Abstract
The impact of bone marrow fibrosis grade on the prognosis of patients with chronic myelomonocytic leukemia (CMML) remains controversial. Therefore, we examined the records of 82 patients diagnosed with CMML at our institution and summarized baseline characteristics and molecular profiles by subgroups of absent or mild (grades 0/1) and moderate (grade 2) fibrosis. Cox proportional hazards models were constructed to assess the prognostic significance of fibrosis grade. Grade 2 fibrosis was identified in 63 patients (76.8%), grade 1 in 16 patients (19.5%), and grade 0 in 3 patients (3.7%). Grade 2 fibrosis was associated with reduced hemoglobin levels (median: 9.75 g/dL vs 11.0 g/dL in grade 0/1; p = 0.04) and increased percentages of ringed sideroblasts (7.5% vs 0%; p = 0.008). In multivariable analysis, grade 2 fibrosis was an independent predictor of poor overall survival (OS; 95% CI: 1.32–6.35; HR: 2.90; p = 0.008), but not event-free survival (EFS; 95% CI: 0.62–2.67; HR: 1.28; p = 0.50). Absolute neutrophil count (ANC) was found to impact OS (95% CI: 1.01–1.09; HR: 1.05; p = 0.009), while both ANC (95% CI: 1.00–1.07; HR: 1.04; p = 0.04) and peripheral blood blast percentage (95% CI: 1.02–1.32; HR: 1.16; p = 0.02) impacted EFS. These results implicate fibrosis grade is an important indicator of prognosis, with high-grade fibrosis predicting inferior survival. Given the prevalence of marrow fibrosis in CMML, fibrosis grading should be incorporated into prognostic assessment and therapeutic decision-making.
Keywords: Bone marrow fibrosis, CMML, Fibrosis grade, Prognosis
Introduction
Chronic myelomonocytic leukemia (CMML) is a clonal hematopoietic malignancy characterized by monocytic proliferation in the bone marrow and peripheral blood. The World Health Organization initially classified CMML as a subtype of myelodysplastic syndrome (MDS) [1], but CMML has since been reclassified as a distinct hematological disorder. Although CMML is categorized as an independent condition, the prognosis of the disease is often difficult to determine owing to the heterogeneity of its clinical features, which overlap with both MDS and myeloproliferative neoplasms (MPN) [2]. For this reason, CMML has also been separated into MDS-type and MPN-type variants. In fact, the clinical heterogeneity of CMML may provide an opportunity for risk stratification to determine prognosis and guide management decisions [3].
Monocytes have been known to differentiate into fibrocytes, and neoplastic fibrocytes were found to induce bone marrow fibrosis in primary myelofibrosis [4]. Therefore, given the nature of CMML as primarily neoplastic monocytic proliferation, monocyte-driven bone marrow fibrosis may be implicated in CMML progression and bone marrow fibrosis may also affect prognosis in CMML.
A number of previous studies have attempted to grade the degree of bone marrow fibrosis [5]. Previous clinical data examining how bone marrow fibrosis influences the survival time or treatment response in patients with CMML or MDS have been controversial. Some studies showed reduced disease-free survival in patients with severe fibrosis compared with mild or moderate fibrosis [6], but other studies have shown no such association [7]. Nonetheless, the presence of bone marrow fibrosis itself has been associated with incidence of specific clinical, laboratory, and genetic features, including splenomegaly, increased ferritin levels, and JAK2 mutations [7,8]. Most importantly, a previous analysis of clinical outcomes in patients with chronic lymphocytic leukemia (CLL) found a significant negative correlation between the grade of bone marrow fibrosis and overall survival (OS) [9]. Bone marrow fibrosis has also been identified in MDS as a predictor of inferior OS and transformation-free survival in low- and intermediate-risk patients with CLL specifically [8]. Based on this premise, the Hannover score also relies on bone marrow fibrosis to infer prognostic implications [10].
Given the widespread use of bone marrow fibrosis as a predictive factor in prognostication, an established relationship between bone marrow fibrosis grade and clinical outcome is likely to augment its contribution to prognostic evaluation. Furthermore, the ambiguity of such a relationship in literature may allude to the correlation of fibrosis with a constellation of other clinico-pathological variables. Therefore, our objectives were to determine the association between bone marrow fibrosis grade at initial diagnosis of de novo CMML and various patient- and disease-related characteristics, and to assess the association of these characteristics with prognosis and clinical outcome.
Methods
Patients
Our evaluation was based on a retrospective analysis of 82 patients diagnosed with CMML who were evaluated at The University of Texas MD Anderson Cancer Center between 2004 and 2017. These patients were identified using an extensive search of our leukemia databases, and the diagnosis of CMML was based strictly on the criteria established by the World Health Organization (WHO) in 2008. These criteria include a persistent peripheral blood monocyte count of more than 1× 109/L, less than 20% blasts in the bone marrow or peripheral blood, hematopoietic dysplastic features in one or more myeloid lineages, and absence of a Philadelphia chromosome or PDGFRA/PDGFRB rearrangement. We included patients that met the criteria for both MDS-CMML and MPN-CMML subtypes, as defined by the previous French-American-British classification [2]; however, patients diagnosed solely with MDS or MPN were excluded from our analysis. Patients previously treated with anti-neoplastic drugs or ionizing radiation (and therefore at risk of developing secondary MDS) and those diagnosed with Philadelphia chromosome-positive chronic granulocytic leukemia or other MPNs were also excluded.
All data were verified and updated by the institution’s physicians and data managers, and data were double-checked to avoid any duplicate cases. The study was approved by the University of Texas MD Anderson Institutional Review Board.
Bone marrow staining
Bone marrow biopsies were obtained from all patients at the time of diagnosis and processed using standard procedures. The core biopsies were assessed for reticulin and trichrome fibrosis by an experienced hematopathologist (T.M.) using the European Consensus [5] for bone marrow fibrosis grading. Grade 0 (absence of fibrosis) was defined by linear, scattered reticulin fibers with no intersections, corresponding to a normal bone marrow; grade 1 (mild fibrosis) was defined as the presence of a loose meshwork of thin reticulin fibers with many intersections, especially in perivascular areas; grade 2 (moderate fibrosis) was defined as the presence of a dense and diffuse meshwork of reticulin fibers, together with extensive intersections, occasionally with only focal collagen bundles and focal osteosclerosis; and grade 3 (severe fibrosis) was defined as the presence of dense reticulin fibers intermingling with focal bundles of collagen and associated with endophytic bone formation. This assessment was performed in cellular areas of the bone marrow. Patients included in the analysis were required to have their bone marrow fibrosis graded independently by an experienced hematopathologist. At the time of analysis, the patient cohort was subdivided into groups characterized by either grade 0/1 or grade 2 bone marrow fibrosis, supported by observable differences between the clinical characteristics of the two subgroups. Severe bone marrow fibrosis (grade 3) was not observed in any of our patients, owing to which it was excluded from the analysis.
Prognostic factors
A number of patient- and disease-related characteristics were recorded at the time of diagnosis and subsequently analyzed to establish their possible association with OS, event-free survival (EFS), and bone marrow fibrosis grade. OS was defined as the time interval between diagnosis and the date of death due to any cause. EFS was defined as the time interval between the date of presentation to our institution and the date of disease progression, relapse, non-response, or death due to any cause.
In addition, cytogenetic analysis was conducted in all patients at the time of diagnosis using the International Prognostic Scoring System (IPSS) and the WHO Classification-based Prognostic Scoring System, hence enabling risk stratification of these patients [11,12]. For molecular profiling and mutational analysis, next-generation sequencing was employed to test for the complete coding sequences of 14 genes (KRAS, NRAS, ASXL1, TET2, TP53, RUNX1, IDH2, DNMT3, FGFR3, MPL, WT1, EZH2, PTPN1, JAK2) in available samples.
Statistical analysis
Patient characteristics were summarized using frequency for categorical variables and median and range for continuous variables. The Wilcoxon rank-sum test was used to assess the difference in patient characteristics between bone marrow fibrosis grade subgroups. The probabilities of OS and EFS were estimated using the Kaplan-Meier method. Cox proportional hazards regression models were fit to assess the association of baseline characteristics with OS and EFS. All statistical analyses were conducted in SAS (Cary, NC) and Splus (Palo Alto, CA). For all analyses, p<0.05 was considered statistically significant.
Results
Clinical and molecular characteristics
Patient baseline demographic and clinical characteristics of the 82 included patients are summarized in Table 1. The median patient age was 69 years (range: 36.2–86.5 years). Only a small fraction of patients presented with hepatomegaly (n = 4; 5%), but splenomegaly was more prevalent (n = 15; 18%). In addition, 39% (n = 32) had transfusion dependency. According to the bone marrow biopsy analysis, 77% of patients (n = 63) were identified with grade 1 bone marrow fibrosis, 20% (n = 16) had grade 2 bone marrow fibrosis, and 4% (n = 3) presented with no bone marrow fibrosis.
Table 1.
Baseline characteristics
| Variable | N = 82a |
|---|---|
| Age, median (range), years | 69 (36.2–86.5) |
| Sex, n (%) | |
| Male | 57 (70) |
| Race, n (%) | |
| Non-white | 4 (5) |
| White | 45 (55) |
| Not specified | 33 (40) |
| White blood cell count, median (range), cells/L | 11.8 ×109 (0.1–207.5 ×109) |
| Hemoglobin level, median (range), g/dL | 10.3 (3.7–15.5) |
| Platelet count, median (range), cells/L | 89 ×109 (1.8–13.86 ×109) |
| Albumin level (n = 80), median (range), g/dL | 4.3 (2.3–5.1) |
| Lactate dehydrogenase level (n = 80), median (range), U/L | 571 (228–2897) |
| Ferritin level (n = 58), median (range), ng/mL | 289 (31–3868) |
| B2-microglobulin level (n = 43), median (range), µg/mL | 3.7 (1.2–33) |
| Absolute neutrophil count, median (range), cells/µL | 59 (0–43.8) |
| Basophils, median (range), % | 0 (0–24.3) |
| Eosinophils (n = 81), median (range), % | 1 (0–25) |
| Hepatomegaly, n (%) | |
| Yes | 4 (5) |
| No | 78 (95) |
| Splenomegaly, n (%) | |
| Yes | 15 (18) |
| No | 66 (80) |
| History of splenectomy | 1 (1) |
| Transfusion dependency, n (%) | |
| Yes | 32 (39) |
| No | 46 (56) |
| Unknown | 4 (5) |
| Treatment, n (%) | |
| HMA | 19 (23) |
| HMA+ combination | 13 (16) |
| TKI | 1 (1) |
| Chemotherapy | 12 (15) |
| Supportive therapy | 37 (45) |
| Bone marrow monocytes, median (range), % | 12 (0–53) |
| Bone marrow fibrosis grade, n (%) | |
| 0 | 3 (4) |
| 1 | 63 (77) |
| 2 | 16 (20) |
| IPSS cytogenetic risk group | |
| Poor | 7(8.5%) |
| Intermediate | 15(18.3%) |
| Good | 57(69.5%) |
| Very Good | 3(3.7%) |
| Ringed sideroblasts (n = 58), median (range), % | 0(0–92) |
| Bone marrow blast %, median (range), n=81 | 5% (0–20%) |
| Peripheral blood blast %, median (range) | 0%(0–14%) |
| Time from diagnosis to treatment (n = 45), median, (range), months | 1.9 (0–59.7) |
Unless otherwise noted.
Abbreviations: HMA=Hypomethylating agent, TKI=Tyrosine kinase inhibitor.
Cytogenetic analysis by IPSS identified poor-risk cytogenetics in a considerable fraction of the patient cohort (n = 7; 8.5%). Nonetheless, the majority of our patients were categorized into the good cytogenetic-risk subgroup (n = 57; 69.5%), followed by intermediate-risk cytogenetics (n = 15; 18.3%) and very good cytogenetics (n = 3; 3.7%).
In addition, genetic aberrations were recognized in a large fraction of the cohort. Among 72 patients with gene aberration data, TET2 mutations dominated at a frequency of 33.3% (n = 24). ASXL1 mutations were also common (n = 21; 29.2%), as were mutations in the NRAS and KRAS genes (NRAS: n = 17, 23.6%; KRAS: n = 14, 19.4%). Mutations in FGFR3, WT1, JAK2, and EZH2 were the least common genetic abnormalities, all occurring at a minimal frequency of 1.4% (n = 1).The association between co-occurring mutations was analyzed and is shown in Figure 1.
Figure 1. Associations between co-occurring mutations. The thickness of each connecting line represents the frequency with which the two mutations co-occurred.
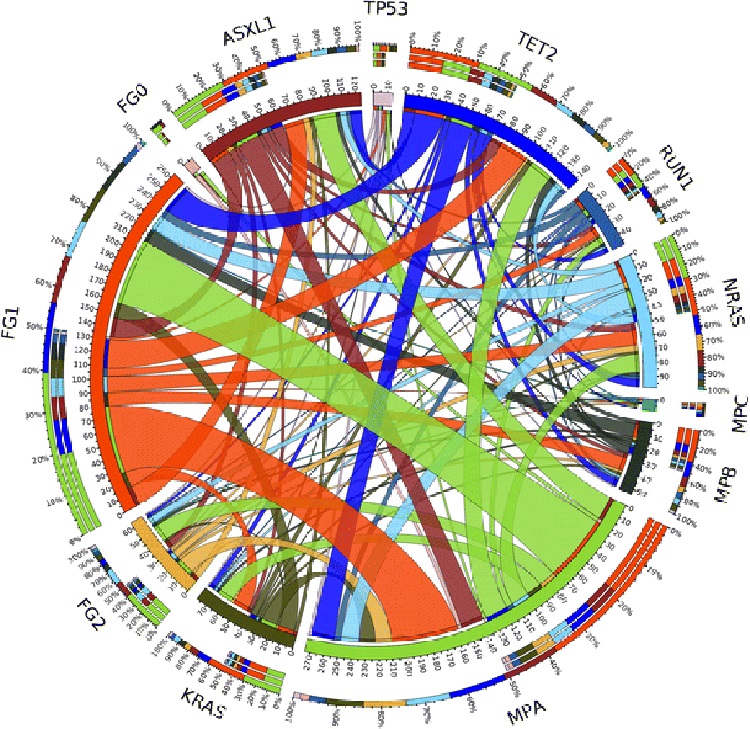
Abbreviations: FG0=grade 0 bone marrow fibrosis; FG1,grade 1 bone marrow fibrosis; FG2=grade 2 bone marrow fibrosis; MPA=0–20% bone marrow monocytes; MPB=21–40% bone marrow monocytes; MPC=41–60% bone marrow monocytes.
Association of bone marrow fibrosis with patient characteristics
Grade 2 bone marrow fibrosis was associated with reduced levels of hemoglobin (p = 0.04) and an increased percentage of ring sideroblasts in aspirate smears (p = 0.008) at the time of diagnosis compared with grade 0 or 1 fibrosis (Table 2a). Age, cytogenetic risk group by IPSS, white blood cell count, peripheral blood percentages of basophils and eosinophils, absolute neutrophil count, platelet count, ferritin levels, lactate dehydrogenase levels, β2-microglobulin levels, percentage of blasts in the peripheral blood and bone marrow, bone marrow cellularity, monocyte percentage in the bone marrow, and time from diagnosis to treatment had no significant impact on bone marrow fibrosis grade (Table 2b).
Table 2a.
Association between bone marrow fibrosis grade and patient characteristics
| Variable | Fibrosis grade | n | Median (range) | p value |
|---|---|---|---|---|
| Age, years | 0,1 | 66 | 68.56 (−0.04–86.46) | 0.19 |
| 2 | 16 | 71.37 (59.48–82.3) | ||
| White blood cell count, cells/L | 0,1 | 66 | 13.7 × 109 (3.3–207.5 ×109) | 0.42 |
| 2 | 16 | 11.25 ×109(0.1–62.9 ×109) | ||
| Hemoglobin, g/dL | 0,1 | 66 | 11 (3.73–15.5) | 0.04 |
| 2 | 16 | 9.75 (5.3–13.4) | ||
| Platelet count, cells/L | 0,1 | 66 | 90 × 109 (9–699 × 109) | 0.48 |
| 2 | 16 | 131.5 × 109 (6–1386 × 109) | ||
| Albumin, g/dL | 0,1 | 64 | 4.3 (2.5–5.1) | 0.05 |
| 2 | 16 | 4.1 (2.3–4.8) | ||
| Lactate dehydrogenase, U/L | 0,1 | 64 | 571 (279–2610) | 0.60 |
| 2 | 16 | 577 (228–2897) | ||
| Ferritin, ng/mL | 0,1 | 48 | 277.5 (39–3820) | 0.24 |
| 2 | 10 | 973 (31–3868) | ||
| B2-microglobulin, µg/mL | 0,1 | 33 | 3.4 (1.2–33) | 0.20 |
| 2 | 10 | 4.5 (2.2–8.5) | ||
| Absolute neutrophil count, cells/µL | 0,1 | 66 | 6.83 (0.35–43.83) | 0.25 |
| 2 | 16 | 5.53 (0–23.03) | ||
| Basophils, % | 0,1 | 66 | 0 (0–4) | 0.45 |
| 2 | 16 | 0 (0–24.3) | ||
| Eosinophils, % | 0,1 | 65 | 1 (0–25) | 0.93 |
| 2 | 16 | 1 (0–21) | ||
| Circulating blasts, % | 0,1 | 66 | 0 (0–14) | 0.98 |
| 2 | 16 | 0 (0–5) | ||
| Bone marrow blasts, % | 0,1 | 66 | 6 (1–20) | 0.20 |
| 2 | 16 | 4.5 (0–18) | ||
| Bone marrow cellularity, % | 0,1 | 66 | 85 (15–100) | 0.26 |
| 2 | 16 | 92.5 (50–100) | ||
| Monocytes, % | 0,1 | 66 | 12 (0–53) | 0.17 |
| 2 | 16 | 9 (2–40) | ||
| Ringed sideroblasts, % | 0,1 | 46 | 0 (0–42) | 0.008 |
| 2 | 12 | 7.5 (0–92) | ||
| Time from diagnosis to treatment, months | 0,1 | 37 | 1.94 (0.03–59.7) | 0.80 |
| 2 | 8 | 2.2 (0.43–16.87) |
Table 2b.
Association between bone marrow fibrosis grade and patient characteristics
| Variable | n (%) | p value | |
|---|---|---|---|
| Grade 0, 1 | Grade 2 | ||
| Sex | |||
| Female | 22 (88) | 3 (12) | 0.37 |
| Male | 44 (77) | 13 (23) | |
| Race | |||
| Non-white | 3 (83) | 1 (50) | 0.61 |
| White | 36 (80) | 9 (20) | |
| Hepatomegaly | |||
| Yes | 2 (50) | 2 (50) | |
| No | 64 (82) | 14 (18) | 0.17 |
| Splenomegaly | |||
| Yes | 11 (73) | 4 (27) | |
| No | 54 (82) | 12 (18) | 0.48 |
| Transfusion dependency | |||
| Yes | 22 (69) | 10 (31) | |
| No | 40 (87) | 6 (13) | 0.09 |
| Treatment | |||
| HMA | 15 (79) | 4 (21) | 0.86 |
| HMA + combination | 10 (77) | 3 (23) | |
| TKI | 1 (100) | 0 (0) | |
| Chemotherapy | 11 (92) | 1 (8) | |
| Supportive therapy | 29 (78) | 8 (22) | |
| IPSS cytogenetic risk group | 0.51 | ||
| Poor | 5 (71) | 2 (29) | |
| Intermediate | 11 (73) | 4(27) | |
| Good or very good | 50 (83) | 10 (17) | |
Abbreviations: HMA=Hypomethylating agents, TKI=Tyrosine kinase inhibitors
Clinical outcomes
In the total cohort, 38% (n = 31) died by the last follow-up; the median OS was 2.8 years (95% confidence interval [CI]: 2.0–7.2 years). Univariate Cox proportional hazards model for OS identified bone marrow fibrosis grade (hazard ratio [HR]: 2.55; 95% CI: 1.18–5.52; p = 0.02), cytogenetic risk group (HR: 0.29; 95% CI: 0.10–0.91; p = 0.03), serum ferritin level (HR: 1.04; 95% CI: 1.01–1.07; p = 0.02), and absolute neutrophil count (HR: 1.05; 95% CI: 1.01–1.08; p = 0.02) as significant prognostic factors (Table 3). After adjusting for other variables, the multivariable model showed that grade 2 fibrosis remained an independent predictor of reduced OS (HR: 2.90; 95% CI: 1.32–6.35; p = 0.008; Figure 2), along with absolute neutrophil count (HR: 1.05; 95% CI: 1.01–1.09; p = 0.009; Table 3).
Table 3.
Univariate and multivariable Cox proportional hazards models for overall survival (n=82; 31 deaths)a
| Variable | HR | 95% CI | p |
|---|---|---|---|
| Univariate | |||
| Age | 0.99 | 0.95–1.02 | 0.46 |
| Sex (male vs female) | 0.85 | 0.39–1.84 | 0.68 |
| White blood cell count | 1.02 | 1.00–1.04 | 0.12 |
| Hemoglobin | 0.91 | 0.80–1.03 | 0.14 |
| Platelet count | 1.00 | 1.00–1.00 | 0.81 |
| Albumin | 0.61 | 0.31–1.17 | 0.14 |
| Lactate dehydrogenase | 1.00 | 1.00–1.00 | 0.48 |
| Ferritin | 1.04 | 1.01–1.07 | 0.02 |
| B2-microglobulin | 0.99 | 0.85–1.15 | 0.90 |
| Absolute neutrophil count | 1.05 | 1.01–1.08 | 0.02 |
| Basophils | 1.05 | 0.96–1.14 | 0.27 |
| Eosinophils | 1.01 | 0.94–1.09 | 0.72 |
| Hepatomegalya | 1.24 | 0.37–4.18 | 0.73 |
| Splenomegalya | 0.94 | 0.40–2.23 | 0.89 |
| Hypomethylating agentsb | 1.20 | 0.47–3.05 | 0.70 |
| Chemotherapyb | 1.46 | 0.50–4.24 | 0.49 |
| Bone marrow fibrosis (grade 2 vs 0/1) | 2.55 | 1.18–5.52 | 0.02 |
| IPSS cytogenetic (Intermediate vs. Poor) | 0.55 | 0.16–1.83 | 0.33 |
| IPSS cytogenetic (Good or Very Good vs. Poor) | 0.29 | 0.10–0.91 | 0.03 |
| Circulating blasts | 1.00 | 0.87–1.16 | 0.97 |
| Bone marrow blasts | 0.98 | 0.90–1.06 | 0.58 |
| Bone marrow cellularity | 1.01 | 0.99–1.04 | 0.27 |
| Bone marrow monocytes | 1.00 | 0.95–1.05 | 0.98 |
| Ringed sideroblasts | 1.00 | 0.98–1.02 | 0.82 |
| Multivariable | |||
| Absolute neutrophil count | 1.06 | 1.02–1.11 | 0.008 |
| Bone marrow fibrosis (grade 2 vs 0/1) | 1.06 | 1.02–1.11 | 0.008 |
| IPSS cytogenetic (Intermediate vs. Poor) | 0.23 | 0.06–0.98 | 0.008 |
| IPSS Cytogenetic (Good or Very Good vs. Poor) | 0.23 | 0.07–0.73 | 0.01 |
Categorical variable (compared with absence of condition).
Compared with no therapy.
Abbreviations: HR, hazard ratio; CI, confidence interval.
Figure 2.
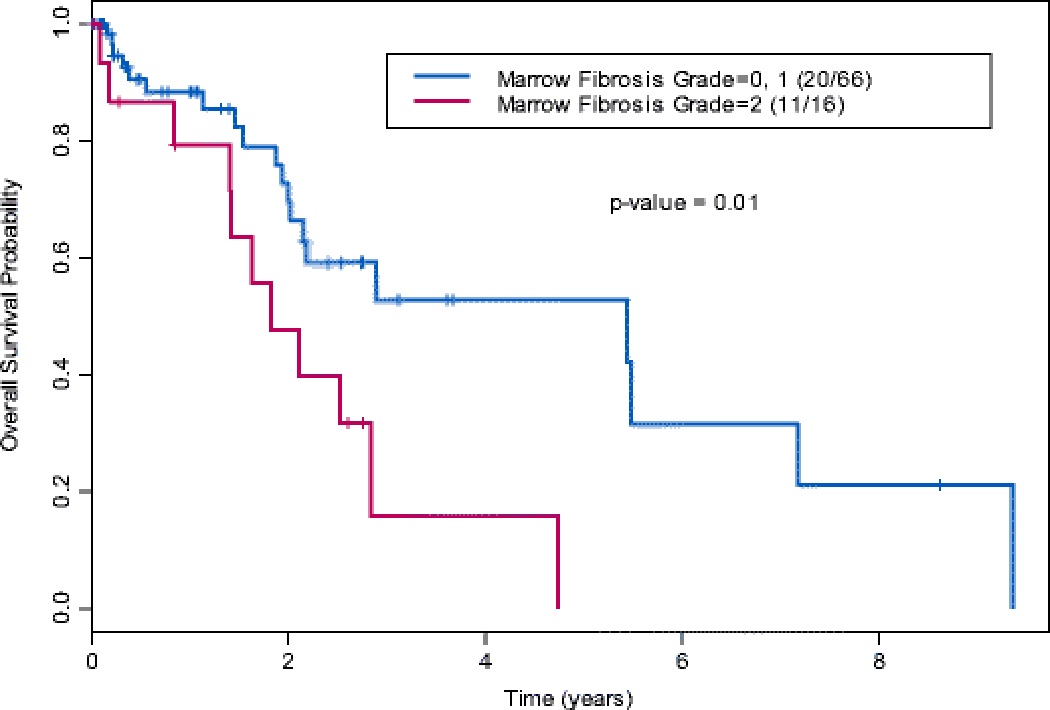
Kaplan-Meier estimates for OS by bone marrow fibrosis grade
In terms of EFS, 45% (n = 37) experienced disease progression, relapse, lack of response, or death. The median EFS was 1.2 years (95% CI: 0.7–1.7 years). In the fitted univariate Cox model for prognostic variables (Table S1), a number of factors were associated with EFS, including serum ferritin level (HR: 1.07; 95% CI: 1.03–1.10; p = 0.0001), absolute neutrophil count (HR: 1.04; 95% CI: 1.01–1.07; p = 0.02), percentage of peripheral blood blasts (HR: 1.18; 95% CI: 1.05–1.33; p = 0.006), and chemotherapy (compared with none; HR: 4.22; 95% CI: 1.51–11.77; p = 0.006. Bone marrow fibrosis grade did not predict inferior EFS (95% CI: 0.62–2.67; HR: 1.28; p = 0.50). (Figure S1). Multivariable analysis showed that absolute neutrophil count (HR: 1.04; 95% CI: 1.00–1.07; p = 0.04) and peripheral blood blast percentage (HR: 1.16; 95% CI: 1.02–1.32; p = 0.02) were the only significant prognostic factors for poor EFS after adjusting for other variables (Table S1).
Discussion
In our cohort, high-grade bone marrow fibrosis was associated with reduced levels of hemoglobin and increased percentages of ring sideroblasts. Along with ANC and poor cytogenetic risk, a higher grade of fibrosis predicted inferior OS, suggesting that bone marrow fibrosis grade is an important indicator of prognosis. However, only the peripheral blood blast percentage, cytogenetic risk, and ANC impacted EFS.
Among the clinical variables analyzed in our study, we found that grade 2 bone marrow fibrosis was associated with reduced levels of hemoglobin and an increased percentage of ringed sideroblasts. The correlation of bone marrow fibrosis with anemia has been delineated in previous studies; both primary bone marrow fibrosis in chronic idiopathic myelofibrosis and fibrosis of the bone marrow secondary to CLL have been known to produce a marked drop in hemoglobin at advanced stages [9,13]. Although similar results in CMML cohorts specifically have been scarce, multiple pathologic factors may be assumed to contribute to the development of an anemic state. Severe bone marrow fibrosis has been associated with poor response to erythropoietin [14]. This, in combination with the replacement of hematopoietic cells with reticulin fibers, is likely to produce disturbances in iron metabolism.
Consistent with this idea, patients with grade 2 fibrosis in our cohort also had relatively high serum ferritin levels and significantly higher percentages of ringed sideroblasts than those with grade 0 or 1 fibrosis. Past literature has suggested the allocation of CMML patients with more than 15% ringed sideroblasts to a distinct subset, CMML-RS, which shares much of its clinical course with classic refractory anemia [15]. Moreover, a case report described the transformation of refractory anemia into CMML as a rare phenomenon and attributed it to an inv(12) cytogenetic abnormality [16]. However, our analysis noted no significant differences in cytogenetic profile or the incidence of splenomegaly or hepatomegaly (otherwise classic features of refractory anemia) between patients with grade 0 or 1 bone marrow fibrosis and those with grade 2 bone marrow fibrosis. Hence, the ringed sideroblasts likely indicate only dysplastic changes in the bone marrow associated with disturbances in iron metabolism [17].
Other studies have linked several other clinical and molecular features with bone marrow fibrosis, including JAK2, CalR, and Mpl mutations, splenomegaly, increased white blood cell and platelet counts, and elevated ferritin levels [7,8,18]. Although our cohort demonstrated a trend of relatively higher platelet counts and elevated ferritin levels with grade 2 bone marrow fibrosis, these differences were not statistically significant, consistent with our recent study; we did not observe any significant change in these variables with the grade (rather than the presence) of bone marrow fibrosis. Our results suggest that bone marrow fibrosis in CMML is induced by neoplastic monocytes and should be considered a disease-related characteristic that progresses independently of the above-mentioned factors.
Generally, the presence of bone marrow fibrosis has been well established as a poor prognostic marker in hematologic malignancies [8,19,20]. However, the impact of bone marrow fibrosis grade on survival is much less concrete in the literature. Interestingly, our analysis delineates grade 2 bone marrow fibrosis as a predictor of reduced OS, but not EFS suggesting that unlike MPN, CMML is an aggressive disease in which the degree of bone marrow fibrosis plays no role in relapse, transformation to acute leukemia, or failure to attain complete remission. Although the adverse impact of severe bone marrow fibrosis on OS has been suggested by previous studies [6,9], other analyses contradicted these findings [7]. It should be remembered, however, that a vast majority of the reports agreeing or disagreeing with this correlation are based on cohorts of MDS patients only, whereas CMML shares many of its clinico-pathologic features with both MDS and MPN.
Bone marrow fibrosis has been historically relevant in the prognostic assessment of MPN; for example, an increased level of reticulin fibrosis in essential thrombocythemia has been shown to predict an increased risk of thrombosis and bleeding [21]. It is thus reasonable to assume that extensive bone marrow fibrosis may similarly alter the clinical course of CMML, given its overlapping myeloproliferative characteristics with MPN. Conversely, bone marrow fibrosis grade had no significant effect on EFS in our cohort; however, this may be attributed to a later starting point for the measurement of EFS (ie, presentation, as opposed to diagnosis for OS). In addition, a number of patients did not receive therapy to induce remission, and were, therefore, excluded from the group of candidates expected to experience events post-remission. Poor-risk cytogenetic group, the percentage of peripheral blood blasts and absolute neutrophil count at diagnosis were the only significant predictors of inferior EFS. This is consistent with previous studies that have outlined blast cell count as one of the most reliable prognostic markers in assessing survival and transformation to leukemia in dysplastic bone marrow neoplasms, with or without concomitant fibrosis [22]. In fact, even a bone marrow blast percentage exceeding 7.5% has been implicated in poor prognosis [23]. Available literature has also underlined the prognostic value of cytogenetic risk stratification among CMML patients, owing to the association between high-risk cytogenetics and decreased survival rates [24].
In terms of the inter-observer reliability of the grading of bone marrow fibrosis, strict evaluation according to WHO criteria yields a reliability of more than 80%. However, our analysis is limited by a lack of routine biochemical staining, due to which the number of evaluable cases from the patient cohort was restricted to a subset. Another limitation in our study is the lack of complete molecular profiling, since the analysis of patients from 2004 pre-dates the use of current gene panels that test for the status of genes commonly mutated in MDS. This is especially relevant to spliceosome mutations such as SF3B1, SRSF2 and SETBP1, which were identified in MDS patients in recent years only and thus could not be included in our analysis.
In conclusion, because fibrosis is induced by monocyte derived cells, the degree of bone marrow fibrosis in CMML rather than an absolute monocyte count is an indicator of the overall tumor mass and clinical outcome. We found that increased bone marrow fibrosis was associated with not only reduced hemoglobin levels and an increased proportion of ringed sideroblasts, but also inferior OS. The role of bone marrow fibrosis as an independent prognostic marker of poor survival, along with peripheral blood blast percentage, absolute neutrophil count and cytogenetic risk, merits the incorporation of bone marrow fibrosis grading into routine clinical workup of CMML patients. Our findings underscore the need for future studies to explore how reversal of bone marrow fibrosis may be addressed in therapeutic management.
Supplementary Material
Acknowledgments:
Department of Scientific Publications, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA
Compliance with Ethical Standards
Funding: This research is supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent: This study has been reviewed by the Institutional Review Board (IRB) and granted a waiver of informed consent.
References:
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. (2008) WHO classification of tumours of haematopoietic and lymphoid tissues IARC: Lyon. [Google Scholar]
- 2.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C (1982) Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 51:189–199. [PubMed] [Google Scholar]
- 3.Padron E, Komrokji R, List AF (2014) The clinical management of chronic myelomonocytic leukemia. Clin Adv Hematol Oncol 12(3):172–178. [PubMed] [Google Scholar]
- 4.Verstovsek S, Manshouri T, Pilling D, Bueso-Ramos CE, Newberry KJ, Prijic S, Knez L, Bozinovic K, Harris DM, Spaeth EL, Post SM, Multani AS, Rampal RK, Ahn J, Levine RL, Creighton CJ, Kantarjian HM, Estrov Z (2016) Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis. J Exp Med 213(9):1723–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A (2005) European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 90(8): 1128–1132. [PubMed] [Google Scholar]
- 6.Della Porta MG, Malcovati L (2011) Myelodysplastic syndromes with bone marrow fibrosis. Haematologica 96(2):180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond D, Jamali M, Wells RA, Zhang L, Mamedov A, Lenis M, Buckstein R (2016) Impact of bone marrow fibrosis in MDS patients treated with azacitidine. Blood 128:4339. [Google Scholar]
- 8.Santos FPS, Schlette E, Kantarjian H, Sargent R, Garcia-Manero G, Verstovsek S, Kadia T, Jabbour E, Pierce S, Borthakur G (2009) Myelodysplastic syndrome with fibrosis: experience of a single-institution with 139 Patients. Blood 114:2775. [Google Scholar]
- 9.Tadmor T, Shvidel L, Aviv A, Ruchlemer R, Bairey O, Yuklea M, Herishanu Y, Braester A, Rahimi-Levene N, Vernea F, Ben-Ezra J, Bejar J, Polliack A, Israeli CLL Study Group (2013) Significance of bone marrow reticulin fibrosis in chronic lymphocytic leukemia at diagnosis: a study of 176 patients with prognostic implications. Cancer 119(10):1853–1859. [DOI] [PubMed] [Google Scholar]
- 10.Maschek H, Gutzmer R, Choritz H, Georgii A (1994) Life expectancy in primary myelodysplastic syndromes: a prognostic score based upon histopathology from bone marrow biopsies of 569 patients. European Journal of Haematology, 1994;53:280–287. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T,Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J (1997) International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89:2079–2088. [PubMed] [Google Scholar]
- 12.Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, Giagounidis A, Hildebrandt B, Bernasconi P, Knipp S, Strupp C, Lazzarino M, Aul C, Cazzola M (2007)Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J ClinOncol 25:3503–3510. [DOI] [PubMed] [Google Scholar]
- 13.Thiele J, Kvasnicka HM (2006) Grade of bone marrow fibrosis is associated with relevant hematological findings-a clinicopathological study on 865 patients with chronic idiopathic myelofibrosis. Ann Hematol 85(4):226–232. [DOI] [PubMed] [Google Scholar]
- 14.Rao DS, Shih MS, Mohini R (1993) Effect of serum parathyroid hormone and bone marrow fibrosis on the response to erythropoietin in uremia. N Engl J Med 328(3):171–175. [DOI] [PubMed] [Google Scholar]
- 15.Such E, Senent L, Nomdedeu B, et al. (2009)Chronic myelomonocytic leukemia (CMML) with more than 15% of ring sideroblasts in bone marrow: an overlapping disorder between CMML and refractory anemia with ring sideroblasts. Blood 114:290.19357397 [Google Scholar]
- 16.Hasegawa Y, Sakai N, Toyama M, Ninomiya H, Abe T (1990) Chronic myelomonocytic leukemia transformed from refractory anemia with ring sideroblasts with a rare abnormal chromosome, inv (12). RinshoKetsueki 31(1):75–79. [PubMed] [Google Scholar]
- 17.Schmitt-Graeff A, Thiele J, Zuk I, Kvasnicka HM (2002) Essential thrombocythemia with ringed sideroblasts: a heterogeneous spectrum of diseases, but not a distinct entity. Haematologica 87(4):392–399. [PubMed] [Google Scholar]
- 18.Lambertenghi-Deliliers G, Orazi A,Luksch R, Annaloro C, Soligo D (1991) Myelodysplastic syndrome with increased marrow fibrosis: a distinct clinico-pathological entity. Br J Haematol 78(2):161–166. [DOI] [PubMed] [Google Scholar]
- 19.Scott BL, Storer BE, Greene JE, Hackman RC, Appelbaum FR, Deeg HJ (2007) Marrow fibrosis as a risk factor for posttransplantation outcome in patients with advanced myelodysplastic syndrome or acute myeloid leukemia with multilineage dysplasia. Biol Blood Marrow Transplant 13(3):345–354. [DOI] [PubMed] [Google Scholar]
- 20.Della Porta MG, Malcovati L, Boveri E, Travaglino E, Pietra D, Pascutto C, Passamonti F, Invernizzi R, Castello A, Magrini U, Lazzarino M, Cazzola M (2009) Clinical relevance of bone marrow fibrosis and CD34-positive cell clusters in primary myelodysplastic syndromes. J ClinOncol 27(5):754–762. [DOI] [PubMed] [Google Scholar]
- 21.Campbell PJ, Bareford D, Erber WN, Wilkins BS, Wright P, Buck G, Wheatley K, Harrison CN, Green AR (2009) Reticulin accumulation in essential thrombocythemia: prognostic significance and relationship to therapy. J Clin Oncol 27(18):2991–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machherndl-Spandl S, Sega W, Bösmüller H, Germing U, Gruber Ch, Nachtkamp K, Reinecke P, Sperr WR, Wimazal F, Müllauer L, Sotlar K, Horny HP, Tüchler H, Valent P, Krieger O (2014) Prognostic impact of blast cell counts in dysplastic bone marrow disorders (MDS and CMML I) with concomitant fibrosis. Ann Hematol 93(1):57–64. [DOI] [PubMed] [Google Scholar]
- 23.Padron E, Garcia-Manero G, Patnaik MM, Itzykson R, Lasho T, Nazha A, Rampal RK, Sanchez ME, Jabbour E, Al Ali NH, Thompson Z, Colla S, Fenaux P, Kantarjian HM, Killick S, Sekeres MA, List AF,Onida F, Komrokji RS, Tefferi A, Solary E (2015) An international data set for CMML validates prognostic scoring systems and demonstrates a need for novel prognostication strategies. Blood Cancer J 5:e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Such E, Cervera J, Costa D, Solé F, Vallespí T, Luño E, Collado R, Calasanz MJ, Hernández-Rivas JM, Cigudosa JC, Nomdedeu B, Mallo M, Carbonell F, Bueno J, Ardanaz MT, Ramos F, Tormo M, Sancho-Tello R, del Cañizo C, Gómez V, Marco V, Xicoy B, Bonanad S, Pedro C, Bernal T, Sanz GF (2011) Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica 96(3):375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


