Abstract
Background/ Aim: A high polar xanthophyll of Fucoxanthin (Fx) is abundantly contained in edible brown algae, and it has chemopreventive effects in mouse cancer models, however, the underlying mechanisms of these effects are not well understood. Thus, we aimed to investigate the effects of Fx on the tumor microenvironment in cancer model mice. Materials and Methods: We investigated the effect of Fx (30 mg/kg body weight) in a variety of cell types within the tumor microenvironment of α mouse preclinical colorectal cancer model and analyzed the mouse saliva in search of predictors for cancer chemopreventive effects. Results: Fx administration significantly decreased the number of colorectal polyps and tended to decrease colonic lesions compared to untreated control mice. In addition, Fx administration showed significantly lower numbers of colorectal cancer stem cells-like CD44high/EpCAMhigh cells, cancer-associated fibroblasts-like αSMAhigh cells, tumor-associated macrophages-like and dendritic cells-like CD206high cells by 0.6-, 0.5- and 0.6-fold, respectively, compared to untreated control mice. Moreover, the treatment also showed significantly lower levels of salivary glycine by 0.5-fold. Conclusion: Our results suggest that salivary glycine may be a predictor representing the chemopreventive effect of Fx in mice.
Keywords: fucoxanthin, carotenoid, glycine, tumor microenvironment, saliva
Abbreviations
AOM: azoxymethane, α-SMA: α-Smooth muscle actin, BSA: bovine serum albumin, BW: body weight, CAFs: cancer-associated fibroblasts, CCSCs: colorectal cancer stem cells, Csps: colonospheres, DCs: dendritic cells, DSS: dextrane sodium sulfate, DW: distilled water, EMT: epithelial-mesenchymal transition, Fx: fucoxanthin, FxOH: fucoxanthinol, TAMs: tumorassociated macrophages, TME: tumor microenvironment.
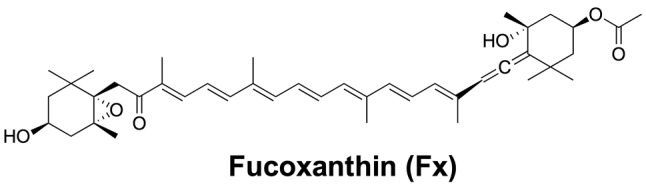
Marine brown algae are a rich source of beneficial lipids, such as highly polar xanthophylls, polyunsaturated fatty acids and sterols, important for human health (1,2). Among the brown algal lipids, a highly polar xanthophyll, fucoxanthin (Fx, Figure 1), is abundantly present in brown algae as it plays an important role in their photosynthesis and photoprotection (3). As shown in Figure 1, this xanthophyll has a unique structure, an allenic bond and a 5,6-monoepoxide. Marine brown algae, such as the Undaria pinnatifida (wakame) and the Hizikia fusiforme (hijiki), are good sources of Fx among Japanese algal foods (1,4). Even in very high doses, oral consumption of Fx has been proven to be safe (5,6).
Figure 1. The Fucoxanthin (Fx) structural formula (designed using ChemDraw Professional 15.1).
Colorectal cancer (CRC) is a cancer affecting many people around the world and is the fourth most common cause of deaths from neoplasia (7). There is now substantial evidence that the consumption of fruits and vegetables rich in carotenoids is associated with CRC prevention. Therefore, many CRC preventive trials using carotenoids have been performed, however, their effects on CRC have been decided as “Limited/not conclusive” (8). As far as we know, there are no epidemiological studies evaluating highly polar xanthophylls such as Fx in CRC prevention. Fx is known to possess a cancer preventive potential in mouse cancer models (9-11). Our group and others have demonstrated that Fx and its metabolite, fucoxanthinol (FxOH), suppress the growth of cancer cells in several cases (12-17), however, the mechanisms underlying the anti-cancerogenic effects of Fx and FxOH have remained unclear.
Recently, CRC stem cells (CCSCs) were recognized as the most important targets for cancer therapy. Self-renewal, drug-resistance, metastasis, sphere formation and tumor formation are the main characteristic multiplexing properties of CCSCs (18). In addition, some of the cell surface proteins, CD44, CD133, CD166, EpCAM and LGR5, are markers associated with these CCSC features (19,20). Among these cells, CD44+/EpCAMhigh-expressing cells are recognized for possessing a hallmark characteristic of the CCSC-like phenotype, such as tumorigenicity (19). Thus, the CCSC-like colonospheres (Csps) that express both CD44 and EpCAM at high levels are considered a good model for studying CCSC. These CD44high/EpCAMhigh colonospheres possess CCSC-like properties, such as spheroid formation and tumorigenicity (21,22). On the other hand, CCSCs may be affected by the tumor microenvironment (TME), stromal cells, including cancer-associated fibroblasts (CAFs) and immune cells, as well as various extracellular matrices that collectively result in enhancing the progression of the tumor and its metastasis (23-25). Despite all these, little information regarding the effect of Fx on TME is currently available.
Intracellular amino acids and carboxylic acids are used for energy metabolism, such as glycolysis, gluconeogenesis and fatty acid synthesis (26). Thus, proteins containing amino and carboxylic acids could be used as prognostic indicators representing the status of somatic cancers (27). Many such metabolite candidates have been isolated from saliva, urine, blood and tissue of CRC patients and animals (27-29). Among these biological samples, saliva may be the most promising one from the point of safety and ease of accessibility. We recently demonstrated that glycine and/or succinic acid are intracellular indicators that can predict sphere disintegration of Csps following FxOH treatment (30). Moreover, these molecules are also useful for predicting the time point of tumor development in Csps-xenograft mice under Fx treatment (31) however, further confirmation and investigation of prognostic metabolites present in saliva from additional cancer models is necessary. In the present study, we have investigated the suppressive effect of Fx on TME at a preclinical cancer condition in a CRC mouse model. Furthermore, we have examined the alteration of metabolite profiles in mouse saliva following Fx treatment. We suggest that Fx may be used as a prognostic marker representing TME suppression and that salivary glycine may be a predictor representing the chemopreventive effect of Fx in mice.
Materials and Methods
Chemicals. Fx oil (5w/v%), mixed with a palm oil (70 w/v%) complex with slight moisture, proteins, carbohydrates and sodium, was provided by the Oryza Oil & Fat Chemical Co. Ltd. (Aichi, Japan). Palm oil alone (75 w/v%) was prepared as a control. Azoxymethane (AOM) (purity, ≥95%) and dextran sodium sulfate (DSS) (M.W.: 36,000-50,000) were obtained from Wako Pure Chemicals (Osaka, Japan) and MP Biomedicals (Solon, OH, USA), respectively. Pilocarpine hydrochloride and urethane were both from Sigma-Aldrich (St. Louis, MO, USA). CD44 conjugated with Alexa Fluor 488 and CD206 conjugated Alexa Fluor 594 antibodies were purchased from BioLegend (San Diego, CA, USA). EpCAM conjugated with an APC antibody was obtained from eBioscience (San Diego, CA, USA). α-Smooth muscle actin (α-SMA) antibody was purchased from Cell Signaling Technology (Danvers, MA, USA). Goat anti-rabbit IgG conjugated with Alexa Fluor 488 and ProLong Gold Antifade conjugated with 4’,6-diamidino-2-phenylindole (DAPI) were from Invitrogen (Carlsbad, CA, USA). All other chemicals and solvents were of analytical grade from Wako Pure Chemicals (Osaka, Japan).
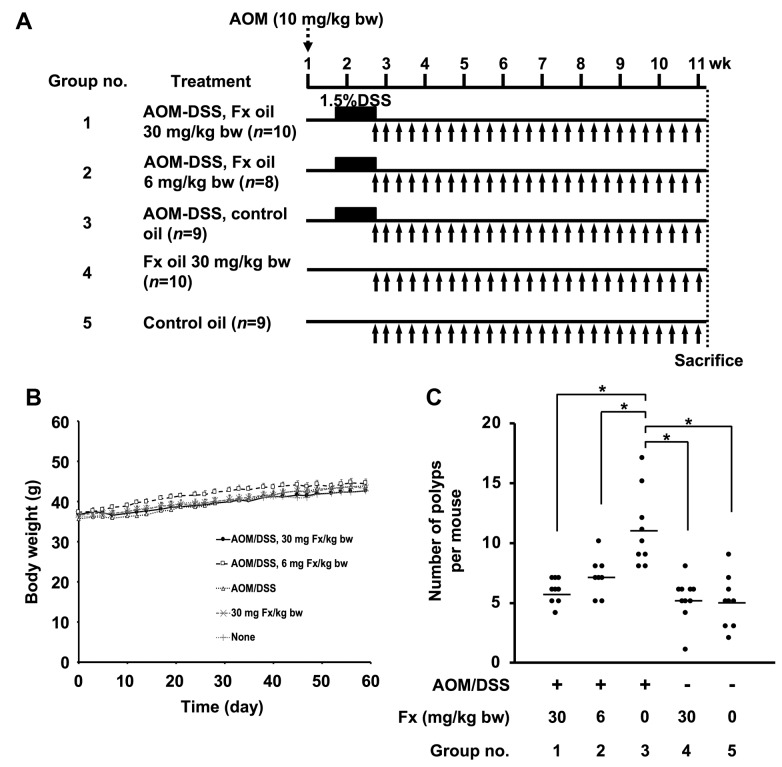
Animal experiments. A detailed protocol is shown in Figure 2A. Male ICR mice were purchased at 5 weeks of age from Sankyo Labo Service (Shizuoka, Japan). All mice were randomly divided into five groups (5 mice/cage, 10 mice per group) and were maintained in a 12-hour light/dark cycle in a humidity- and temperature-controlled room. Solid food (Grade: MF, Oriental Yeast Co. Ltd.) and water were given ad libitum until sacrifice (about 10 weeks later). After a week of acclimation, mice in groups 1, 2 and 3 were treated with a single IP injection of AOM (10 mg/kg of body weight). Groups 4 and 5 were injected with saline only (IP). One week later, mice in groups 1-3 received 1.5 w/v% DSS in drinking water for 1 week. Mice in groups 4 and 5 were given normal water during that time. Mice in groups 1, 2 and 4 were given Fx oil at 30, 6 and 30 mg/kg, respectively, using a stomach needle every 1 or 3 days during the final 8 weeks. Mice in groups 3 and 5 were given the equivalent volume of palm oil. Mice were anaesthetized using urethane (1.0 g/kg, IP) for 20 minutes and their saliva (<500 μl) was collected for 20 min following an injection of pilocarpine (5 mg/kg, SC) 40 min before sacrifice. Mice were sacrificed by isoflurane inhalation. Subsequently, the large bowel of each mouse was excised, was cut open longitudinally and was fixed in 10% formalin/PBS for at least 48 hours. Polyp number and size on the colonic mucosa were measured using a micrometer caliper. The polyp size (mm3) was expressed as the formula of a (mm) × b2 (mm)/2 (a, long range: b, short range). Histopathologic examinations were confirmed in hematoxylin and eosin-stained sections made from paraffin-embedded blocks prepared by Morphotechnology (Sapporo, Japan). The experiments were performed according to the Institutional Ethics Review Committee for Animal Experimentation in the Health Sciences, University of Hokkaido, and the Guidelines for Animal Experiments in the Health Sciences, University of Hokkaido (32).
Figure 2. Effects of fucoxanthin on body weight and number of colorectal polyps in AOM/DSS mice. (A) Experimental protocol with fucoxanthin (Fx) in AOM/DSS mice. Fx oil (fucoxanthin-5™) was administrated at 30 or 6 mg Fx/kg every 2-3 days for 8 weeks (black arrows). The control groups with or without AOM/DSS treatment was given the equivalent volume (μL) of control oil (without Fx). (B) Body weight changes from the day after DSS intake to the day of final Fx administration. (C) Polyps numbers. Means±SE (n=8-10). (*) p<0.05 by one-way ANOVA with post-hoc Tukey-Kramer test.
Fluorescence immunohistochemistry. Paraffin-embedded sections from the mouse colonic mucosa were stained for co-localization of CD44 together with EpCAM, or α-SMA together with CD206, using immunofluorescence. Sections were deparaffinized and antigen-retrieval was performed with the use of 1 mM EDTA buffer (pH 9.0) at 95˚C for 20 min. Sections were then washed with distilled water, PBS and tris buffer saline containing 0.1% polyoxyethylene (20) sorbitan monolaurate (TBST) and were subsequently blocked in a 5% BSA/TBST solution at room temperature for 1 h. For detecting CCSC-like cells, sections (section-A) were then incubated with the anti-CD44 antibody conjugated with Alexa Fluor 488 and the anti-EpCAM antibody conjugated with the APC antibody at a dilution of 1:50 in 1% BSA/TBST overnight at 4˚C. For detecting CAFs, TAMs and dendric cells (DC)-like cells, other sections (section-B) were incubated with the α-SMA primary antibody diluted 1:50 in 1%BSA/TBST overnight at 4˚C, washed with TBST, and were then incubated with a goat anti-rabbit IgG conjugated with Alexa Fluor 488 at a dilution of 1:100 in PBST for 1 hour at room temperature and in the dark. Section-B slides were then probed with anti-CD206 conjugated with Alexa Fluor 594 antibody overnight at 4˚C. Both sections-A and -B were washed with TBST and PBS and were finally stained with ProLong Gold Antifade reagent with DAPI. Immunofluorescence imaging was performed using a Nikon TE2000 confocal microscope equipped with an EZ-C1 software (Nikon, Melville, NY, USA). The number of CD44high/EpCAMhigh, α-SMAhigh and CD206high cells was evaluated per set tissue area (mm2).
Gas Chromatography-Mass Spectrometry. A 100 μl aliquot of collected saliva was dispensed, and 0.05 μg of 2-isopropylmalic acid (Sigma-Aldrich, St. Louis, MA, USA) was spiked as an internal standard. The aqueous components were extracted with 0.5 ml of CH3OH/CHCl3/DW (2.5:1:1, v/v/v), centrifuged at 16,000 g for 5 min and the upper phase was washed with 0.5 ml of DW. The upper extracts were dried and were subsequently derivatized using methoxyamine hydrochloride and N-methyl-N(trimethylsilyl)-trifluoroacetate. GC-MS was achieved with the use of a GCMS-QP5000 spectrometer (Shimadzu, Kyoto, Japan) equipped with a Rxi-5ms column (30 mx0.25 mm i.d., film thickness: 0.25 μm, RESTEK Co. Ltd., GmbH, Bad Homburg, Germany). Samples (1 μl) were injected with helium (0.5 ml/min) as the carrier gas, with a split ratio of 1:3. Column temperature program was as follows: i) 80˚C for 2 min, ii) 80-330˚C for 4 min, and iii) 330˚C for 8 min. The ion source and interface temperatures were 230 and 250˚C, respectively. The identification of the low-molecular weight metabolites was confirmed by comparing them to authentic standards. Metabolite contents were expressed in picomoles (pmol) per 100 μl of saliva.
Statistical analyses. All the results are expressed as mean±SE values. Significant differences for multiple comparison tests were performed by one-way ANOVA followed by a Tukey-Kramer post-hoc test. Differences were considered statistically significant at p<0.05.
Results
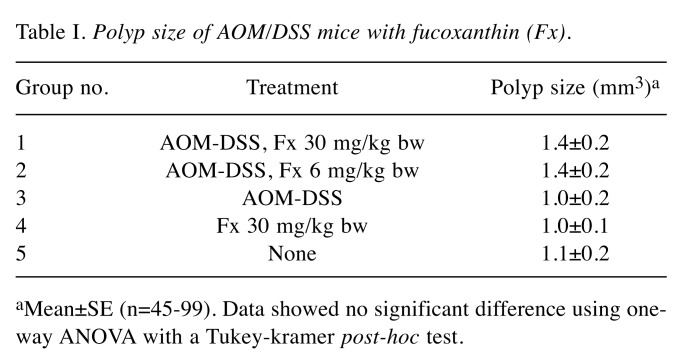
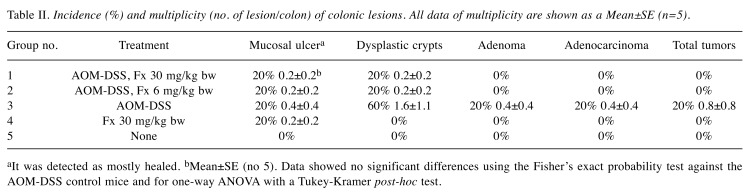
Suppression of colorectal polyp formation in AOM/DSS mice by Fx. No differences in clinical signs and food intake were observed by the administration of Fx to the preclinical cancer AOM/DSS mice for 8 weeks. There were no significant differences in the body weight of mice among the five groups during the Fx treatment period (Figure 2B). Interestingly, the number of polyps in Fx-treated mice (groups 1 and 2) was significantly lower compared to the polyps of mice in group 3 (p=0.000021, p=0.003832, respectively), and was comparable to the number of polyps in groups 4 and 5: i) group 1: 5.7±0.4, ii) group 2: 7.1±0.6, iii group 3, 1:1.0±1.1. iv) group 4: 5.2±0.6, and v) group 5: 5.0±0.7 polyps (Figure 2C). No significant difference was observed in the polyp size among the five groups (Table I). Pathology examination revealed that the incidence and/or multiplicity of mucosal ulcer, dysplastic crypts, adenoma and adenocarcinoma tended to be lower when Fx was administered at 30 (group 1) and/or at 6 (group 2) mg/kg, compared to the control mice (group 3) (Table II).
Table I. Polyp size of AOM/DSS mice with fucoxanthin (Fx).
aMean±SE (n=45-99). Data showed no significant difference using oneway ANOVA with a Tukey-kramer post-hoc test.
Table II. Incidence (%) and multiplicity (no. of lesion/colon) of colonic lesions. All data of multiplicity are shown as a Mean±SE (n=5).
aIt was detected as mostly healed. bMean±SE (no 5). Data showed no significant differences using the Fisher’s exact probability test against the AOM-DSS control mice and for one-way ANOVA with a Tukey-Kramer post-hoc test.
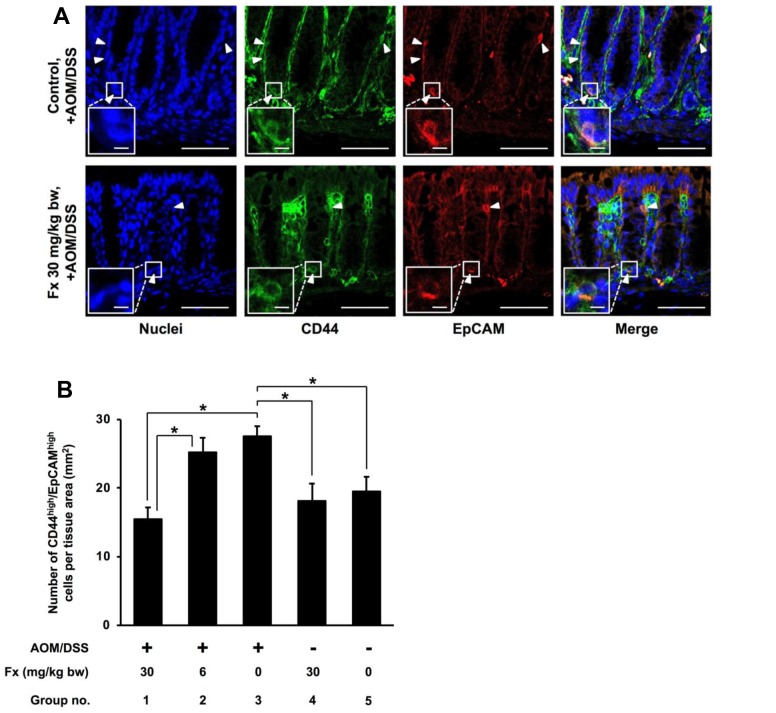
Effect of Fx on colorectal cancer stem cell-like cells in AOM/DSS mice. We evaluated the number of CCSC-like CD44high/EpCAMhigh cells in colonic mucosal crypts for AOM/DSS mice by fluorescent immunohistochemistry (Figure 3A and B). CCSC-like cells showed a remarkably strong fluorescence for both CD44 and EpCAM proteins (CD44high/EpCAMhigh) in the colonic crypt cells. The CD44high/EpCAMhigh cells in group-1 were significantly lower (0.6-fold, p=0.001087) compared to those cells in control AOM/DSS mice (group 3), and to the cells from groups 4 and 5. A significant difference in the number of CD44high/ EpCAMhigh cells was observed between groups 1 and 2 (p=0.017064), but none was found between groups 2 and 3.
Figure 3. Detection of colorectal cancer stem cells-like CD44high/EpCAMhigh cells in AOM/DSS mice with or without Fx administration. The blocked section was co-probed with CD44 conjugated with Alexa Fluor 488 and EpCAM conjugated with APC primary antibodies. The tissue was stained with ProLong Gold Antifade reagent containing DAPI. The nuclei (blue fluorescence), CD44 (green fluorescence), and EpCAM (red fluorescence) were observed by confocal microscopy. (A) Arrow heads show cells having high expression of CD44 and EpCAM: CD44high/EpCAMhigh cells. Long and short bars are 50 and 5 μm, respectively. (B) The number of CD44high/EpCAMhigh cells per tissue area (mm2) was counted under confocal microscopy. Means±SE (n=6). (*) p<0.05 by one-way ANOVA with post-hoc Tukey-Kramer test.
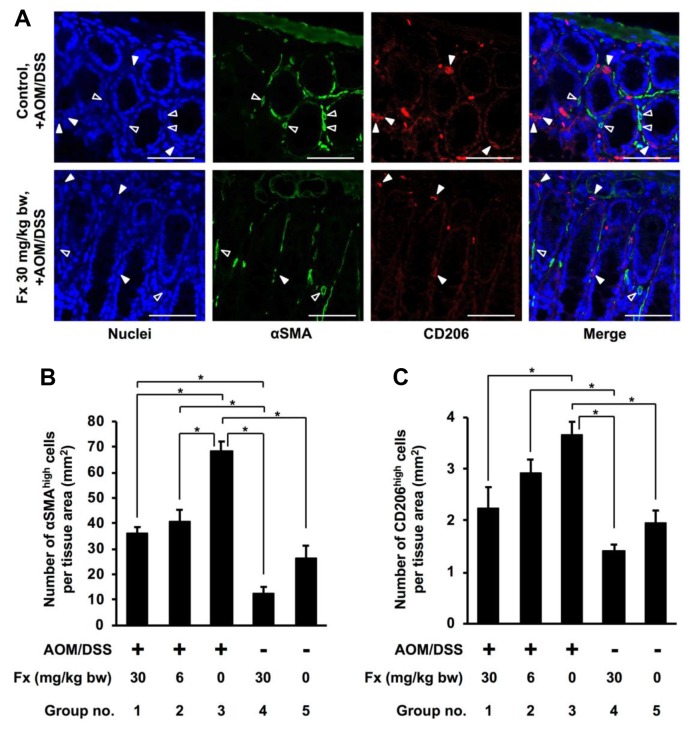
Effect of Fx on cancer-associated fibroblasts-, tumor-associated macrophage- and dendritic cell-like cells in AOM/DSS mice. Next, we investigated the accumulation of CAF-like α-SMAhigh cells, and TAM- and DC-like CD206high cells in the colonic mucosal stroma of AOM/DSS mice (Figures 4A-C). CAF-like cells showed a remarkably strong fluorescence of α-SMA (α-SMAhigh). Inflammatory tumor-associated macrophages (TAMs) or dendritic cells (DC)-like cells with a remarkably strong fluorescence of the CD206 protein (CD206high) were seen surrounding the colonic crypts. The α-SMAhigh and CD206high cells existed in the surrounding area of the mucosal crypts (Figure 4A). The α-SMAhigh cells in groups 1 and 2 that were treated with Fx, were significantly lower by 0.5- and 0.6-fold, respectively, compared to those in group 3 of the control AOM/DSS mice (p=0.000169, p=0.000920, respectively). The number of α-SMAhigh cells in groups 1 and 2 was, however, significantly higher compared to cells in group 4 (p=0.000910, p=0.000127, respectively). The CD206high cells treated with 30 mg/kg of FX in group 1 were significantly decreased by 0.6-fold compared to those cells in the control AOM/DSS mice (group 3) (p=0.010422), whereas no difference in cell numbers was found between groups 4 and 5. Moreover, significant difference was observed between groups 2 and 4 (p=0.010799), but not between groups 2 and 3.
Figure 4. Detection of cancer-associated fibroblasts cells-like αSMAhigh cells, and tumor-associated macrophages- and dendritic cells-like CD206high cells in AOM/DSS mice with or without Fx administration. The nuclei (blue fluorescence), α-SMA (green fluorescence), and CD206 (red fluorescence) were observed under a confocal microscope. (A) Arrow heads show cells having high expression of αSMA (αSMAhigh cells, open arrowheads) and CD206 (CD206high cells, closed arrowheads). Bars are 50μm. The number of (B) CD44high/EpCAMhigh cells and (C) CD206high cells per tissue area (mm2) were counted under the confocal microscope. Means±SE (n=6). (*) p<0.05 by one-way ANOVA with post-hoc Tukey-Kramer test.
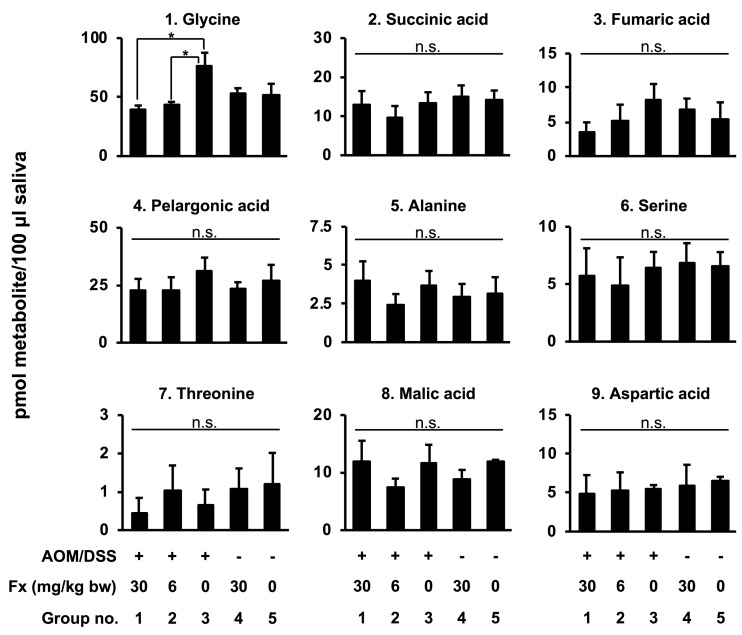
Changes of salivary metabolites in AOM/DSS mice by Fx. Quantitative data of the salivary metabolite profiles in Fx-administrated AOM/DSS mice were examined by GC-MS (Figure 5). Of the nine metabolites detected, only glycine was significantly lower in the Fx-treated mice from groups 1 and 2 compared to group 3 control mice (p=0.020104, p=0.043912, respectively), while it was at similar levels for the mice in groups 4 and 5: i) group 1: 39.3±3.6, ii) group 2: 43.4±2.6, iii) group 3: 76.0±11.6, iv) group 4: 52.4±5.1, v) group 5: 52.0±9.3 pmol.
Figure 5. Effects of Fx on salivary metabolite content in AOM/DSS mice. Mouse saliva was collected after pilocarpine injection under anesthesia, and metabolites were analyzed by GC-MS. Mean±SE (n=6). (*) p<0.05 by one-way ANOVA with post-hoc Tukey-Kramer test.
Discussion
Our results suggest that Fx administration suppresses the formation of colonic polyps and TME in AOM/DSS mice during a cancer preclinical condition. In addition, glycine was found to be a prognostic indicator representing the chemopreventive efficacy of Fx in this cancer mouse model. This is the first report suggesting a salivary indicator related to TME attenuation in cancer mouse models treated with Fx. In this study, many CCSC-like CD44high/EpCAMhigh cells developed in the colorectal crypts of the AOM/DSS control mice that received no treatment (group 3), but were significantly lower in the same mouse model treated with
30 mg/kg Fx (group 1).
As the formation of TME could change the progression of tumorigenesis, we evaluated how the treatment with Fx may affect the number of CAF-, TAM- and DC-like cells. Accumulating evidence has demonstrated that CAFs are largely composed of elements of TME and facilitate cancer stemness, tumor growth, invasion, metastasis, epithelial-mesenchymal transition (EMT), angiogenesis, as well as alterations in the extracellular matrix, tumor immunity and TME metabolome (33-35). CAFs are characterized by high levels of α-SMA that is distinctive for the myofibroblast differentiation phenotype and is regarded as a prognostic marker of human CRC (36,37). We found that Fx treatment at both doses of 30 and 6 mg/kg was accompanied by an accumulation of CAF-like α-SMAhigh cells in the mucosal stroma of neighboring colonic crypts to a similar degree. Other groups have previously indicated that α-SMA-positive CAFs spread to adjacent CRC tissue of AOM/DSS-induced and/or in genetically-modified mouse cancer models, as compared to control mice (38,39). Targeting cellular components of CAFs can contribute to the reduction of tumor development and CCSC function. For example, IKKβ, a potential activator of a pro-tumorigenic form of NF-ĸB, has been shown to originate from CAFs in colorectal cancer mouse models (38). Oral administration of regorafenib, a multikinase inhibitor, has been found to attenuate the interaction between human KM125M CRC cells and stromal cells (including CAFs), and inhibit the metastasis of CRC cells, angiogenesis and lymphangiogenesis in nude mice (40). Both genetic and pharmacological inhibitions of a fibroblast activation protein (FAP), which can be used as a cell surface CAF marker, suppressed tumor growth derived from mouse CT26 CRC cells in an allogeneic transplant mouse model (41). Treatment by a vitamin A analogue of retinoic acid appears to inhibit the migration and EMT of pancreatic cancer cells through low levels of secreted IL-6 from CAFs (42). Curcumin, a phenol contained in turmeric, disrupts the cell-cell interactions of CCSCs-like Csps from human HCT116 CRC cells and normal human MRC-5 fibroblast cells mediated by the suppression of the TGF-β3, NFĸB, matrix metalloprotease-13, CCSC cell surface markers and of EMT-related molecules (43).
In this study, we have also detected TAM- and DC-like cells, using a cell surface marker of CD206 in mouse colonic mucosa, which is commonly overexpressed in TAMs and DCs (44,45). TAMs, a type of M2-phenotype macrophage, and DCs, are both pivotal components of TME and promote cancer initiation, tumor development, invasion, inflammation, angiogenesis, while they can cause immunosuppression (24,25,44,46). CRC initiation and development are elicited by many molecular regulations from TAMs and DCs via their chemokines, cytokines, modifications of extracellular matrix (24,25). In this study, Fx administration, particularly at the high dose of 30 mg/kg had a suppressive effect on the number of TAMs- and DCs-like CD206high cells present in the mucosal stroma of the mice.
Limited molecular information regarding TAMs and DCs is currently available for the Fx-treated TME. A synthetic retinoid derivative, fenretinide, suppresses TAMs polarization involved in CRC progression through the inhibition of STAT6 phosphorylation, and thus prevents CRC in Apcmin/+ mice (47). Isoliquiritigenin, a flavonoid from licorice, has been found to also suppress CRC development in AOM/DSS mice with the attenuation of TAMs polarization mediating the downregulation of PGE2 and IL-6 (48). Finally, STAT3-inactivated DCs promote anti-tumor immune responses and suppress tumor growth in an allogeneic mouse model injected with mouse MC38 CRC cells, compared to mice injected with control DCs (49).
Many salivary metabolites obtained from oral, breast and pancreatic cancer patients are shown to be much higher as compared to healthy individuals (50), however, to date, there is little evidence on the role of these metabolites isolated from the saliva of cancer models. Our previous studies suggest that the low levels of glycine in the tumor and the high levels of glycine, glutamic acid and succinic acid in the saliva, could be metabolite indicators predicting a delay in the tumor onset in Csps-xenograft mice treated with Fx (31). In the present study only glycine, among the nine metabolites identified in the saliva of AOM/DSS mice, was significantly lower when mice were treated with Fx. Thus, salivary glycine could be a candidate marker indicating the chemopreventive efficacy of Fx. It is possible that the changes observed in salivary metabolites may be related to the dose and the duration of Fx administration, the saliva sampling method, the mouse strain, and the cancer model.
In summary, we found that Fx significantly suppresses the number of colorectal polyps and the formation of TME, as evaluated by the different numbers of CCSCs-, CAFs-, TAMs- and DCs-like cells in AOM/DSS mice at a cancer preclinical condition, with or without treatment with Fx, along with low levels of salivary glycine. Our results imply that salivary glycine may be a predictor associated with the chemopreventive efficacy of Fx in this cancer model. Further studies are needed to confirm the diagnostic usefulness of salivary glycine and possibly other such metabolites for cancer chemoprevention in additional cancer animal models following their treatment with Fx
Conflicts of Interest
The Authors declare no competing financial interest.
Authors Contributions
M. Terasaki conceived and designed the study. M. Terasaki, S. Masaka, C. Fukada, M. Houzaki and T. Tanaka performed the experiments. M. Terasaki and M. Mutoh wrote the paper. T. Endo, H. Maeda and K. Miyashita reviewed the edited the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
This work was supported in part by the Japan Society for the Promotion of Science KAKENHI (no. 16K07880).
References
- 1.Terasaki M, Hirose A, Narayan B, Baba Y, Kawagoe C, Yasui H, Saga N, Hosokawa M, Miyashita K. Evaluation of recoverable functional lipid components of several brown seaweeds (phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. J Phycol. 2009;45(4):974–980. doi: 10.1111/j.1529-8817.2009.00706.x. PMID: 27034228 doi: 10.1111/j.1529-8817.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 2.Miyashita K, Nishikawa S, Beppu F, Tsukui T, Abe M, Hosokawa M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J Sci Food Agric. 2011;91:1166–1174. doi: 10.1002/jsfa.4353. PMID: 21433011 doi: 10.1002/jsfa.4353. [DOI] [PubMed] [Google Scholar]
- 3.Mikami K, Hosokawa M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int J Mol Sci. 2013;14:13763–13781. doi: 10.3390/ijms140713763. PMID: 23820585 doi: 10.3390/ijms140713763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terasaki M, Narayan B, Kamogawa H, Nomura M, Stephen NM, Kawagoe C, Hosokawa M, Miyashita K. Carotenoid profile of edible Japanese seaweeds: an improved HPLC method for separation of major carotenoids. J Aquatic Food Prod Tech. 2012;21:468–479. doi: https://doi.org/10.1080/10498850.2011. 610025. [Google Scholar]
- 5.Beppu F, Niwano Y, Tsukui T, Hosokawa M, Miyashita K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J Toxicol Sci. 2009;34:501–510. doi: 10.2131/jts.34.501. PMID: 19797858. [DOI] [PubMed] [Google Scholar]
- 6.Iio K, Okada Y, Ishikura M. Single and 13-week oral toxicity study of fucoxanthin oil from microalgae in rats. Shokuhin Eiseigaku Zasshi. 2011;52:183–189. doi: 10.3358/shokueishi.52.183. PMID: 21720124. [DOI] [PubMed] [Google Scholar]
- 7.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: source, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. PMID: 25220842 doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund/American Institute for Cancer Research, Food, Nutrition, Physical Activity and the Prevention of Cancer:a Global Perspective. AICR. 2007:280–288. ISBN: 978-0-9722522-2-5. [Google Scholar]
- 9.Kim JM, Araki S, Kim DJ, Park CB, Takasuka N, Baba-Toriyama H, Ota T, Nir Z, Khachik F, Shimidzu N, Tanaka Y, Osawa T, Uraji T, Murakoshi M, Nishino H, Tsuda H. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis. 1998;19:81–85. doi: 10.1093/carcin/19.1.81. PMID: 9472697. [DOI] [PubMed] [Google Scholar]
- 10.Nishino H, Murakoshi M, Tokuda H, Satomi Y. Cancer prevention by carotenoids. Arch Biochem Biophys. 2009;483:165–168. doi: 10.1016/j.abb.2008.09.011. PMID: 18848517 doi: 10.1016/j.abb.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Mei CH, Zhou SC, Zhu L, Ming JX, Zeng FD, Xu R. Antitumor effects of Laminaria extract fucoxanthin on lung cancer. Mar Drugs. 2017;15:39–50. doi: 10.3390/md15020039. PMID: 28212270 doi: 10.3390/md15020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K, Hosokawa M, Kasajima H, Hatanaka K, Kudo K, Shimoyama N, Miyashita K. Anticancer effects of fucoxanthin and fucoxanthinol on colorectal cancer cell lines and colorectal cancer tissues. Oncol Lett. 2015;10:1463–1467. doi: 10.3892/ol.2015.3380. PMID: 26622691 doi: 10.3892/ol.2015.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terasaki M, Maeda H, Miyashita K, Mutoh M. Induction of anoikis in human colorectal cancer cells by fucoxanthinol. Nutr Cancer. 2017;69:1043–1052. doi: 10.1080/01635581.2017.1339814. PMID: 28990814 doi: 10.1080/ 01635581.2017.1339814. [DOI] [PubMed] [Google Scholar]
- 14.Das SK, Hashimoto T, Kanazawa K. Growth inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is associated with down-regulation of cyclin D. Biochim Biophys Acta. 2008;1780:743–749. doi: 10.1016/j.bbagen.2008.01.003. PMID: 18230364 doi: 10.1016/ j.bbagen.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Jin Y, Qiu S, Shao N, Zheng J. Fucoxanthin and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically promotes apoptosis of human cervical cancer cells by targeting PI3K/Akt/NF-ĸB signaling pathway. Med Sci Monit. 2018;24:1–18. doi: 10.12659/MSM.905360. PMID: 29291370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu RX, Yu RT, Liu Z. Inhibition of two gastric cancer cell lines induced by fucoxanthin involves downregulation of Mcl-1 and STAT3. Hum Cell. 2018;31:50–63. doi: 10.1007/s13577-017-0188-4. PMID: 29110251 doi: 10.1007/s13577-017-0188-4. [DOI] [PubMed] [Google Scholar]
- 17.Kotake-Nara E, Terasaki M, Nagao A. Characterization of apoptosis induced by fucoxanthin in human promyelocytic leukemia cells. Biosci Biotechnol Biochem. 2005;69:224–227. doi: 10.1271/bbb.69.224. PMID: 15665492 doi: 10.1271/bbb.69.224. [DOI] [PubMed] [Google Scholar]
- 18.Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci. 2018;6:31–143. doi: 10.3390/medsci6020031. PMID: 29652830 doi: 10.3390/medsci6020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. PMID: 17548814 doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Alea MP, Richel DJ, Stassi G, Medema JP. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. PMID: 18765800 doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanwar SS, Yu Y, Nautiyal J, Patel BB, Majumdar AP. The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol Cancer. 2010;9:212–224. doi: 10.1186/1476-4598-9-212. PMID: 20691072 doi: 10.1186/1476-4598-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terasaki M, Maeda H, Miyashita K, Tanaka T, Miyamoto S, Mutoh M. A marine bio-functional lipid, fucoxanthinol, attenuates human colorectal cancer stem-like cell tumorigenicity and sphere formation. J Clin Biochem Nutr. 2017;61:25–32. doi: 10.3164/jcbn.16-112. PMID: 28751806 doi: 10.3164/jcbn.16-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318–326. doi: 10.1038/nature10212. PMID: 21677748 doi: 10.1038/nature10212. [DOI] [PubMed] [Google Scholar]
- 24.Mariani F, Sena P, Roncucci L. Inflammatory pathways in the early steps of colorectal cancer development. World J Gastroenterol. 2014;20:9716–9731. doi: 10.3748/wjg.v20.i29.9716. PMID: 25110410 doi: 10.3748/wjg.v20.i29.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legitimo A, Consolini R, Failli A, Orsini G, Spisni R. Dendritic cell defects in the colorectal cancer. Hum Vaccin Immunother. 2014;10:3224–3235. doi: 10.4161/hv.29857. PMID: 25483675 doi: 10.4161/hv.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 324:1029–1033. doi: 10.1126/science.1160809. PMID: 19460998 doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida M, Hatano N, Nishiumi S, Irino Y, Izumi Y, Takenawa T, Azuma T. Diagnosis of gastroenterological diseases by metabolome analysis using gas chromatography-mass spectrometry. J Gastroenterol. 2012;47:9–20. doi: 10.1007/s00535-011-0493-8. PMID: 22041921 doi: 10.1007/s00535-011-0493-8. [DOI] [PubMed] [Google Scholar]
- 28.Dazard Dazard, JE JE, Sandlers Y, Doerner SK, Berger NA, Brunengraber H. Metabolomics of ApcMin/+ mice genetically susceptible to intestinal cancer. BMC Syst Biol. 2014;8:72–92. doi: 10.1186/1752-0509-8-72. PMID: 24954394 doi: 10.1186/1752-0509-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshie T, Nishiumi S, Izumi Y, Sakai A, Inoue J, Azuma T, Yoshida M. Regulation of the metabolite profile by an APC gene mutation in colorectal cancer. Cancer Sci. 2012;10:1010–1021. doi: 10.1111/j.1349-7006.2012.02262.x. PMID: 22380946 doi: 10.1111/j.1349-7006.2012.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terasaki M, Mima M, Kudoh S, Endo T, Maeda H, Hamada J, Osada K, Miyashita K, Mutoh M. Glycine and succinic acid are effective indicators of the suppression of epithelial-mesenchymal transition by fucoxanthinol in colorectal cancer stem-like cells. Oncol Rep. 2018;40:412–424. doi: 10.3892/or.2018.6398. PMID: 29693702 doi: 10.3892/or.2018.6398. [DOI] [PubMed] [Google Scholar]
- 31.Terasaki M, Matsumoto N, Hashimoto R, Endo T, Maeda H, Hamada J, Osada K, Miyashita K, Mutoh M. Fucoxanthin administration delays occurrence of tumors in xenograft mice by colonospheres, with an anti-tumor predictor of glycine. J Clin Nutr Biochem. 2019:52–58. doi: 10.3164/jcbn.18-45. doi: https://doi.org/10.3164/jcbn.18-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Center for Experimental Animals. Center for Experimental Animals: The guidelines for the care and use of laboratory animals. Health Sciences University of Hokkaido. 2009 [Google Scholar]
- 33.Chen WJ, Ho CC, Chang YL, Chen HY, Lin CA, Ling TY, Yu SL, Yuan SS, Chen YJ, Lin CY, Pan SH, Chou HY, Chen YJ, Chang GC, Chu WC, Lee YM, Lee JY, Lee PJ, Li KC, Chen HW, Yang PC. Cancer-associated fibroblasts regulate the platicity of lung cancer stemnessviaparacrine signaling. Nat Commun. 2014;5:3472–3488. doi: 10.1038/ncomms4472. PMID: 24668028 doi: 10.1038/ ncomms4472. [DOI] [PubMed] [Google Scholar]
- 34.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev. 2006;6:392–401. doi: 10.1038/nrc1877. PMID: 16572188 doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 35.Valencia T, Kim JY, Abu-Baker S, Moscat-Pardos J, Ahn CS, Reina-Campos M, Duran A, Castilla EA, Metallo CM, Diaz-Meco MT, Moscat J. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell. 2014;26:121–135. doi: 10.1016/j.ccr.2014.05.004. PMID: 25002027 doi: 10.1016/j.ccr.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med. 2002;126:829–836. doi: 10.5858/2002-126-0829-ISOMIN. PMID: 12088453 doi: 10.1043/0003-9985(2002)126<0829: ISOMIN>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Ikuta D, Miyake T, Shimizu T, Sonoda H, Mukaisho KI, Tokuda A, Ueki T, Sugihara H, Tani M. Fibrosis in metastatic lymph nodes is clinically correlated to poor prognosis in colorectal cancer. Oncotarget. 2018;9:29574–29586. doi: 10.18632/oncotarget.25636. PMID: 30038705 doi: 10.18632/oncotarget.25636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koliaraki V, Pasparakis M, Kollias G. IKKβ in intestinal mesenchymal cells promotes initiation of colitis-associated cancer. J Exp Med. 2015;212:2235–2251. doi: 10.1084/jem.20150542. PMID: 26621453 doi: 10.1084/jem.20150542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres S, Bartolomé RA, Mendes M, Barderas R, Fernandez-Aceñero MJ, Peláez-García A, Peña C, Lopez-Lucendo M, Villar-Vázquez R, de Herreros AG, Bonilla F, Casal JI. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin Cancer Res. 2013;19:6006–6019. doi: 10.1158/1078-0432.CCR-13-1130. PMID: 24025712 doi: 10.1158/1078-0432.CCR-13-1130. [DOI] [PubMed] [Google Scholar]
- 40.Takigawa H, Kitadai Y, Shinagawa K, Yuge R, Higashi Y, Tanaka S, Yasui W, Chayama K. Multikinase inhibitor regorafenib inhibits the growth and metastasis of colon cancer with abundant stroma. Cancer Sci. 2016;107:601–608. doi: 10.1111/cas.12907. PMID: 26865419 doi: 10.1111/cas.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos AM, Jung J, Aziz N, Kissil JL, Puré E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119:3613–3625. doi: 10.1172/JCI38988. PMID: 19920354 doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan J, Zhang H, Wen Z, Gu Y, Cheng Y, Sun Y, Zhang T, Jia C, Lu Z, Chen J. Retinoic acid inhibits pancreatic cancer cell migration and EMT through the downregulation of IL-6 in cancer associated fibroblast cells. Cancer Lett. 2014;345:132–139. doi: 10.1016/j.canlet.2013.12.006. PMID: 24334138 doi: 10.1016/j.canlet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Buhrmann C, Kraehe P, Lueders C, Shayan P, Goel A, Shakibaei M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: potential role of EMT. PLos One. 2014;9:e107514–e107514. doi: 10.1371/journal.pone.0107514. PMID: 25238234 doi: 10.1371/journal.pone.0107514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katara GK, Kulshrestha A, Jaiswal MK, Pamarthy S, Gilman-Sachs A, Beaman KD. Inhibition of vacuolar ATPase subunit in tumor cells delays tumor growth by decreasing the essential macrophage population in the tumor microenvironment. Oncogene. 2016;35:1–58-1065. doi: 10.1038/onc.2015.159. PMID: 25961933 doi: 10.1038/ onc.2015.159. [DOI] [PubMed] [Google Scholar]
- 45.Steinman RM, Jacques B. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. PMID: 17898760 doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng Q, Wang H, Chen J, Wang H. Tumor-associated macrophages recruit CCR6+regulatory T cells and promote the development of colorectal cancerviaenhancing CCL20 production in mice. PLos One. 2011;6:e19495–e19495. doi: 10.1371/journal.pone.0019495. PMID: 21559338 doi: 10.1371/ journal.pone. 0019495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong R, Gong Y, Meng W, Yuan M, Zhu H, Ying M, He Q, Cao J, Yang B. The involvement of M2 macrophage polarization inhibition in fenretinide-mediated chemoprevention effects on colon cancer. Cancer Lett. 2017;388:43–53. doi: 10.1016/j.canlet.2016.11.029. PMID: 27913199 doi: 10.1016/j.canlet.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 48.Zhao H, Zhang X, Chen X, Li Y, Ke Z, Tang T, Chai H, Guo AM, Chen H, Yang J. Isoliquiritigenin, a flavonoid from licorice, blocks M2 macrophage polarization in colitis-associated tumorigenesis through downregulating PGE2 and IL-6. Toxicol Appl Pharmacol. 2014;279:311–321. doi: 10.1016/j.taap.2014.07.001. PMID: 25026504 doi: 10.1016/j.taap.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Iwata-Kajihara T, Sumimoto H, Kawamura N, Ueda R, Takahashi T, Mizuguchi H, Miyagishi M, Takeda K, Kawakami Y. Enhanced cancer immunotherapy using STAT3-depleted dendritic cells with high Th1-inducing ability and resistance to cancer cell-derived inhibitory factors. J Immunol. 2011;187:27–36. doi: 10.4049/jimmunol.1002067. PMID: 21632716 doi: 10.4049/ jimmunol. 1002067. [DOI] [PubMed] [Google Scholar]
- 50.Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010;6:78–95. doi: 10.1007/s11306-009-0178-y. PMID: 20300169 doi: 10.1007/s11306-009-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]