Abstract
Background.
Studies of the effects of postoperative atrial fibrillation (poAF) on long-term survival are conflicting, likely because of comorbidities that occur with poAF and the patient populations studied. Furthermore, the effects of poAF duration on long-term survival are poorly understood.
Methods.
We utilized a prospectively collected data-base on outcomes of cardiac surgery at a large tertiary care institution between August 2001 and December 2010 with survival follow-up through June 2015 to analyze long-term survival of patients with poAF. In addition, we identified patient- and procedure-related variables associated with poAF, and estimated overall comorbidity burden using the Elixhauser comorbidity index. Survival was compared between patients with poAF (n [ 513) and a propensity score matched control cohort, both for all patients and separately for subgroups of patients with poAF lasting less than 2 days (n [ 218) and patients with prolonged poAF (n [ 265).
Results.
Patients with poAF were older and had a higher burden of comorbidities. Survival was significantly worse for patients with poAF than for the matched control group (hazard ratio 1.43, 95% confidence interval: 1.11 to 1.86). That was driven by decreased survival among patients with prolonged poAF (hazard ratio 1.97, 95% confidence interval: 1.37 to 2.80), whereas survival of patients with poAF for less than 2 days was not significantly different from that of matched controls (hazard ratio 0.91, 95% confidence interval: 0.60 to 1.39).
Conclusions.
After close matching based on comorbidity burden, prolonged poAF is still associated with decreased survival. Therefore, vigilance is warranted in monitoring and treating patients with prolonged poAF after cardiac surgery.
Postoperative atrial fibrillation (poAF) is observed in 26% to 32% of patients after isolated coronary artery bypass graft (CABG) surgery, and 30% to 50% of patients after isolated valve or combined valve and CABG surgery [1]. Several clinical risk factors for poAF have been identified, including older age [2, 3], male sex [2], and obesity [4]. Comorbidities such as history of prior atrial fibrillation (AF) [5], hypertension [3], congestive heart failure [5], chronic obstructive lung disease [5], and chronic kidney disease [6] have also been associated with poAF. Furthermore, valve repair or replacement and an increased aortic cross-clamp time also contribute to an increased risk of poAF [2, 5]. More recently, several genetic variants associated with ambulatory AF have also been associated with the risk of poAF, indicating a genetic predisposition to poAF [7, 8]. Therefore, poAF remains a complex disorder associated with risk factors of numerous etiologies.
An association between the occurrence of poAF and higher long-term mortality for patients with poAF has been reported [2, 9, 10], but not consistently [11], and seems to vary depending on type of surgery [12]. Many of these studies, however, are retrospectively designed and have a relatively short observation time. Furthermore, practices controlling for confounding variables associated with both poAF and long-term mortality vary widely. The majority of these studies only control for variables related directly to the cardiac surgical procedure and omit quantification of important aspects of the overall health of the patient, including cancer and neurologic comorbidities that can also be associated with both poAF and mortality. This overall comorbidity burden can be quantified using indices that have high correlation with both short-term and long-term outcomes over a wide range of populations and conditions [13–16].
We investigated the long-term survival of patients having poAF after cardiac surgery with adjustments for the validated Elixhauser index for overall quantification of preoperative comorbidity burden and also with adjustments for clinical variables associated with poAF or worse overall survival. Propensity score matching was also performed to minimize confounder effects. We hypothesized that both the occurrence of poAF after heart surgery and its duration were associated with worse long-term survival, even after adjustment for patient characteristics, overall patient comorbidity burden, and procedure-specific variables associated with both poAF and worse long-term mortality.
Material and Methods
Patients and Clinical Variables
We analyzed a prospective cohort of 1,712 cardiac surgical patients from the Identification of Genomic Predictors of Adverse Events After Cardiac Surgery (CABGGenomics)/ Perigren study (http://clinicaltrials.gov/show/NCT00281164), enrolled between August 2001 and December 2010 at Brigham and Women’s Hospital, Boston, Massachusetts. We included study variables for occurrence of AF, hospital diagnoses, and mortality. Patients with a baseline hematocrit less than 25% and patients who had undergone bone marrow transplant were excluded from enrollment in the primary cohort. The study protocol was approved by the Institutional Review Board, and all patients provided written informed consent.
Included patients were not in AF on the day of surgery but inclusion of patients with a prior self-reported history of AF was permitted. We defined poAF as any episode of AF of any duration diagnosed by treating clinicians using electrocardiography or telemetry, occurring postoperatively during the primary surgical hospitalization. Patients were monitored for the onset of poAF until postoperative day 7 or until hospital discharge if hospital discharge occurred before post-operative day 7 [8]. For patients in whom poAF developed, the first day and the last day that poAF occurred (continuous or intermittent poAF during the 7 days) was registered, and the interval between these times was reported as poAF duration. After studying the distribution of poAF duration and before survival analysis, patients were further classified into two equalsized subgroups: (1) duration of poAF less than 2 days; or (2) duration of poAF 2 days or longer, or poAF present at hospital discharge (regardless of duration). Although no institutionwide treatment protocol for poAF was present during the study period, AF duration of 48 hours was a frequently used trigger for long-term anticoagulation therapy; therefore, we chose this cutoff period [17]. Survival of the subset of patients discharged with poAF was studied separately.
For each patient, information on preadmission medications, preoperative testing, operative variables, and postoperative course was prospectively collected. Furthermore, all diagnoses-related group codes and International Classification of Diseases, ninth revision, diagnoses between 90 days preoperative and 30 days postoperative were collected for calculation of the van Walraven modification of the Elixhauser comorbidity index [16, 18]. That provides a single numeric index to represent comorbidities from 30 different disease subgroups from the original Elixhauser index [16]. The standard index was calculated using the comorbidities package in R (version 3.0.3; The R Foundation for Statistical Computing, Vienna, Austria). All-cause mortality was obtained from the Social Security Death Index.
Statistical Analysis
Statistical analysis was performed in R. A two-tailed p value of less than 0.05 was considered statistically significant. Clinical variables were compared between patients who did and patients who did not have poAF using two-sample Student’s t tests, X2 tests with Yates’ continuity correction, or Fisher’s exact tests as appropriate. Missing values were imputed using the MissForest package in R, which applies random forest theory to impute iteratively both continuous and categoric missing values [19].
For the group with poAF, a propensity score matched (PSM) control group was created using the MatchIt package in R with the default nearest neighbor method that gives a 100% match [20]. Propensity score matching was based on observed variables that were associated either with poAF or with long-term survival. Adequacy of the match of the two groups was additionally assessed by calculating standardized differences of the matched variables, where a standardized difference of less than 0.1 is indicative of good matching [21].
Overall survival was visualized using Kaplan-Meier curves, and was compared between patients with and patients without poAF using the log rank test. Survival between the poAF and the PSM control group was compared using the Klein and Moeschberger test [21]. A Cox proportional hazards model was applied to assess the contribution of various covariates on long-term mortality. The hazard ratio (HR) for long-term mortality of the poAF cohort compared with the PSM cohort was estimated by a Cox proportional hazards model stratified by the case/control pairs. The proportional hazard assumption of all Cox models was tested by the cox.zph test in R.
Results
Patient Demographics
Of 1,712 patients included, a total of 1,709 patients had adequate information on poAF, comorbidities, and long-term mortality. Of these, 513 patients (30%) who had poAF were identified. Table 1 compares patient characteristics for patients who did and patients who did not have poAF. Patients who had poAF were on average older and had a higher burden of comorbidities as indicated by higher Elixhauser comorbidity indices. Patients who had poAF had longer cardiopulmonary bypass times, with a greater proportion of patients undergoing concomitant valve surgery.
Table 1.
Patient Characteristics
| Characteristics | No PoAF (n = 1,196) | PoAF (n = 513) | p Value |
|---|---|---|---|
| Age, years | |||
| <50 | 111 (9) | 16 (3) | … |
| 50–59 | 335 (28) | 69 (13) | … |
| 60–69 | 390 (33) | 161 (31) | … |
| 70–79 | 297 (25) | 194 (38) | … |
| >85 | 63 (5) | 73 (14) | <0.001 |
| Female | 273 (23) | 106 (21) | 0.36 |
| Body mass index, kg/m2 | 29.4 ± 5.5 | 29.1 ± 5.6 | 0.45 |
| Medical history | |||
| Prior atrial fibrillation | 83 (7) | 64 (12) | <0.001 |
| Hypertensiona | 855 (72) | 413 (81) | <0.001 |
| Diabetes mellitus | 403 (34) | 162 (32) | 0.42 |
| Myocardial infarction | 463 (39) | 216 (42) | 0.21 |
| COPD | 55 (5) | 25 (5) | 0.89 |
| ECI score | |||
| 0–3 | 464 (39) | 142 (28) | … |
| 4–5 | 519 (43) | 229 (45) | … |
| 6–12 | 213 (18) | 142 (28) | <0.001 |
| Preoperative assessment | |||
| Creatinine >1.5 mg/dL | 75 (6) | 38 (7) | 0.45 |
| LVEF < 40%a | 144 (13) | 66 (14) | 0.85 |
| Preoperative medications | |||
| ACE/AR inhibitorsa | 550 (46) | 249 (48) | 0.38 |
| Aspirin | 945 (79) | 390 (76) | 0.19 |
| Beta-blocker | 929 (78) | 411 (80) | 0.29 |
| Calcium-channel inhibitora | 159 (13) | 82 (16) | 0.17 |
| Diuretic | 281 (23) | 146 (28) | 0.03 |
| Statin | 915 (77) | 375 (73) | 0.15 |
| Operative characteristics | |||
| CPB duration, minutes | 110 ± 46 | 123 ± 60 | <0.001 |
| CABG only | 1,055 (88) | 415 (81) | … |
| Any valve surgery | 141 (12) | 98 (19) | <0.001 |
| Aortic valve surgery | 81 (7) | 59 (12) | 0.002 |
| Replacement | 66 (6) | 48 (9) | 0.005 |
| Other | 15 (1) | 11 (2) | 0.25 |
| Mitral valve surgery | 65 (5) | 42 (8) | 0.04 |
| Repair | 27 (2) | 22 (4) | 0.03 |
| Replacement | 22 (2) | 11 (2) | 0.82 |
| Other | 16 (1) | 9 (2) | 0.66 |
| Tricuspid valve surgery | 14 (1) | 4 (2) | 0.61 |
| Replacement | 4 (0.3) | 1 (0.2) | 1.00 |
| Repair | 4 (0.3) | 1 (0.2) | 1.00 |
| Other | 6 (0.5) | 2 (0.4) | 1.00 |
| Pulmonary valve | |||
| Reconstruction | 1 (0.1) | 0 (0.0) | 1.00 |
| Ablation/maze | 27 (2) | 8 (2) | 0.45 |
Variable contains 1 or more patients with missing data.
Values are n (%) or mean ± SD.
ACE = angiotensin-converting enzyme;AR = angiotensin receptor; CABG = coronary artery bypass graft; COPD = chronic obstructive pulmonary disease; CPB = cardiopulmonary bypass; ECI = Elixhauser comorbidity index; LVEF = left ventricular ejection fraction; PoAF = postoperative atrial fibrillation.
For the patients with poAF, information on onset and duration of poAF was available for 483 of the 513 patients. The poAF occurred most commonly on postoperative day 2 (n = 176, 36%) and day 3 (n = 127, 26%). A total of 265 patients (55% of the poAF group) either had poAF duration of 2 days or longer or were discharged with poAF.
Long-Term Survival of Patients With PoAF
Median follow-up for survival was 8.5 years (interquartile range [IQR]: 5.9 to 10.9). The duration of follow-up was shorter for patients who had poAF, 7.6 years (IQR: 5.2 to 10.5), compared with patients who did not have poAF, 8.8 years (IQR: 6.1 to 11.1; p < 0.001). Univariate and multivariate Cox proportional hazards model analysis of the effects of clinical variables on long-term mortality is shown in Table 2.
Table 2.
Univariate and Multivariate Predictors of Long-Term Survival
| Predictors | Univariate HR (95% CI) | p Value | Multivariate HR (95% CI) | p Value |
|---|---|---|---|---|
| PoAF | 1.89 (1.57 2.28) | <0.001 | 1.28 (1.06 1.56) | 0.01 |
| Age, years | ||||
| <50 | 1.00 | 1.00 | ||
| 50–59 | 1.33 (0.73 – 2.43) | 0.35 | 1.33 (0.72 – 2.43) | 0.36 |
| 60–69 | 2.11 (1.19 – 3.75) | 0.01 | 2.09 (1.17 – 3.74) | 0.01 |
| 70–79 | 4.34 (2.47 – 7.61) | <0.001 | 3.74 (2.11 – 6.60) | <0.001 |
| >85 | 8.98 (5.00 – 16.10) | <0.001 | 7.46 (4.12 – 13.53) | <0.001 |
| Female | 1.34 (1.09 – 1.64) | 0.01 | 0.96 (0.77 – 1.19) | 0.72 |
| Body mass index, kg/m2 | 0.99 (0.97 – 1.01) | 0.33 | … | |
| Medical history | ||||
| Prior atrial fibrillation | 2.23 (1.69 – 2.95) | <0.001 | 1.49 (1.11 – 1.99) | 0.01 |
| Hypertensiona | 0.87 (0.70 – 1.08) | 0.58 | … | |
| Diabetes mellitus | 1.50 (1.24 – 1.80) | <0.001 | 1.40 (1.15 – 1.70) | <0.001 |
| Myocardial infarction | 1.47 (1.22 – 1.77) | <0.001 | 1.32 (1.09 – 1.60) | 0.005 |
| COPD | 2.69 (1.94 – 3.75) | <0.001 | 2.09 (1.48 – 2.95) | <0.001 |
| ECI score | ||||
| 0–3 | 1.00 | 1.00 | ||
| 4–5 | 1.75 (1.40 – 2.20) | <0.001 | 1.30 (1.03 – 1.65) | 0.03 |
| 6–12 | 2.82 (2.19 – 3.62) | <0.001 | 1.63 (1.23 – 2.17) | <0.001 |
| Preoperative assessment | ||||
| Creatinine >1.5 mg/dL | 2.36 (1.78 – 3.13) | <0.001 | 1.50 (1.12 – 2.02) | 0.01 |
| LVEF < 40%a | 2.01 (1.60 – 2.52) | <0.001 | 1.67 (1.32 – 2.12) | <0.001 |
| Preoperative medications | ||||
| ACE/AR inhibitorsa | 0.98 (0.81 – 1.17) | 0.79 | … | |
| Aspirin | 1.17 (0.94 – 1.45) | 0.15 | … | |
| Beta-blocker | 1.23 (0.99 – 1.53) | 0.06 | … | |
| CCI | Nonproportional | |||
| Diuretic | 1.74 (1.43 – 2.12) | <0.001 | 1.26 (1.02 – 1.57) | 0.04 |
| Statin | 0.79 (0.64 – 0.97) | 0.02 | 0.77 (0.62 – 0.95) | 0.01 |
| Operative characteristics | ||||
| CPB duration | Nonproportional | |||
| CABG surgery only | 1.00 | 1.00 | ||
| Any valve surgery | 1.61 (1.22 – 2.12) | <0.001 | 1.08 (0.80 – 1.45) | 0.63 |
Variable contains 1 or more patients with missing data, imputed before multivariate analysis.
ACE = angiotensin-converting enzyme; AR = angiotensin receptor; CABG = coronary artery bypass graft; CCI = calcium-channel inhibitor; CI = confidence interval; COPD = chronic obstructive pulmonary disease; CPB = cardiopulmonary bypass; ECI = Elixhauser comorbidity index; HR = hazard ratio;LVEF = left ventricular ejection fraction; PoAF = postoperative atrial fibrillation.
In univariate analysis, poAF was found to be significantly associated with increased mortality (HR 1.89, 95% confidence interval [CI]: 1.57 to 2.28; Fig 1). Age, female sex, Elixhauser comorbidity index, preoperative diagnosis of AF, diabetes mellitus, myocardial infarction, chronic obstructive pulmonary disease, valve or concomitant valve and CABG surgery (compared with CABG only), and usage of diuretics were also associated with increased mortality, whereas preoperative use or statins was protective. In multivariate modeling of all significant variables from the univariate model, poAF of any duration was significantly associated with higher hazard of mortality (HR 1.28, 95% CI: 1.06 to 1.56; Table 2), even after accounting for age, sex, prior AF, comorbidity burden, medications, and variables associated with surgery. A few variables, cardiopulmonary bypass time in particular, did not meet the requirements of proportionality and could not be analyzed with Cox proportional hazards modeling.
Fig 1.
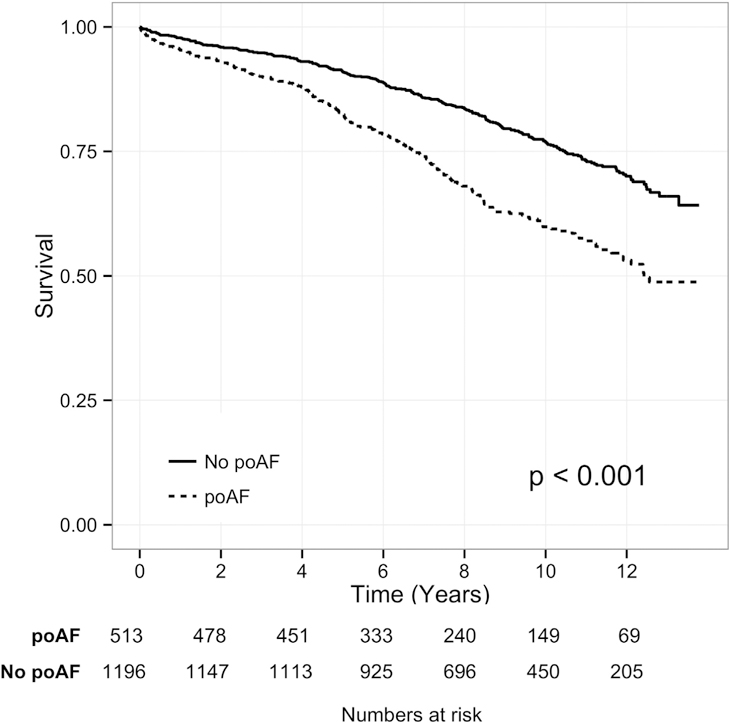
Long-term survival of patients having postoperative atrial fibrillation (poAF [dotted line]) and patients not having poAF (No poAF [solid line]). The p value reflects the log odds test of difference in overall survival.
Survival of Patients With PoAf and PSM Control Group
To compensate for the shortcomings of multivariate adjustment by Cox proportional hazards modeling, and to provide a more direct comparison of survival for patients with poAF compared with patients without poAF, a PSM control group was created. The propensity score analysis included all variables with univariate association with either poAF or long-term mortality (variables are listed in Table 3). Comparison of the poAF group and the PSM control group revealed good balancing between the two groups (Table 3), with standardized difference between the poAF and PSM group less than 0.1 for each matching variable, indicative of adequate matching [21]. Of note, an equal percentage of patients in both the poAF group and the PSM control group had a history of prior AF. Median follow-up for survival was 8.5 years (IQR: 5.9 to 10.9), 7.6 years (IQR: 5.2 to 10.5.1) for patients who had poAF compared with 8.1 years (IQR: 5.7 to 10.6) for the PSM controls (p = 0.10).
Table 3.
Comparison of Postoperative Atrial Fibrillation and Propensity Matched Score Control Group
| Variables | PSM Control (n = 513) | PoAF (n = 513) | P Value | Std. Diff. |
|---|---|---|---|---|
| Age, years | ||||
| <50 | 13 (3) | 16 (3) | … | 0.03 |
| 50–59 | 61 (12) | 69 (13) | … | 0.05 |
| 60–69 | 170 (33) | 161 (31) | … | −0.04 |
| 70–79 | 208 (41) | 194 (38) | … | −0.06 |
| >85 | 61 (12) | 73 (14) | 0.63 | 0.07 |
| Female | 111 (22) | 106 (21) | 0.76 | −0.02 |
| Medical history | ||||
| Prior atrial fibrillation | 59 (12) | 64 (12) | 0.70 | 0.03 |
| Hypertensiona | 410 (80) | 415 (81) | 0.75 | −0.02 |
| Diabetes mellitus | 164 (32) | 162 (32) | 0.95 | −0.01 |
| Myocardial infarction | 230 (45) | 216 (42) | 0.41 | −0.06 |
| COPD | 26 (5) | 25 (5) | 1.00 | −0.01 |
| ECI score | ||||
| 0–3 | 145 (28) | 142 (28) | … | −0.01 |
| 4–5 | 231 (45) | 229 (45) | … | −0.01 |
| 6–12 | 137 (28) | 142 (28) | 0.94 | 0.02 |
| Preoperative assessment | ||||
| Creatinine >1.5 mg/dL | 38 (7) | 38 (7) | 1.00 | … |
| LVEF < 40%a | 62 (12) | 66 (13) | 0.78 | −0.02 |
| Preoperative medications | ||||
| Diuretic | 144 (28) | 146 (28) | 0.94 | −0.01 |
| Statin | 380 (74) | 375 (73) | 0.78 | −0.02 |
| Operative characteristics | ||||
| CPB duration, minutes | 119 51 | 123 59 | 0.28 | 0.07 |
| CABG only | 429 (84) | 415 (81) | … | … |
| Any valve surgery | 84 (16) | 98 (19) | 0.29 | 0.07 |
| Any aortic valve surgery | 50 (10) | 59 (11) | 0.42 | 0.06 |
| Any mitral valve surgery | 40 (8) | 42 (8) | 0.91 | 0.01 |
| Ablation/maze | 15 (3) | 8 (2) | 0.21 | −0.09 |
Variable contains 1 or more patients with imputed data. Values are n (%).
CABG = coronary artery bypass graft; COPD = chronic obstructive pulmonary disease; CPB = cardiopulmonary bypass; ECI = Elixhauser comorbidity index; LVEF = left ventricular ejection fraction; PoAF = postoperative atrial fibrillation; PSM = propensity score matched; Std. Diff. = standardized difference.
Figure 2 compares the survival between the poAF group and the PSM control group. Overall, long-term survival was significantly worse for the poAF group compared with the PSM control group (Klein test, p = 0.006), and the hazard for mortality stratified by case-control pairs was also significantly higher for the poAF group (HR 1.43, 95% CI: 1.11 to 1.86
Fig 2.
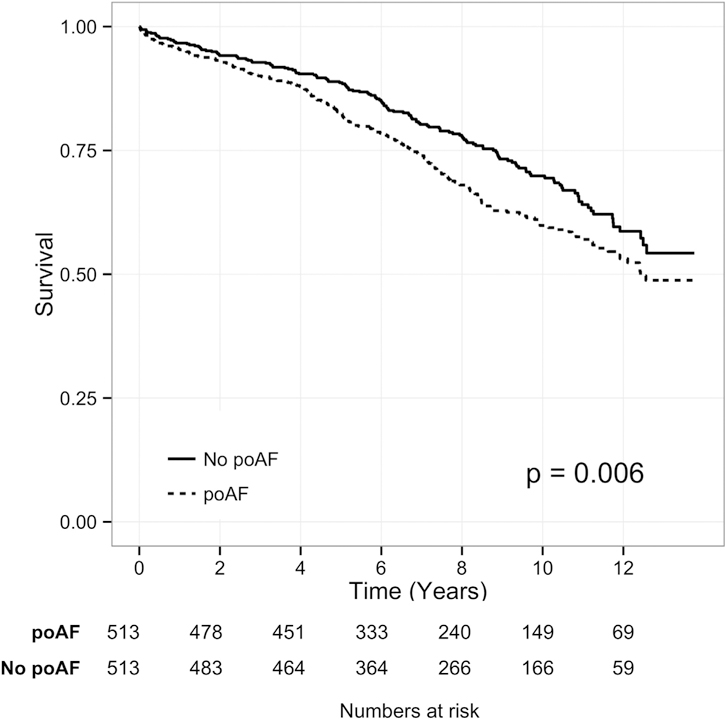
Long-term survival of patients having postoperative atrial fibrillation (poAF [dotted line]) and a propensity score matched control group (No poAF [solid line]). The p value reflects the Klein test of difference in overall survival between each patient with poAF and their PSM control patient.
Duration of PoAF and Survival
Finally, patients for whom the duration of poAF duration (n = 483) was available were divided into two subgroups: (1) patients with duration of poAF less than 2 days (n = 218); and (2) patients with duration of poAF of 2 days or more or present at discharge (n = 265). Patient characteristics for each group were similar, and are shown in the Supplemental Table. The group with the shorter poAF duration had both shorter mean intensive care unit stay (2 versus 4 days, p < 0.001) and shorter hospital stay (7 versus 12 days, p < 0.001). Although no formal treatment protocol for poAF existed during the study period, patients with shorter poAF duration were less likely to be treated with amiodarone during the primary hospitalization and less likely to be discharged on a regimen of warfarin or antiarrhythmic medications (Table 4).
Table 4.
Inhospital and Discharge Treatment of Two Subsets of Postoperative Atrial Fibrillation Patients
| Treatment | PoAF <2 Days (n = 218) | PoAF ≥ 2 Days (n = 265) | p Value |
|---|---|---|---|
| In-hospital medications | |||
| Amiodarone | 26 (12) | 82 (31) | <0.001 |
| Beta-blocker | 210 (96) | 259 (98) | 0.52 |
| Calcium-channel inhibitor | 51 (23) | 75 (28) | 0.26 |
| Other antiarrhythmic | 52 (24) | 91 (34) | 0.02 |
| Discharge medications | |||
| Beta-blocker | 203 (93) | 240 (91) | 0.39 |
| Calcium-channel inhibitor | 36 (17) | 50 (19) | 0.58 |
| Other antiarrhythmic drugsa | 26 (12) | 73 (28) | <0.001 |
| Warfarin | 41 (19) | 171 (64) | <0.001 |
Including amiodarone.
Values are n (%).
PoAF = postoperative atrial fibrillation.
The survival of each patient was compared with the survival of a PSM control patient (Fig 3). Although the survival of the patients with poAF duration less than 2 days did not differ significantly from that of their PSM controls (Klein test, p = 0.67, HR 0.91, 95% CI: 0.60 to 1.39; Fig 3A), the survival of the group with longer duration of poAF (n = 265) was significantly worse than that of their PSM controls (Klein test, p < 0.001, HR 1.97, 95% CI: 1.37 to 2.80; Fig 3B). Furthermore, poAF patients discharged in AF (n = 53) had significantly worse survival than their PSM controls (Klein test, p = 0.02, HR 2.30, 95% CI: 1.10 to 4.83). Finally, within the subset of patients with poAF, the hazard of mortality increased with each day in poAF (HR 1.16 per day, 95% CI: 1.08 to 1.23, p < 0.001).
Fig 3.
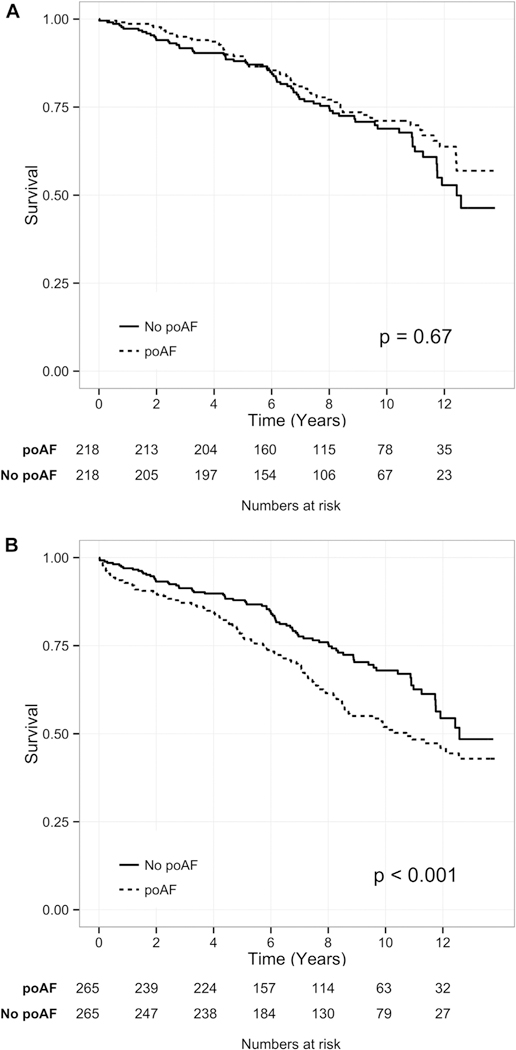
Long-term survival of patients with postoperative atrial fibrillation (poAF [dotted lines]) of different duration compared with propensity score matched patients without poAF (No poAF [solid lines]). (A) Although there was no change in survival between patients with poAF of less than 2 days’ duration and their matched patients, (B) there was a significantly worse survival for patients with poAF of more than 2 days’ duration or present at discharge compared with matched patients without poAF. The p value reflects the Klein test of difference in overall survival between each patient with poAF and the propensity score matched control patient.
Comment
In this analysis of a large, prospectively collected cohort of cardiac surgery patients who underwent surgery at a single tertiary care center, poAF was associated with worse long-term mortality, even after careful propensity score matching for possible confounding variables. This difference in long-term survival was most pronounced in the subset of patients who had poAF for 2 or more days or were discharged still in AF.
Patient- and procedure-related variables that were associated with poAF, including older age, a preoperative history of hypertension or AF, valve surgery, and prolonged cardiopulmonary bypass time, have been identified in several other studies [1, 2, 22]. However, a novel aspect of our study is the association between the Elixhauser comorbidity index and poAF, demonstrating a higher overall comorbidity burden for patients with poAF. This finding is likely explained by both preoperaive arrhythmia and several predictors of poAF being included in the Elixhauser comorbidity index.
The available literature on the association between poAF and long-term survival appears contradictory, with several publications indicating worse overall survival for patients who have poAF [2, 9, 10], and others indicating no difference [11]. Comparing survival between patients who have and patients who do not have poAF can be challenging due to different baseline patient comorbidities and surgical course. In a database with an adequate number of patients, propensity score matching can be used to account for differences in baseline characteristics and comorbidity burden. By using propensity score matching and the Elixhauser comorbidity index, we were able to account for additional comorbid states not traditionally available in studies involving cardiac surgery patients.
After propensity score matching, there was still a significantly higher hazard for mortality for patients with poAF compared with their PSM paired control counterpart. In addition, we were able to quantify the poAF burden by separating the poAF patients and matched controls into two groups based on duration. Analyzing these groups separately revealed that long-term survival was worse only for patients who had poAF lasting longer than 2 days or were discharged with the dysrhythmia. Patients in that group had a 97% higher hazard of long-term mortality compared with their matched controls. It is likely that a portion of this hazard is due to the poAF itself, although insufficient matching for unmeasured covariates could also contribute to the difference.
Several explanations are possible for why both the overall occurrence and the prolonged poAF are associated with worse overall survival, although it should be stressed that we cannot definitively conclude that prolonged poAF accounts for the observed increased mortality. Assuming a portion of the group with prolonged poAF continues to have either paroxysmal or chronic AF after the episode, poAF may contribute to long-term risk of heart failure or embolic stroke, and the side effects of medications and treatment for these diseases may also contribute to adverse outcomes [23, 24].
A key strength of the study design included the prospective collection of data with rigorous definitions of the study outcomes. Limitations of the database include the relative homogeneity of the inclusion criteria per the design of the original genomic study. Consequently, the population consists of mostly Caucasian patients undergoing nonemergent cardiac surgery in a single tertiary care center. That limits the generalizability of the results. Because patients came from a large referral base, we were not able to collect complete follow-up after hospital discharge. We were able to collect mortality information, but cause of death was not determined.
The results of this study call for increased attention on the subgroup of patients with prolonged poAF after cardiac surgery. An immediate follow-up on this initial observation would be further quantification of poAF burden with a prolonged follow-up for arrhythmia after hospital discharge to identify what is the critical duration associated with worse outcome. It would be interesting to see whether patients with poAF of longer duration are subsequently more likely to have AF, and whether the subset of patients who convert to sinus rhythm within 4 to 6 weeks has improved prognosis compared with patients who not. Although current guidelines for ambulatory patients with AF suggest that it is appropriate to treat patients with poAF similarly [25], there is less evidence to support this recommendation in surgical populations, and there is likely a high variability in the postoperative management of patients with poAF. Further investigation should focus on determining the duration of poAF where benefits of AF treatment modalities such as anticoagulation and rate and rhythm controlling medications and procedures are maximized to optimize the postoperative outcomes of patients undergoing cardiac surgery.
Supplementary Material
Acknowledgments
The authors wish to thank the Perioperative Genomics Center staff, James Gosnell, RN, Kujtim Bodinaku, MD, and Svetlana Gorbatov, MPH, for their assistance with database acquisition. This work was supported by grants from the National Heart, Lung, and Blood Institute (HL65962, HL068774, and HL092217).
Footnotes
The Supplemental Table can be viewed in the online version of this article [http://dx.doi.org/10.1016/j.athoracsur.2016.05.016] on http://www.annalsthoracicsurgery.org.
References
- 1.Kaireviciute D, Aidietis A, Lip GY. Atrial fibrillation following cardiac surgery: clinical features and preventative strategies. Eur Heart J 2009;30:410–25. [DOI] [PubMed] [Google Scholar]
- 2.Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg 1997;226:501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation 1996;94:390–7. [DOI] [PubMed] [Google Scholar]
- 4.Sun X, Boyce SW, Hill PC, et al. Association of body mass index with new-onset atrial fibrillation after coronary artery bypass grafting operations. Ann Thorac Surg 2011;91: 1852–8. [DOI] [PubMed] [Google Scholar]
- 5.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004;291:1720–9. [DOI] [PubMed] [Google Scholar]
- 6.Anderson RJ, O’Brien M, MaWhinney S, et al. Renal failure predisposes patients to adverse outcome after coronary artery bypass surgery. VA Cooperative Study #5. Kidney Int 1999;55:1057–62. [DOI] [PubMed] [Google Scholar]
- 7.Sigurdsson MI, Muehlschlegel JD, Fox AA, et al. Genetic variants associated with atrial fibrillation and PR interval following cardiac surgery. J Cardiothorac Vasc Anesth 2015;29:605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Body SC, Collard CD, Shernan SK, et al. Variation in the 4q25 chromosomal locus predicts atrial fibrillation after coronary artery bypass graft surgery. Circ Cardiovasc Genet 2009;2:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helgadottir S, Sigurdsson MI, Ingvarsdottir IL, Arnar DO, Gudbjartsson T. Atrial fibrillation following cardiac surgery: risk analysis and long-term survival. J Cardiothorac Surg 2012;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol 2004;43:742–8. [DOI] [PubMed] [Google Scholar]
- 11.Saxena A, Shi WY, Bappayya S, et al. Postoperative atrial fibrillation after isolated aortic valve replacement: a cause for concern? Ann Thorac Surg 2013;95:133–40. [DOI] [PubMed] [Google Scholar]
- 12.Mariscalco G, Engstrom KG. Postoperative atrial fibrillation is associated with late mortality after coronary surgery, but not after valvular surgery. Ann Thorac Surg 2009;88:1871–6. [DOI] [PubMed] [Google Scholar]
- 13.Gutacker N, Bloor K, Cookson R. Comparing the performance of the Charlson/Deyo and Elixhauser comorbidity measures across five European countries and three condi-tions. Eur J Pub Health 2015;25(Suppl 1):15–20. [DOI] [PubMed] [Google Scholar]
- 14.Ladha KS, Zhao K, Quraishi SA, et al. The Deyo-Charlson and Elixhauservan Walraven comorbidity indices as predictors of mortality in critically ill patients. BMJ Open 2015;5: e008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy FH, Kobrin D, Rame JE, et al. Increasing frequency of left ventricular assist device exchanges in the United States. Ann Thorac Surg 2015;100(5):1660–4. [DOI] [PubMed] [Google Scholar]
- 16.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009;47:626–33. [DOI] [PubMed] [Google Scholar]
- 17.Echahidi N, Pibarot P, O’Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol 2008;51:793–801. [DOI] [PubMed] [Google Scholar]
- 18.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36: 8–27. [DOI] [PubMed] [Google Scholar]
- 19.Stekhoven DJ, Buhlmann P. MissForest—non-parametric missing value imputation for mixed-type data. Bioinformatics 2012;28:112–8. [DOI] [PubMed] [Google Scholar]
- 20.Ho DE, Imal K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;12:1–28. [Google Scholar]
- 21.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malaisrie SC, Lee R, Kruse J, et al. Atrial fibrillation ablation in patients undergoing aortic valve replacement. J Heart Valve Dis 2012;21:350–7. [PubMed] [Google Scholar]
- 23.Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation 2009;119:2516–25. [DOI] [PubMed] [Google Scholar]
- 24.Horwich P, Buth KJ, Legare JF. New onset postoperative atrial fibrillation is associated with a long-term risk for stroke and death following cardiac surgery. J Card Surg 2013;28:8–13. [DOI] [PubMed] [Google Scholar]
- 25.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. A report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


