Abstract
Widespread clinical implementation of dynamic CT myocardial perfusion has been hampered by its limited accuracy and high radiation dose. The purpose of this study was to evaluate the accuracy and radiation dose reduction of a dynamic CT myocardial perfusion technique based on first pass analysis (FPA). To test the FPA technique, a pulsatile pump was used to generate known perfusion rates in a range of 0.96–2.49 mL/min/g. All the known perfusion rates were determined using an ultrasonic flow probe and the known mass of the perfusion volume. FPA and maximum slope model (MSM) perfusion rates were measured using volume scans acquired from a 320-slice CT scanner, and then compared to the known perfusion rates. The measured perfusion using FPA (PFPA), with two volume scans, and the maximum slope model (PMSM) were related to known perfusion (PK) by PFPA = 0.91PK + 0.06 (r = 0.98) and PMSM = 0.25PK — 0.02 (r = 0.96), respectively. The standard error of estimate for the FPA technique, using two volume scans, and the MSM was 0.14 and 0.30 mL/min/g, respectively. The estimated radiation dose required for the FPA technique with two volume scans and the MSM was 2.6 and 11.7–17.5 mSv, respectively. Therefore, the FPA technique can yield accurate perfusion measurements using as few as two volume scans, corresponding to approximately a factor of four reductions in radiation dose as compared with the currently available MSM. In conclusion, the results of the study indicate that the FPA technique can make accurate dynamic CT perfusion measurements over a range of clinically relevant perfusion rates, while substantially reducing radiation dose, as compared to currently available dynamic CT perfusion techniques.
Keywords: CT, Dynamic perfusion, Phantom, Myocardial perfusion
Introduction
Computed tomography (CT) angiography is a well-established, noninvasive method used to detect coronary artery stenoses. However, CT angiography is limited in its ability to determine whether an intermediate lesion (35–75 % diameter stenoses) is the cause of ischemia [1, 2]. Although multimodal techniques such as PET/CT and SPECT/CT can provide functional information, these techniques are limited by the cost and availability of radiotracers, preventing their widespread clinical implementation. Therefore, it would be clinically useful to combine the anatomic information of CT angiography with the physiologic information of CT myocardial perfusion into a single, low-dose procedure to better stratify a patient’s risk.
Several different techniques of dynamic CT perfusion have been reported [3–7], most of which are based on some variation of the Mullani–Gould method [8]. These techniques, such as the maximum slope model, monitor the mean enhancement of a small volume of myocardium over time. The resulting contrast pass curve is then used to measure myocardial perfusion using fitting parameters from different models. While such techniques have shown positive correlation with coronary fractional flow reserve [7] and microsphere measurements [9, 10], overall they tend to underestimate perfusion [10, 11]. This problem of under-estimation stems fundamentally from the rapid transit time of contrast through the myocardium. Specifically, these techniques operate under the assumption that no contrast leaves the myocardial tissue volume of interest over the time of measurement. However, these techniques generally make measurements using small volumes of interest (VOI), over many cardiac cycles; therefore they are inherently subject to contrast loss from the small VOIs, especially at hyperemia. In addition, such techniques require multiple volume scans over many cardiac cycles to generate perfusion measurements, leading to cumulative radiation doses of up to 30 mSv for combined rest and stress scans [12]. Therefore, the issues of measurement accuracy and radiation dose need to be addressed before dynamic CT perfusion can be implemented as a clinical standard.
To resolve such limitations, much larger VOIs, defined as an entire perfusion bed of an artery or major arterial branch, distal to a stenosis [13, 14], could be used to make vessel-specific perfusion measurements. Further, measurement in large VOIs would improve the signal-to-noise-ratio (SNR). Unfortunately, CT technology has been limited by the total myocardial volume coverage per cardiac cycle. Hence, dynamic CT perfusion techniques have been limited in the accurate quantification of myocardial perfusion [15, 16]. However, with recent advances in CT technology, the coverage has been extended, allowing the entire heart to be imaged within a fraction of a second. Such advances have enabled implementation of the first pass analysis (FPA) technique for dynamic CT myocardial perfusion measurement. By increasing the VOI size, SNR is improved, and the problem of contrast loss from VOIs over the time of measurement is eliminated. Hence, the FPA technique is able to measure the total volume of contrast material that has entered a given perfusion bed between two volume scans. As a result, the complicated problems of myocardial contrast dynamics and blood flow quantification can be distilled down into the much simpler concept of conservation of mass.
In this study, the ability of the FPA technique to accurately measure myocardial perfusion over a range of known, clinically relevant perfusion rates was evaluated in a cardiac phantom using a 320-slice CT scanner. The possibility of radiation dose reduction was also investigated by assessing the accuracy of FPA perfusion measurements using a limited number of volume scans.
Materials and methods
Perfusion model
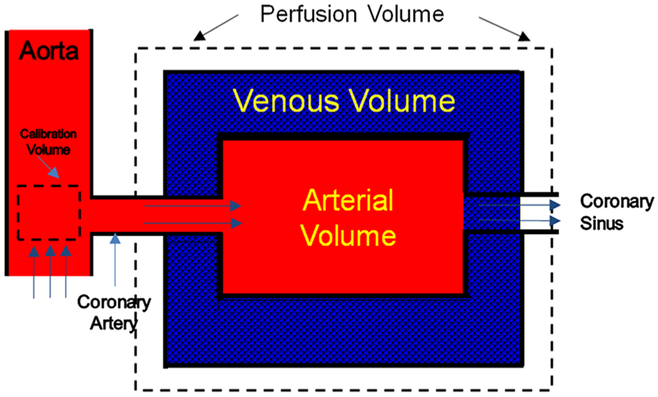
The proposed FPA perfusion measurement technique is based on a first pass distribution model [17–20] and the principle of conservation of mass. Specifically, any myocardial perfusion volume supplied by a coronary artery may be modeled as a compartment with a unique entrance and exit vessel, as seen in Fig. 1. Given this model, no assumptions about the compartment shape, internal structure, vascular permeability, or nature of the exit conduits need to be made. In order to measure flow (Q) through the compartment, it is necessary to determine the volume (ΔV) of blood entering the compartment within a certain time interval (Δt), as well as the input blood iodine concentration (Cin) using (see Appendix):
| (1) |
Fig. 1.
Single compartment model used in the first-pass analysis technique showing calibration and perfusion volumes
It is possible to calculate by determining the change in the integrated Hounsfield units (HU) of all voxels within the compartment per unit time. Additionally, Cin may be estimated from the integrated HU per unit volume (HU/cm3) of a calibration VOI close to the compartment entrance. Thus, using the proposed FPA technique, the flow through any large myocardial tissue compartment, or perfusion volume of interest, can be calculated as the average slope of the integrated HU in the VOI per unit time, divided by the maximum input concentration from the calibration volume, assuming no contrast agent has exited the myocardial VOI before measurements are completed. Further, because the mass of the myocardial VOI is known, the perfusion rate can be derived directly from the calculated flow rate. As a result, many intra-compartment parameters, such as capillary permeability and extraction, do not need to be considered when determining perfusion. Thus, such a technique eliminates the need to acquire multiple volume scans over many cardiac cycles and has the potential to substantially reduce the radiation dose of dynamic CT perfusion.
Cardiac perfusion phantom
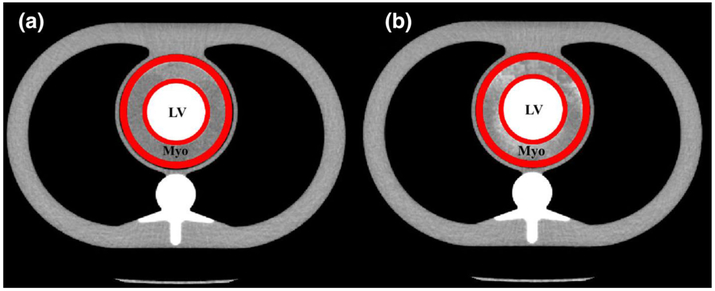
The cardiac perfusion phantom was designed to model the pulsatile mixing dynamics of the heart with a femoral contrast injection site [21]. A photograph and a schematic of the cardiac perfusion phantom are shown in Fig. 2. Proximally, the components of the phantom served to generate a realistic stroke volume and cardiac output, and ensured sufficient mixing prior to the myocardial compartment. Distally, a myocardial compartment with a coronary vessel input was constructed from a 10 cm diameter polymethyl methacrylate (PMMA) tube filled with small diameter plastic beads of different sizes. The beads simulated myocardial tissue packing, and reduced the interstitial fluid distribution volume within the myocardial compartment, such that the measured density of the simulated myocardial tissue was 0.97 g/mL. Another PMMA chamber with a diameter of 5.7 cm was also placed in the center of the myocardial compartment, and was filled with contrast media with an iodine concentration of 20 mg/mL to simulate a contrast-filled ventricle. Further, the total volume of the simulated myocardial tissue was designed to approximate the total tissue volume of the myocardium. Lastly, the entire myocardial compartment was imaged inside an average adult-sized anthropomorphic thorax to generate realistic radiation dose, X-ray attenuation, and image noise properties (Cardio; QRM, Mohrendorf, Germany) [22]. Overall, the design of the myocardial compartment simulates a first pass distribution model. An axial cross section of the myocardial compartment before and after contrast injection is shown in Fig. 3.
Fig. 2.
(a) A photograph of the cardiac perfusion phantom inside the anthropomorphic thorax along with the pulsatile pump, (b) a schematic diagram of the cardiac perfusion phantom
Fig. 3.
Myocardial compartment (Myo) around the contrast filled left ventricle (LV). The VOI (denoted in red) is overlaid before (a) and after (b) contrast infusion
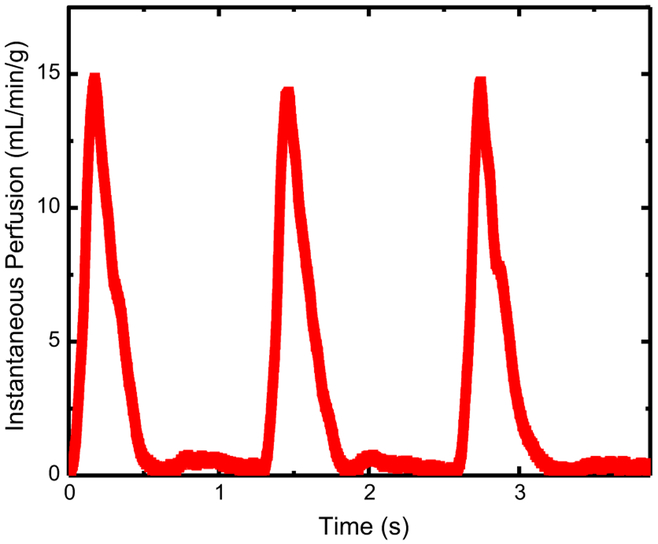
Water was circulated through the phantom using a pulsatile pump (Harvard Apparatus, Holliston, MA). The stroke volume and cardiac output were held constant at 100 mL per stroke and 5 L/min total through the system. Separate input and output reservoirs were used to eliminate recirculation of contrast material. An ultrasonic flow probe (Transonic Systems Inc., Ithaca, NY) was used to determine instantaneous flow through the myocardial compartment, and all known flow rates were continuously recorded (MP150, Biopac Systems Inc., Goleta, CA). The known flow rates were converted to perfusion using the known mass of the perfusion volume. The known perfusion was used as the reference standard for comparison to the measured CT perfusion. An example of the pulsatile flow profile through the phantom can be found in Fig. 4, and is characteristic of in vivo systolic and diastolic flow properties. In order to modulate the perfusion rate through the myocardial compartment, variable resistance was applied downstream of the perfusion compartment and at the termination of the distal aorta (see Fig. 2). A range of known perfusion rates between 0.96 and 2.49 mL/min/g were evaluated. The minimum and maximum perfusion rates are approximately representative of perfusion in resting and stress conditions, and correspond to a coronary flow reserve (CFR) of 2.6. The range of perfusion rates evaluated is also approximately representative of perfusion deficits caused by stenoses of increasing severity in stress conditions.
Fig. 4.
Pulsatile flow profile corresponding to an average perfusion rate of 2.49 mL/min/g
CT imaging protocol
CT imaging was performed using a 320-slice CT scanner (Aquilion One, Toshiba America Medical Systems, Tustin, CA). An ECG emulator was used to generate a heart rate that matched the frequency of the pulsatile pump, allowing the cardiac phantom to be imaged using a prospective, ECG-gated cardiac perfusion protocol. The CT imaging parameters were: 320 × 0.5 mm detector collimation, 100 kVp tube voltage, 200 mA current, and 0.35 s rotation time. For each perfusion measurement approximately 20 volume scans were acquired following a 15 mL bolus injection of contrast material (Isovue 370, Bracco Diagnostics, Princeton, NJ) and a 15 mL bolus injection of saline. All injections were made using a power injector (Empower CTA, Acist Medical Systems, Eden Prairie, MN) at a rate of 5 mL/s. While transient increases in flow, on the order of 5–10 %, were measured immediately after contrast injection, all flow rates equilibrated well before CT perfusion measurements were made. Hence, any effects of the contrast injection on measured perfusion rates were negligible.
CT images were reconstructed from full projection data sets with a slice thickness of 0.5 mm using a medium-smooth FC03 kernel with beam hardening corrections. The voxel size was 0.43 × 0.43 × 0.5 mm3 and images were reconstructed at 75 % of the ECG cycle, as is customary in diastolic imaging.
Perfusion measurement using the FPA technique
All FPA perfusion measurements were made using a VOI with an outer diameter of 9 cm, an inner diameter of 5.7 cm, and a slab thickness of 1.5 cm, defined inside the myocardial compartment. The contrast filled ventricle was excluded from the VOI. Given these dimensions and the packing fraction of the beads, the total volume inside the VOI was 57 mL, corresponding to approximately 55 grams of simulated myocardial tissue. Example images of the phantom before and after contrast infusion are shown in Fig. 3. For each perfusion measurement, the integrated HU (sum of all voxel HU) within the myocardial VOI was used to generate the tissue time attenuation curve (TAC).
To obtain the input concentration Cin in Eq. 1, a calibration VOI with a cross-sectional area of approximately 2 cm2 and a thickness of 0.3 cm was defined directly upstream from the myocardial compartment. The integrated HU within this calibration VOI was determined and divided by the known VOI volume to yield the arterial input function (AIF). Perfusion was calculated by measuring the change in iodinated blood volume within the known time interval and dividing by the myocardial mass.
Perfusion measurement using the maximum slope model
For comparison purposes, perfusions rates were also measured using the maximum slope model. In this case, a number of small VOIs, measuring approximately 0.3 cm3, were defined inside the myocardial compartment. The VOI size was chosen based on previously reported maximum slope model implementations [9, 10, 23]. The average HU in the small VOIs, instead of the integrated HU, was determined and plotted as a function of time. Curve fitting was performed, and maximum slope model perfusion was calculated using the following equation [11].
| (2) |
Radiation dose reduction
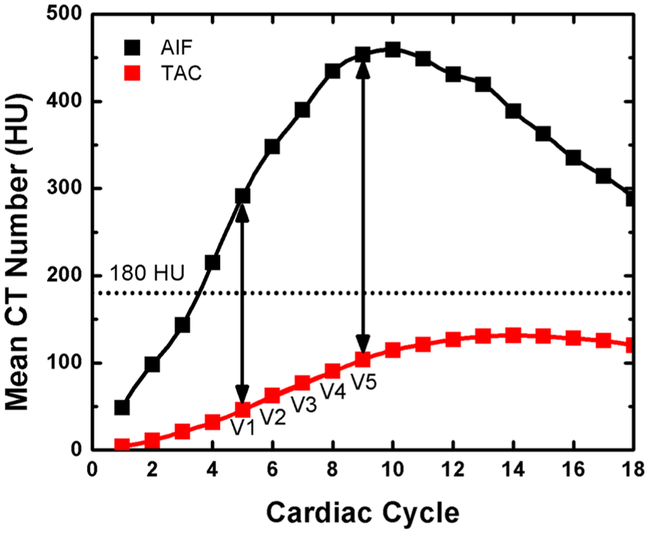
The radiation dose reduction capacity of the FPA technique was evaluated by successively reducing the number of volume scans per perfusion measurement from the original 20 volume scans, in order to determine the minimum number of volume scans necessary for accurate perfusion measurement. A small VOI inside the aorta is normally monitored to determine the start of image acquisition using a preset HU. In order to simulate a clinical protocol, a threshold of 180 HU was set for the AIF. After a threshold of 180 HU was reached in the AIF, five volume scans over five consecutive cardiac cycles (V1–V5) were used for perfusion measurements. The first-pass analysis perfusion calculations were performed based on two (V1 and V5), three (V1, V3 and V5), and five (V1-V5) volume scans (see Fig. 5). The initial volume scan (V1) used was one cardiac cycle after a triggering threshold of 180 HU in the AIF. The input concentration Cin used for FPA perfusion measurement was always acquired from the final volume scan (V5) and approximates the maximum of the arterial input function. The total dose-length-product (DLP) was determined for each measurement, and converted to an effective dose (ED) in mSv using an ED/DLP conversion factor (k = 0.015) [24]. The reduced radiation dose was calculated using the radiation dose per volume scan multiplied by the number of volume scans used for perfusion measurement.
Fig. 5.
Example arterial input function (AIF) and the corresponding myocardial tissue time attenuation curve (TAC) showing the five volume scans (V1–V5) used for FPA perfusion calculations
Results
Perfusion measurements
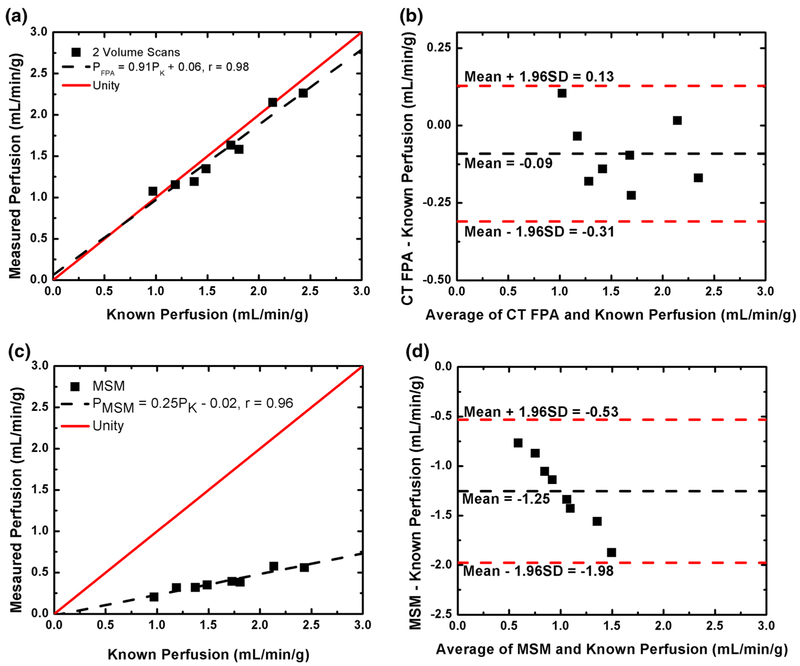
FPA and maximum slope model measurements were made for known flow rates between 60 and 140 mL/min corresponding to known perfusion rates between 0.96 and 2.49 mL/min/g. An example arterial input function for the 2.49 mL/min/g perfusion measurement is shown in Fig. 5. The corresponding FPA tissue time attenuation curve (TAC) is also shown in Fig. 5, where the upslope of the TAC between V1 and V5 is proportional to the average perfusion rate. For both the AIF and TAC, clinically realistic enhancement was achieved. FPA and maximum slope model perfusion measurements versus known perfusion are shown in Table 1 and Fig. 6. The results show an excellent correlation between the known and measured perfusion using 2, 3, and 5 volume scans with no significant difference between the results from 2 to 5 volume scans. On the other hand, the maximum slope model showed a significant, systematic underestimation of the known perfusion.
Table 1.
Summary of the linear regression analysis between different CT perfusion methods and known perfusion measurements
| Method | Slope | Intercept | Pearson’s r | SEE (mL/min/g) | Dose (mSv) |
|---|---|---|---|---|---|
| 2 Scans | 0.91 | 0.06 | 0.98 | 0.14 | 2.64 |
| 3 Scans | 0.91 | 0.06 | 0.98 | 0.14 | 3.96 |
| 5 Scans | 0.92 | 0.05 | 0.98 | 0.13 | 6.60 |
| MSM | 0.25 | −0.02 | 0.97 | 0.30 | 11.69–17.51 |
The associated radiation dose is also included
Fig. 6.
Two-volume scan FPA perfusion (a) and maximum slope model (MSM) perfusion (c) versus known perfusion with respective Bland–Altman plots (b, d)
Radiation dose reduction
FPA perfusion measurements were calculated using two, three, and five volume scans. The average difference between FPA perfusion measurements made using two volume scans versus five volume scans was 0.00 ± 0.02 mL/min/g, suggesting the FPA technique can yield accurate perfusion measurements using as few as two volume scans. The radiation dose incurred per volume scan was 1.32 mSv, resulting in a total radiation dose of 2.64, 3.96, and 6.6 mSv for two, three, and five volume scans, respectively. Furthermore, depending on the perfusion rate, the radiation dose for the maximum slope model was in the range of 11.69–17.51 mSv. Based on the radiation dose of a two volume scan FPA acquisition compared to the radiation dose of the maximum slope model, the FPA technique offers more than a fourfold reduction in radiation dose, and is more accurate in perfusion quantification.
Discussion
Existing CT perfusion techniques
Existing dynamic CT perfusion techniques, such as the maximum slope model, use a relative index of myocardial blood flow to measure perfusion. Previous reports have indicated that CT perfusion is positively correlated with coronary fractional flow reserve (FFR) and microsphere measurements [7, 9, 10]. However, in these reports perfusion was always underestimated, as illustrated by a less-than-unity slope and non-zero offset, when compared to reference standard microsphere measurements [9, 10]. Such perfusion underestimation is due to the use of small VOIs (~ 1 mL) to generate myocardial tissue time attenuation curves. Smaller VOIs are subject to shorter transit times of contrast, and as a result are highly susceptible to contrast loss and perfusion underestimation, especially at hyperemia [12]. Such problems of underestimation were also described in a recent simulation study [11] and are in agreement with the maximum slope model perfusion results from this study (see Fig. 6).
The FPA technique differs from previously reported dynamic CT perfusion techniques in that it does not suffer from perfusion underestimation and can determine perfusion with near unity slope and minimal offset when compared to reference standard ultrasonic flow probe measurements. Specifically, it takes advantage of whole organ CT scanners to prospectively image the entire heart within a fraction of a second. The extended coverage allows perfusion measurement of the entire perfusion bed for an arterial tree or a major branch, eliminating the problem of contrast loss by increasing the transit time window; a requirement that is not satisfied by most dynamic CT perfusion techniques. Additionally, measurements in large perfusion beds are much less sensitive to image noise, making it easier to extract accurate perfusion information from fewer volume scans.
In the current study, a single, large VOI, which encompassed the entire myocardial tissue volume, was used for perfusion measurements. However, clinical implementation of this technique will require vessel-specific VOIs determined from CT angiographic images [13, 14]. Such VOIs will be defined as the entire perfusion bed of an artery or major arterial branch, distal to a stenosis, and will allow for vessel-specific perfusion measurements to be made.
Perfusion measurement using the FPA technique
The results of the study indicate that the proposed FPA technique accurately measures perfusion using CT image data over a range of clinically relevant perfusion rates. FPA derived perfusion measurements had a standard deviation of 0.08 mL/min/g with an average RMS error of 0.083 mL/min/g. Maximum slope model derived perfusion measurements had a standard deviation of 0.03 mL/min/g and an average RMS error of 1.30 mL/min/g. The results indicate that the FPA technique is both accurate and reproducible (see Table 1). The results also indicate that the maximum slope model greatly underestimates perfusion (see Fig. 6), which is in agreement with previous reports [10, 11]. Overall, the accuracy and reproducibility of the perfusion results validate the FPA technique and its underlying assumptions. Additionally, the FPA technique has previously been validated for flow measurement using invasive 2D coronary angiographic images [17–20], further supporting its potential as a CT perfusion technique.
Dose reduction
A major limitation of existing dynamic CT perfusion techniques is the high radiation dose required. Previous reports indicate that the average radiation dose delivered during a single dynamic CT perfusion stress scan is approximately 9–12 mSv [5–7, 9, 10, 23, 25–32]. In the cases where both rest and stress perfusion scans are acquired, the total radiation dose is further increased and can be as high as 30 mSv [5–7, 9, 25, 27, 28]. Therefore, there is a specific need for dose reduction before dynamic CT perfusion techniques can be implemented as a routine clinical standard.
The results from this study indicate that the proposed FPA technique has the potential to substantially reduce radiation dose in addition to improving the accuracy of perfusion measurements. The reduction in radiation dose is accomplished by minimizing the number of volume scans necessary for accurate perfusion measurement. Compared to current dynamic CT perfusion techniques, which require approximately 15 volume scans for perfusion measurement [12], the FPA technique requires as few as two volume scans for perfusion measurement, resulting in more than a fourfold reduction in radiation dose, as well as more accurate perfusion quantification.
The FPA technique acquires both CT angiography and dynamic CT perfusion data during the same low-dose protocol, which further reduces the total radiation dose and contrast loading to the patient. Furthermore, the FPA technique can be used in conjunction with standard dose reduction methods such as tube voltage optimization, tube current modulation, and iterative reconstruction techniques [28, 33] to further reduce the radiation dose.
Clinical application and study limitations
While the FPA technique was validated in a static cardiac phantom, there are a few limitations for in vivo application of this technique. The first limitation is the effect of potential motion artifacts on perfusion measurement. To address such motion artifacts, prospective ECG-gating can be used to minimize cardiac motion during data acquisition. Additionally, image processing techniques, such as deformable image registration based on mutual information, can be used to further reduce motion artifacts. Another potential limitation is beam hardening due to large volumes of contrast pooling inside the ventricles. The phantom was designed to simulate contrast within the left ventricle. However, the associated artifacts can be minimized by applying beam-hardening corrections available with CT scanners, as well as additional corrections that take into account the dynamic nature of contrast in the heart [34, 35]. A single VOI, which encompassed the entire myocardial tissue compartment, was also used in the phantom study. Since the setup was used to develop and validate the FPA technique, less emphasis was placed on dividing the myocardial tissue compartment into multiple VOIs of complex shape. However, the accuracy of the FPA technique does not depend on the shape of a compartment. Hence, in the current phantom study, the shape of the compartment was chosen to be cylindrical for convenience. That being said, myocardial segmentation and multiple VOIs need to be used in vivo for relevant perfusion measurement. CT angiography data can be used to automatically generate vessel-specific VOIs [13, 14], allowing the perfusion in each coronary artery perfusion bed or major coronary branch to be determined. Another potential limitation of the study is the time-to-peak of each arterial input function and the corresponding tissue time attenuation curve. In vivo, the time-to-peak of these functions is relatively short due to the hyperemic transit time of blood from coronary artery to coronary sinus [36, 37]. Fortunately, since the FPA technique only requires a minimum of two volume scans, as long as those volume scans are acquired during the upslope of the myocardial tissue time attenuation curve in vivo, and include the peak of the arterial input function, absolute myocardial perfusion can be measured independent of the time-to-peak. The actual timing and total number of volume scans will require more investigation, and will be determined in future in vivo studies. Lastly, full projection data reconstruction was used in the study, resulting in reduced temporal resolution as compared with partial scan reconstructions. This was done to avoid the previously reported partial scan artifacts [36] that limit the quantitative nature of CT perfusion.
Conclusions
The results of the phantom study indicate the FPA technique can be used to make accurate dynamic CT perfusion measurements over a range of clinically relevant perfusion rates using a minimum of two volume scans. This technique has the potential to substantially reduce the radiation dose as compared with existing dynamic CT perfusion techniques by reducing the total number of volume scans necessary for accurate perfusion measurement.
Acknowledgments
The authors would like to thank Drs. Ding and Cho for their helpful suggestions on the manuscript.
Appendix
In order to measure perfusion through a compartment, it is necessary to determine the volume [V(t)] of contrast material entering the compartment within a certain time interval, and this volume can be described as:
| (3) |
where Qi(t) and Qo(t) are the incoming and outgoing flow rates, and Ci(t) and Co(t) are the incoming and outgoing concentrations of contrast agent, respectively. Equation 3 represents the fluid dynamic form of mass conservation indicating that the total amount of contrast material in the compartment equals the amount that has entered minus the amount that has exited. The term tmin denotes the minimum transit time of contrast material through the compartment, from entrance to exit. Hence, if V(t) is calculated before any contrast material has exited the vascular compartment, at t < tmin, the outgoing contrast concentration is zero [i.e. Co(t) = 0] and the latter integral can be ignored.
| (4) |
The derivative of both sides of Eq. 4, divided by the input iodine concentration, Cin(t), yields:
| (5) |
Integrating from t to t + Δt and dividing by Δt to give the time-averaged value of Eq. 5 over the sampling period, the final form of flow derived via the proposed first pass analysis (FPA) technique is:
| (6) |
where Qave is the calculated flow, V is the rate of change of contrast volume in the vascular compartment per unit time, and Cin is the maximum input concentration of incoming contrast material at the time of measurement. The measured flow can be further simplified as:
| (7) |
Footnotes
Conflict of interest Author Benjamin P. Ziemer declares that he has no conflict of interest. Author Logan Hubbard declares that he has no conflict of interest. Author Jerry Lipinski declares that he has no conflict of interest. Author Sabee Molloi has received research grants from Toshiba America Medical Systems and Philips Medical systems.
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Di Carli M, Czernin J, Hoh CK, Gerbaudo VH, Brunken RC, Huang SC, Phelps ME, Schelbert HR (1995) Relation among stenosis severity, myocardial blood flow, and flow reserve in patients with coronary artery disease. Circulation 91:1944–1951 [DOI] [PubMed] [Google Scholar]
- 2.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, Koolen JJ (1996) Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 334:1703–1708 [DOI] [PubMed] [Google Scholar]
- 3.Klocke FJ, Simonetti OP, Judd RM, Kim RJ, Harris KR, Hedjbeli S, Fieno DS, Miller S, Chen V, Parker MA (2001) Limits of detection of regional differences in vasodilated flow in viable myocardium by first-pass magnetic resonance perfusion imaging. Circulation 104:2412–2416 [DOI] [PubMed] [Google Scholar]
- 4.Christian TF, Frankish ML, Sisemoore JH, Christian MR, Gentchos G, Bell SP, Jerosch-Herold M (2010) Myocardial perfusion imaging with first-pass computed tomographic imaging: measurement of coronary flow reserve in an animal model of regional hyperemia. J Nuclear Cardiol 17:625–630 [DOI] [PubMed] [Google Scholar]
- 5.Mahnken AH, Klotz E, Pietsch H, Schmidt B, Allmendinger T, Haberland U, Kalender WA, Flohr T (2010) Quantitative whole heart stress perfusion ct imaging as noninvasive assessment of hemodynamics in coronary artery stenosis: preliminary animal experience. Invest Radiol 45:298–305 [DOI] [PubMed] [Google Scholar]
- 6.Bamberg F, Becker A, Schwarz F, Marcus RP, Greif M, von Ziegler F, Blankstein R, Hoffmann U, Sommer WH, Hoffmann VS (2011) Detection of hemodynamically significant coronary artery stenosis: incremental diagnostic value of dynamic ct-based myocardial perfusion imaging. Radiology 260:689–698 [DOI] [PubMed] [Google Scholar]
- 7.Rossi A, Uitterdijk A, Dijkshoorn M, Klotz E, Dharampal A, Van Straten M, Van Der Giessen WJ, Mollet N, Van Geuns R-J, Krestin GP (2013) Quantification of myocardial blood flow by adenosine-stress ct perfusion imaging in pigs during various degrees of stenosis correlates well with coronary artery blood flow and fractional flow reserve. Eur Heart J Cardiovasc Imaging 14:331–338 [DOI] [PubMed] [Google Scholar]
- 8.Mullani NA, Gould KL (1983) First-pass measurements of regional blood flow with external detectors. J Nucl Med 24:577. [PubMed] [Google Scholar]
- 9.Bamberg F, Hinkel R, Schwarz F, Sandner TA, Baloch E, Marcus R, Becker A, Kupatt C, Wintersperger BJ, Johnson TR, Theisen D, Klotz E, Reiser MF, Nikolaou K (2012) Accuracy of dynamic computed tomography adenosine stress myocardial perfusion imaging in estimating myocardial blood flow at various degrees of coronary artery stenosis using a porcine animal model. Invest Radiol 47:71–77 [DOI] [PubMed] [Google Scholar]
- 10.Bamberg F, Hinkel R, Marcus RP, Baloch E, Hildebrandt K, Schwarz F, Hetterich H, Sandner TA, Schlett CL, Ebersberger U, Kupatt C, Hoffmann U, Reiser MF, Theisen D, Nikolaou K (2014) Feasibility of dynamic CT-based adenosine stress myocardial perfusion imaging to detect and differentiate ischemic and infarcted myocardium in an large experimental porcine animal model. Int J Cardiovasc Imaging 30:803–812 [DOI] [PubMed] [Google Scholar]
- 11.Bindschadler M, Modgil D, Branch KR, La Riviere PJ, Alessio AM (2014) Comparison of blood flow models and acquisitions for quantitative myocardial perfusion estimation from dynamic ct. Phys Med Biol 59:1533–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi A, Merkus D, Klotz E, Mollet N, de Feyter PJ, Krestin GP (2014) Stress myocardial perfusion: imaging with multidetector ct. Radiology 270:25–46 [DOI] [PubMed] [Google Scholar]
- 13.Le H, Wong JT, Molloi S (2008) Estimation of regional myocardial mass at risk based on distal arterial lumen volume and length using 3d micro-ct images. Comput Med Imaging Graph 32:488–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le HQ, Wong JT, Molloi S (2008) Allometric scaling in the coronary arterial system. Int J Cardiovasc Imaging 24:771–781 [DOI] [PubMed] [Google Scholar]
- 15.Lipton M, Higgins C, Farmer D, Boyd D (1984) Cardiac imaging with a high-speed cine-ct scanner: preliminary results. Radiology 152:579–582 [DOI] [PubMed] [Google Scholar]
- 16.Wolfkiel CJ, Ferguson J, Chomka E, Law W, Labin I, Tenzer M, Booker M, Brundage B (1987) Measurement of myocardial blood flow by ultrafast computed tomography. Circulation 76:1262–1273 [DOI] [PubMed] [Google Scholar]
- 17.Molloi S, Zhou Y, Kassab GS (2004) Regional volumetric coronary blood flow measurement by digital angiography: in vivo validation. Acad Radiol 11:757–766 [DOI] [PubMed] [Google Scholar]
- 18.Molloi S, Qian YJ, Ersahin A (1993) Absolute volumetric blood flow measurements using dual-energy digital subtraction angiography. Med Phys 20:85–91 [DOI] [PubMed] [Google Scholar]
- 19.Molloi S, Ersahin A, Tang J, Hicks J, Leung CY (1996) Quantification of volumetric coronary blood flow with dual-energy digital subtraction angiography. Circulation 93:1919–1927 [DOI] [PubMed] [Google Scholar]
- 20.Molloi S, Bednarz G, Tang J, Zhou Y, Mathur T (1998) Absolute volumetric coronary blood flow measurement with digital subtraction angiography. Int J Cardiac Imaging 14:137–145 [DOI] [PubMed] [Google Scholar]
- 21.Chiribiri A, Schuster A, Ishida M, Hautvast G, Zarinabad N, Morton G, Otton J, Plein S, Breeuwer M, Batchelor P, Schaeffter T, Nagel E (2013) Perfusion phantom: an efficient and reproducible method to simulate myocardial first-pass perfusion measurements with cardiovascular magnetic resonance. Magn Reson Med 69:698–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulzheimer S, Kalender WA (2003) Assessment of calcium scoring performance in cardiac computed tomography. Eur Radiol 13:484–497 [DOI] [PubMed] [Google Scholar]
- 23.Bamberg F, Marcus RP, Becker A, Hildebrandt K, Bauner K, Schwarz F, Greif M, von Ziegler F, Bischoff B, Becker HC, Johnson TR, Reiser MF, Nikolaou K, Theisen D (2014) Dynamic myocardial ct perfusion imaging for evaluation of myocardial ischemia as determined by mr imaging. JACC Cardiovasc Imaging 7:267–277 [DOI] [PubMed] [Google Scholar]
- 24.AAPM. The measurement, reporting, and management of radiation dose in ct. AAPM; 2008 [Google Scholar]
- 25.George RT, Silva C, Cordeiro MA, DiPaula A, Thompson DR, McCarthy WF, Ichihara T, Lima JA, Lardo AC (2006) Multidetector computed tomography myocardial perfusion imaging during adenosine stress. J Am Coll Cardiol 48:153–160 [DOI] [PubMed] [Google Scholar]
- 26.Bastarrika G, Ramos-Duran L, Rosenblum MA, Kang DK, Rowe GW, Schoepf UJ (2010) Adenosine-stress dynamic myocardial ct perfusion imaging: initial clinical experience. Invest Radiol 45:306–313 [DOI] [PubMed] [Google Scholar]
- 27.Ho KT, Chua KC, Klotz E, Panknin C (2010) Stress and rest dynamic myocardial perfusion imaging by evaluation of complete time-attenuation curves with dual-source ct. JACC Cardiovasc Imaging 3:811–820 [DOI] [PubMed] [Google Scholar]
- 28.Greif M, von Ziegler F, Bamberg F, Tittus J, Schwarz F, D’Anastasi M, Marcus RP, Schenzle J, Becker C, Nikolaou K, Becker A (2013) Ct stress perfusion imaging for detection of haemodynamically relevant coronary stenosis as defined by ffr. Heart 99:1004–1011 [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Qin L, Shi X, Zeng Y, Jing H, Schoepf UJ, Jin Z (2012) Adenosine-stress dynamic myocardial perfusion imaging with second-generation dual-source ct: comparison with conventional catheter coronary angiography and spect nuclear myocardial perfusion imaging. Am J Roentgenol 198:521–529 [DOI] [PubMed] [Google Scholar]
- 30.Weininger M, Schoepf UJ, Ramachandra A, Fink C, Rowe GW, Costello P, Henzler T (2012) Adenosine-stress dynamic real-time myocardial perfusion ct and adenosine-stress first-pass dual-energy myocardial perfusion ct for the assessment of acute chest pain: initial results. Eur J Radiol 81:3703–3710 [DOI] [PubMed] [Google Scholar]
- 31.Huber AM, Leber V, Gramer BM, Muenzel D, Leber A, Rieber J, Schmidt M, Vembar M, Hoffmann E, Rummeny E (2013) Myocardium: dynamic versus single-shot ct perfusion imaging. Radiology 269:378–386 [DOI] [PubMed] [Google Scholar]
- 32.Rossi A, Dharampal A, Wragg A, Davies LC, van Geuns RJ, Anagnostopoulos C, Klotz E, Kitslaar P, Broersen A, Mathur A, Nieman K, Hunink MG, de Feyter PJ, Petersen SE, Pugliese F (2014) Diagnostic performance of hyperaemic myocardial blood flow index obtained by dynamic computed tomography: does it predict functionally significant coronary lesions? Eur Heart J Cardiovasc Imaging 15:85–94 [DOI] [PubMed] [Google Scholar]
- 33.Einstein AJ (2013) Multiple opportunities to reduce radiation dose from myocardial perfusion imaging. Eur J Nucl Med Mol Imaging 40:649–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitagawa K, George RT, Arbab-Zadeh A, Lima JA, Lardo AC (2010) Characterization and correction of beam-hardening artifacts during dynamic volume ct assessment of myocardial perfusion. Radiology 256:111–118 [DOI] [PubMed] [Google Scholar]
- 35.Stenner P, Schmidt B, Allmendinger T, Flohr T, Kachelrie M (2010) Dynamic iterative beam hardening correction (dibhc) in myocardial perfusion imaging using contrast-enhanced computed tomography. Invest Radiol 45:314–323 [DOI] [PubMed] [Google Scholar]
- 36.Haridasan V, Nandan D, Raju D, Rajesh GN, Sajeev CG, Vinayakumar D, Muneer K, Babu K, Krishnan MN (2013) Coronary sinus filling time: a novel method to assess microcirculatory function in patients with angina and normal coronaries. Indian Heart J 65:142–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pijls NH, Uijen GJ, Hoevelaken A, Arts T, Aengevaeren WR, Ros HS, Fast JH, Van Leeuwen KL, Van der Werf T (1990) Mean transit time for the assessment of myocardial perfusion by videodensitometry. Circulation 81:1331–1340 [DOI] [PubMed] [Google Scholar]