous reports (Lanquillon et al., 2000, O'Brien et al., 2018, Hiles et al., 2012). Similarly, lack of association between change in CRP with clinical improvement in this report is consistent with previous reports (Lanquillon et al., 2000, O'Brien et al., 2018). However, this is the first report to systematically evaluate any sex-differences in the association of changes in CRP and depression severity with antidepressant treatment. It is noteworthy that some findings in this report differ from previous reports that either failed to find significant reduction in CRP or reported increased CRP with antidepressant treatments (Tuglu et al., 2003, Hamer et al., 2011). Lack of difference in treatment outcomes based on sex differed from those reported in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study (Young et al., 2009). Notably in the STAR*D study, females had greater baseline depression severity and were younger in age than males, which differs from this report. Additionally, the STAR*D reports that reduction in depression severity between males and females was no longer significant after adjusting for baseline differences including baseline depressive symptom severity.
Sex differences in immune system are well recognized (Klein and Flanagan, 2016). Females exhibit higher levels of inflammatory markers (Klein and Flanagan, 2016) and mount greater pro-inflammatory response to immune challenge with endotoxin (van Eijk et al., 2007) than males. Additionally, adaptive immune system in females is biased towards T-helper type 2 (Th2) cells while being biased towards Th1 in males (Klein and Flanagan, 2016). Modulation of adaptive immune response, specifically the Th1/Th2 balance (Eyre et al., 2016, Martino et al., 2012), by antidepressant medications may underlie the sex-differences in the association of baseline CRP levels with antidepressant treatment outcomes.
There are several limitations of this report. As sex differences in treatment outcomes on the basis of CRP was not the primary objective of EMBARC study, this report may not have been adequately powered to detect this difference. Thus, these findings need replication. The inclusion and exclusion criteria of EMBARC may further limit the generalizability of these findings. Selection of CRP as the biomarker of inflammation, while clinically pragmatic, may have been inadequate to fully capture the immune changes associated with antidepressant treatment. Finally, this report did not include treatments such as nortriptyline and bupropion-SSRI combination (Jha et al., 2017, Uher et al., 2014), which have been shown to be more effective than SSRI monotherapy in patients with elevated CRP.
In conclusion, elevated levels of CRP prior to treatment initiation are associated with poorer acute-phase antidepressant and placebo outcomes in female but not in male patients with MDD. Future studies should take sex into account in evaluating the impact of elevated CRP in treatment outcomes for depression and measurement of CRP in clinical practice may inform treatment selection in female patients with MDD.
Supplementary Material
Highlights.
Higher baseline CRP predicts worse acute-phase antidepressant outcomes.
This association is limited to female patients with major depressive disorder.
Reduction in CRP with antidepressant treatment did not differ on the basis of sex.
Baseline-to-week-8 changes in depression and CRP were not significantly correlated.
Acknowledgements
The authors thank the clinical staff at each site for their assistance with this project; all of the study participants; Drs. Gadad, Furman, Gordillo and Mason for CRP assay; and Ms. Taryn Mayes for administrative support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Drs. Minhajuddin, Chin-Fatt, and Carmody report no potential conflicts of interest. Dr. Jha has received contract research support from Acadia Pharmaceuticals and Janssen Research. Dr. Greer has received research funding from NARSAD and honoraria and/or consulting fees from H. Lundbeck A/S and Takeda. Dr. Trivedi is or has been an advisor/consultant and received fee from (lifetime disclosure): Abbott Laboratories Inc., Akzo (Organon Pharmaceuticals Inc.), Allergan Sales LLC, Alkermes, Acadia Pharmaceuticals Inc., AstraZeneca, Axon Advisors, Brintellix, Bristol-Myers Squibb Company, Cephalon Inc., Cerecor, Eli Lilly & Company, Evotec, Fabre Kramer Pharmaceuticals Inc., Forest Pharmaceuticals, GlaxoSmithKline, Global Medical Education Inc., Health Research Associates, Johnson & Johnson, Lundbeck, MedAvante Medscape, Medtronic, Merck, Mitsubishi Tanabe Pharma Development America Inc., MSI Methylation Sciences Inc., Nestle Health Science-PamLab Inc., Naurex, Neuronetics, One Carbon Therapeutics Ltd., Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals Inc., Pfizer Inc., PgxHealth, Phoenix Marketing Solutions, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Products Ltd., Sepracor, SHIRE Development, Sierra, SK Life and Science, Sunovion, Takeda, Tal Medical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, and Wyeth-Ayerst Laboratories. In addition, he has received grants/research support from: Agency for Healthcare Research and Quality, Cyberonics Inc., National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Diabetes and Digestive and Kidney Diseases, Johnson & Johnson; and he receives royalties from Janssen Research and Development LLC.
References
- Castren E & Hen R, 2013. Neuronal plasticity and antidepressant actions. Trends Neurosci, 36, 259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1992. A power primer. Psychological bulletin, 112, 155. [DOI] [PubMed] [Google Scholar]
- De Menezes ST, De Figueiredo RC, Goulart AC, Nunes MA, I, M. B., Viana MC & Barreto SM, 2017. Lack of association between depression and C-reactive protein level in the baseline of Longitudinal Study of Adult Health (ELSA-Brasil). J Affect Disord, 208, 448–454. [DOI] [PubMed] [Google Scholar]
- Eyre HA, Lavretsky H, Kartika J, Qassim A & Baune BT, 2016. Modulatory Effects of Antidepressant Classes on the Innate and Adaptive Immune System in Depression. Pharmacopsychiatry, 49, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, Le NA, Feinberg R, Tansey MG & Miller AH, 2018. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams JBW, Spitzer RL, and Gibbon M, 2007. Structured Clinical Interview for DSM-IV-TR Axis I Disorders Clinical Trials Version (SCID-CT) New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Hamer M, Batty GD, Marmot MG, Singh-Manoux A & Kivimaki M, 2011. Anti-depressant medication use and C-reactive protein: Results from two population-based studies. Brain, behavior, and immunity, 25, 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry, 23, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, Felger JC & Miller AH, 2018. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology, 95, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, De Malmanche T & Attia J, 2012. Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: a meta-analysis. Psychological Medicine, 42, 2015–2026. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM & Suls J, 2009. Associations of Depression With C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosomatic Medicine, 71, 171–186. [DOI] [PubMed] [Google Scholar]
- Jha MK, Miller AH, Minhajuddin A & Trivedi MH, 2018. Association of T and non-T cell cytokines with anhedonia: Role of gender differences. Psychoneuroendocrinology, 95, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A, Mayes TL, Rush AJ & Trivedi MH, 2017. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology, 78, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH JR., Grundy SM & De Lemos JA, 2005. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol, 46, 464–9. [DOI] [PubMed] [Google Scholar]
- Klein SL & Flanagan KL, 2016. Sex differences in immune responses. Nature Reviews Immunology, 16, 626. [DOI] [PubMed] [Google Scholar]
- Köhler-Forsberg O, Buttenschøn HN, Tansey KE, Maier W, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A & Rietschel M, 2017. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain, behavior, and immunity, 62, 344–350. [DOI] [PubMed] [Google Scholar]
- Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh Y-HE & Cahill M, 2017. Sex-specific transcriptional signatures in human depression. Nature medicine, 23, 1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U & Vedder H, 2000. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology, 22, 370–9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Al-Sayegh H, Jabrah R, Wang W, Yan F & Zhang J, 2014. Association between C-reactive protein and depression: modulated by gender and mediated by body weight. Psychiatry Research, 219, 103–108. [DOI] [PubMed] [Google Scholar]
- Marcus SM, Kerber KB, Rush AJ, Wisniewski SR, Nierenberg A, Balasubramani GK, Ritz L, Kornstein S, Young EA & Trivedi MH, 2008. Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the Sequenced Treatment Alternatives to Relieve Depression study. Compr Psychiatry, 49, 238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, Wisniewski SR, Balasubramani GK, Trivedi MH & Rush AJ, 2005. Gender differences in depression: findings from the STAR*D study. J Affect Disord, 87, 141–50. [DOI] [PubMed] [Google Scholar]
- Martino M, Rocchi G, Escelsior A & Fornaro M, 2012. Immunomodulation Mechanism of Antidepressants: Interactions between Serotonin/Norepinephrine Balance and Th1/Th2 Balance. Current Neuropharmacology, 10, 97–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta NN, St Clair C, Farouk S, Braunstein S, Schutta M, Iqbal N, Rader D, Reilly MP, Qasim AN & Budharaju V, 2011. Gender Differences in the Association of C-Reactive Protein with Coronary Artery Calcium in Type-2 Diabetes. Clinical endocrinology, 74, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Trivedi MH & Jha MK, 2017. Is C-reactive protein ready for prime time in the selection of antidepressant medications? Psychoneuroendocrinology, 84, 206. [DOI] [PubMed] [Google Scholar]
- Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM & De Lemos JA, 2013. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring), 21, E439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien SM, Scott LV & Dinan TG, 2018. Antidepressant therapy and C-reactive protein levels. British Journal of Psychiatry, 188, 449–452. [DOI] [PubMed] [Google Scholar]
- Olfson M & Marcus SC, 2009. National patterns in antidepressant medication treatment. Archives of general psychiatry, 66, 848–856. [DOI] [PubMed] [Google Scholar]
- Petkova E, Ogden RT, Tarpey T, Ciarleglio A, Jiang B, Su Z, Carmody T, Adams P, Kraemer HC, Grannemann BD, Oquendo MA, Parsey R, Weissman M, McGrath PJ, Fava M & Trivedi MH, 2017. Statistical Analysis Plan for Stage 1 EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care) Study. Contemp Clin Trials Commun, 6, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH & Keller MB, 2003. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry, 54, 573–583. [DOI] [PubMed] [Google Scholar]
- Tayefi M, Shafiee M, Kazemi-Bajestani SMR, Esmaeili H, Darroudi S, Khakpouri S, Mohammadi M, Ghaneifar Z, Azarpajouh MR, Moohebati M, Heidari-Bakavoli A, Parizadeh MR, Nematy M, Safarian M, Ebrahimi M, Ferns GA, Mokhber N & Ghayour-Mobarhan M, 2017. Depression and anxiety both associate with serum level of hs-CRP: A gender-stratified analysis in a population-based study. Psychoneuroendocrinology, 81, 63–69. [DOI] [PubMed] [Google Scholar]
- Trajković G, Starčević V, Latas M, LeŠtarević M, Ille T, Bukumirić Z & Marinković J, 2011. Reliability of the Hamilton Rating Scale for Depression: a meta-analysis over a period of 49 years. Psychiatry research, 189, 1–9. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, McGrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML, Oquendo MA, Bruder G, Pizzagalli D, Toups M, Cooper C, Adams P, Weyandt S, Morris DW, Grannemann BD, Ogden RT, Buckner R, McInnis M, Kraemer HC, Petkova E, Carmody TJ & Weissman MM, 2016. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): Rationale and design. J Psychiatr Res, 78, 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK & Fava M, 2006. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry, 163, 28–40. [DOI] [PubMed] [Google Scholar]
- Tuglu C, Kara SH, Caliyurt O, Vardar E & Abay E, 2003. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology, 170, 429–433. [DOI] [PubMed] [Google Scholar]
- Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A & McGuffin P, 2014. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry, 171, 1278–86. [DOI] [PubMed] [Google Scholar]
- Van Eijk LT, Dorresteijn MJ, Smits P, Van der Hoeven JG, Netea MG & Pickkers P, 2007. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers*. Critical Care Medicine, 35, 1464–1469. [DOI] [PubMed] [Google Scholar]
- Vetter ML, Wadden TA, Vinnard C, Moore RH, Khan Z, Volger S, Sarwer DB & Faulconbridge LF, 2013. Gender differences in the relationship between symptoms of depression and high-sensitivity CRP. International Journal Of Obesity, 37, S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wium-Andersen MK & Nielsen SF, 2013. Elevated C-Reactive Protein Levels, Psychological Distress, and Depression in 73a131 Individuals. JAMA psychiatry, 70, 176–184. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen MK, Orsted DD & Nordestgaard BG, 2014. Elevated C-reactive protein, depression, somatic diseases, and all-cause mortality: a mendelian randomization study. Biol Psychiatry, 76, 249–57. [DOI] [PubMed] [Google Scholar]
- Young EA, Kornstein SG, Marcus SM, Harvey AT, Warden D, Wisniewski SR, Balasubramani GK, Fava M, Trivedi MH & Rush AJ, 2009. Sex differences in response to citalopram: A STAR*D report. Journal of Psychiatric Research, 43, 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.