Abstract
Objective:
Further prospective study is needed to elucidate the etiology and natural history of systemic lupus erythematosus (SLE) development. The clinical complexity of this heterogeneous disease makes study design challenging. Our objective was to ascertain useful screening factors for identifying at-risk individuals for follow-up rheumatologic assessment or inclusion in prospective studies.
Methods:
We attempted to re-contact 3823 subjects with a family history of SLE, who did not meet American College of Rheumatology (ACR) SLE classification at a baseline study visit; 436 agreed to follow-up participation an average of 6.3 years after baseline. Fifty-six of these individuals had transitioned to classified SLE (≥ 4 cumulative ACR criteria, verified by medical record review) by the time of follow-up. Generalized estimating equations assessed associations between our dichotomous outcome of transitioning to SLE with baseline characteristics, including anti-nuclear antibody (ANA) positivity, Connective Tissue Disease Screening Questionnaire SLE score (SLE-CSQ), and number of ACR criteria. We analyzed predictive accuracy of characteristics on transitioning.
Results:
ANA positivity, SLE-CSQ categorization of possible or probable SLE (SLE-CSQ+), and greater number of ACR criteria at baseline were each associated with transitioning to SLE classification. Being ANA positive and having confirmed immunologic ACR criteria at baseline had the highest PPV and specificity for transitioning to SLE. SLE-CSQ+ had a better PPV, NPV, sensitivity and specificity than ANA positivity.
Conclusion:
Given limited resources, identifying individuals for follow-up based on the SLE portion of the CSQ questionnaire could be an efficient way to identify family members at highest risk of disease transition.
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by autoantibody production and chronic inflammation that can lead to tissue and end-organ damage. The etiology and pathogenesis of SLE remains poorly understood, but a combination of genetic and environmental risk factors are likely required. More than 100 loci have been identified through candidate gene and genome-wide association studies that individually confer a modest risk of SLE 1, 2; genetic factors alone are not usually sufficient for development of SLE, and environmental factors likely play an additional role 3–7. SLE is characterized by a prolonged pre-classification phase during which time individuals develop and accumulate pathogenic RNA- and DNA-protein binding autoantibody specificities 8, as well as symptoms/signs of SLE, but do not meet American College of Rheumatology (ACR) 9 disease classification 10, 11. Several pre-classification cohorts to evaluate potential SLE risk factors have focused on clinical and serological or cellular features of SLE through retrospective medical chart review 12, retrospective sample analysis from SLE patients taken prior to disease classification 8, 13, and prospectively following cohorts of individuals with undifferentiated connective tissue disease (UCTD) 14–21 or incomplete lupus erythematosus (ILE) 22–24 who develop SLE. However, these studies have yet to determine the individuals at highest risk of transitioning to SLE classification.
While beneficial to understanding the etiology of SLE, the retrospective nature of monitoring individuals close to transition to SLE may not allow one to study the full pathogenesis of disease, particularly those early in the disease process in absence of immune modifying agents that may confound the detection of pathogenic features and mechanisms of SLE. Additionally, in prevention trials it may be too difficult for mild medications to re-set an immune system with ongoing inflammation and organ involvement; in addition, such trials may require therapies with too high of toxicity and cost for use in otherwise “healthy” individuals. Prospective cohorts of at-risk individuals to identify risk factors of disease development have been demonstrated in type 1 diabetes 25, 26 and rheumatoid arthritis 27. Use of a similar cohort in the study of SLE will allow for a more complete understanding of the etiologic process in those most at risk of transitioning to SLE.
Family members of individuals with SLE are at increased risk of SLE themselves. First-degree relatives have a 17-fold greater risk of SLE than the general population 28. Risk of SLE in siblings is estimated to be up to 29-fold greater than that of the general population 29, suggesting family members of individuals with SLE would be a useful at-risk population to follow for natural history or prevention studies. However, additional data are needed to inform the study design in terms of who to target for follow-up in order to identify the greatest number of SLE cases as efficiently as possible. Using data from the baseline and follow-up visits of participants in the Lupus Family Registry and Repository (LFRR) 30, we elucidated study participant characteristics that identify lupus relatives at highest risk of disease transition for inclusion in natural history studies or prevention trials.
Materials and methods
Participant recruitment
All protocols were approved by the Institutional Review Boards at the Oklahoma Medical Research Foundation, the Medical University of South Carolina, and the University of Colorado Denver. Study participants were recruited from the LFRR 30, which included individuals in the Systemic Lupus Erythematosus in Gullah Health (SLEIGH) 31 cohort. The LFRR is an established research registry of SLE patients and their family members that is designed to characterize the genetic and environmental risk factors of SLE. The proband for the LFRR is defined as the first SLE patient in the family to enter the study. Upon enrollment in the LFRR (baseline visit occurred between 1992 and 2011), detailed demographic, environmental, clinical, and therapeutic data were collected by questionnaires, and a blood sample was obtained. Consent to obtain medical records was provided by study participants; medical record review was conducted in SLE patients and in those unaffected family members who self-reported SLE signs or symptoms, to determine whether they met SLE disease classification as determined by the American College of Rheumatology (ACR) 9 SLE classification criteria. In 6.7% of the family members, the LFRR did not obtain medical records to confirm SLE classification of the SLE patient proband due to family estrangement or study-related decisions based on competing priorities.
Individuals who reported having a relative with SLE and who did not meet ≥ 4 ACR criteria for SLE at their prior (baseline) visit were invited to enroll in a follow-up study to gather information regarding interim development of signs and symptoms consistent with SLE. Follow-up contact occurred between March 2010 and May 2012 and the mean time between baseline and follow-up was 6.3 ± 3.9 years. Two phases of recruitment letters were sent to LFRR participants. For the first phase, we identified 3544 individuals who reported a family member with SLE classification that was confirmed by medical record review and sent letters asking them to participate in a follow-up visit. For the second phase, we identified 279 individuals for whom their family member’s SLE classification was not confirmed (which is why they were not contacted in the first phase), preferentially selected those that had 2–3 ACR criteria at their baseline visit, and sent letters asking them to participate in follow-up. A single letter was sent to each address. If an address came back as invalid, open access online resources were searched for a valid address. Individuals who responded to the letter completed a short telephone interview to confirm their eligibility, to determine willingness to complete required questionnaires and provide blood samples, and to answer questions about signs and symptoms of SLE.
Of the 3823 family members who were sent letters, we received no response from 3147 (82.6%), a notification of an invalid address (with no access to an updated address) in 66 (1.7%), and a request for no further contact in 2 (0.001%). Of the 608 (15.9%) that participated in the screening interview, 165 (4.3%) declined (either actively or passively) to participate; 7 (0.2%) were determined to be ineligible due to < 6 months between their baseline and follow-up visits; and 436 (375 from the first phase and 61 from the second phase) returned the questionnaires and samples (Figure 1).
Figure 1. Flow diagram of recruitment.
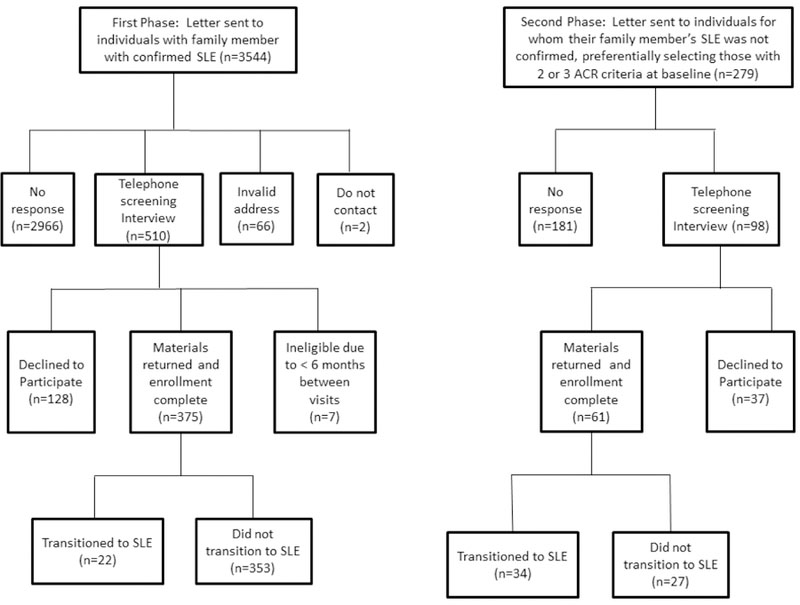
Individuals who reported a family member with SLE were sent letters requesting participation in a follow-up study examining factors associated with transitioning to SLE. The first wave of letters were sent to individuals with family member with SLE that was confirmed by medical records. The second wave of letters were sent to individuals for whom their family member’s SLE was not confirmed, preferentially selecting individuals who had 2 or 3 confirmed ACR criteria at their baseline visit.
Compared to those who did not provide follow-up information, family members enrolled in the follow-up study were younger, had shorter time to follow-up, and were more likely to be female and European American (p<0.0001 for all) (Table 1). Those who enrolled in the follow-up study were more likely to have one or more baseline ACR criteria, and a higher proportion scored as possible or probable SLE on the CSQ at baseline than those who did not provide follow-up information (Table 1).
Table 1.
Baseline Characteristics of Study Subjects by Their Participation in Follow-up
| Characteristic Collected at the Baseline Visit (N, %) | Study Subjects Who Who Participated in Follow-up N=436 |
Study Subjects Who Did Not Participate in Follow-up N=3387 |
p-value |
|---|---|---|---|
| Age at baseline in years: mean (median) ± sd | 47.2 (48.0) ± 15.3 | 51.7 (52.0) ± 17.2 | <0.0001 |
| Time between contact in years: mean (median) ± sd | 6.1 (5.0) ± 3.9 | 8.0 (8.0) ± 4.1 | <0.0001 |
| Sex: Female | 365 (83.7%) | 2265 (66.9%) | <0.0001 |
| Race: European American | 323 (73.9%) | 1793 (52.9%) | <0.0001 |
| African American | 64 (14.7%) | 835 (24.7%) | |
| Native American | 21 (4.8%) | 185 (5.5%) | |
| Asian/Pacific Islander | 17 (3.9%) | 101 (3.0%) | |
| Hispanic | 11 (2.5%) | 446 (13.2%) | |
| Unknown | 0 (0.0%) | 27 (0.8%) | |
| Relationship to SLE Proband: | <0.0001 | ||
| First Degree Relative (FDR): | |||
| Sister | 195 (44.7%) | 1119 (33.0%) | |
| Brother | 31 (7.1%) | 521 (15.4%) | |
| Child | 29 (6.6%) | 158 (4.7% ) | |
| Parent | 147 (33.7%) | 1406 (41.5%) | |
| Second Degree Relative (SDR) | |||
| or more distant relative | 34 (7.8%) | 32 (0.9%) | |
| ANA Positive | 226 (51.8%) | 1704 (50.3%) | 0.55 |
| SLE-CSQ Categories at Baselinea: | <0.0001 | ||
| Unlikely SLE | 253 (58.0%) | 2444 (63.9%) | |
| Possible SLE | 45 (10.3%) | 304 (9.0%) | |
| Probable SLE | 138 (31.7%) | 639 (18.9%) | |
| Number of ACR Criteria at Baselineb: | <0.0001 | ||
| 0 | 144 (33.0%) | 3027 (89.4%) | |
| 1 | 154 (35.3%) | 47 (1.4%) | |
| 2 | 97 (22.3%) | 246 (7.2%) | |
| 3 | 41 (9.4%) | 67 (2.0%) | |
ANA, anti-nuclear antibodies; CSQ, Connective Tissue Disease Screening Questionnaire; ACR, American College of Rheumatology.
SLE-CSQ categories are based on responses to the SLE portion of the CSQ questionnaire. Twenty-eight questions were grouped into 12 categories. If the person indicated that < 3 categories were true, then a person was classified as unlikely SLE. If three of the 12 categories were marked as true, then the person was classified as possible SLE. If the person indicated that at least four of the 12 categories were true, then the person was classified as probable SLE.
Confirmed ACR criteria by medical record.
Data collection
Identical questionnaires and blood tests were completed at baseline and follow-up. Detailed demographic, environmental, clinical, and therapeutic information was collected by questionnaire. In addition, participants completed the Connective Tissue Disease Screening Questionnaire (CSQ) 32, a validated 30-item instrument designed to identify individuals with potential connective tissue disease. The SLE portion of the CSQ (SLE-CSQ) was scored using an algorithm based on ACR classification criteria 32. Twenty-eight questions were grouped into 12 categories. If < 3 SLE-CSQ categories were reported, then a person was categorized as unlikely SLE. If 3 of the 12 categories were reported, then a person was categorized as possible SLE. If 4 or more of the 12 categories were reported, then a person was categorized as probable SLE.
Boxes containing sample collection tubes were sent to all participants. Participants could then have their blood drawn at their personal physician’s office, designated collection sites, or at the LFRR study site. Samples were then mailed using pre-paid boxes to the OMRF Biorepository. To ensure identical sample processing, samples collected at the LFRR site were left out at room temperature overnight to simulate the shipping process of the other samples. Blood samples were processed for serum, plasma, peripheral blood mononuclear cells and DNA 30.
Autoantibody assays
Antinuclear antibodies (ANA) (positive titer ≥1:120) were detected using an indirect immunofluorescence of HEp-2 cells and anti-dsDNA autoantibodies (positive titer ≥1:30) were detected using Crithidia luciliae according to manufacturer instructions (INOVA Diagnostics, San Diego, California, USA). Precipitin levels of Ro, La, Sm, nRNP, and ribosomal P autoantibodies were detected by immunodiffusion and anti-cardiolipin IgG and IgM responses by ELISA as previously described 33. All autoantibody assays were performed in the CAP/CLIA certified OMRF Clinical Immunology Laboratory.
Definition of Outcomes
Consent to obtain medical records at follow-up was provided by study participants. Medical records were obtained and reviewed if 1) the individual reported a diagnosis of SLE, 2) the individual was scored as having possible or probable SLE based on the SLE-CSQ 32, or 3) the individual self-reported having ≥ 3 ACR SLE criteria. Information regarding clinical and laboratory features for each case was obtained by medical record review and collected on a standard data collection form by a rheumatologist or a rheumatology-trained nurse or physician assistant. Clinical manifestations evaluated in this protocol were determined according to SLE classification criteria set by the ACR9, using stringent documentation requirements. Evidence of SLE ACR classification criteria were categorized as: 0 = no evidence; 1 = patient-reported evidence; 2 = physician reported evidence not confirmed by physical examination findings, and 3 = physician observed evidence documented in the medical record. Only SLE ACR criteria meeting the category 3 designation were considered as confirmed ACR criteria for this study. At follow-up, 147 out of the 436 participants had medical record review.
As per ACR guidelines9, medical record verified ACR classification criteria accumulated towards the total number of ACR criteria present; classification criteria did not need to be concurrently present. Individuals were determined to have transitioned to SLE between baseline and follow-up if they had ≥ 4 cumulative ACR criteria confirmed by medical record review at follow-up.
Statistical analyses
All analyses were performed in SAS 9.4 (Cary, NC). Normality of variables was assessed. T-tests for continuous variables and chi-square tests for categorical variables were used to determine differences between individuals who transitioned to SLE and individuals who did not transition to SLE. Correlation between CSQ score and number of ACR criteria and CSQ score and ANA titer was determined by Spearman rank. Within this cohort, individuals within the same family could be enrolled; family size ranged from 1–6 family members, with 23% of our population having at least one other family member enrolled.
Generalized estimating equations, accounting for correlation within families, were used to assess univariate associations between our dichotomous outcome of transitioning to SLE at follow-up with possible demographic and clinical screening characteristics. For Number of ACR criteria, the bottom 2 categories (0 criteria and 1 criterion) were combined for the reference group as no individuals in the transitioned group had 0 ACR criteria at baseline. Odds ratios and 95% confidence intervals were calculated. Select significant characteristics were examined alone and in combination to determine their utility as screening characteristics for following individuals in a prospective study for transitioning to SLE. The positive predictive value (PPV), negative predictive value (NPV), sensitivity and specificity for these characteristics were calculated.
Results
Fifty-six family members (12.8%) had ≥ 4 cumulative ACR classification criteria at the time of follow-up, indicative of transitioning to classified SLE since the baseline visit. Twenty-two (5.9%) transitioned from those enrolled in the first phase of recruitment, and 34 (55.7%) transitioned from those enrolled in the second phase.
Sex, race, age at baseline, attained education at baseline, and time between baseline and follow-up were similar in those who transitioned to SLE compared to those who did not transition in both recruitment phases (Table 2). There was no significant difference in transitioned status by relationship to SLE proband. Education status differed by transition status in the first recruitment phase, but not the second recruitment phase. A larger proportion of ANA positive individuals, individuals classified as possible or probable SLE on the CSQ, and those with a larger number of confirmed ACR criteria at baseline transitioned to SLE in the first recruitment phase, but not the second recruitment phase (Table 2).
Table 2.
Baseline Characteristics of Follow-up Study Population and Odds Ratio of Transitioning to SLE Stratified by Enrollment in First or Second Phase of Recruitmenta.
| First Phase of Recruitment (N=375) | Second Phase of Recruitment (N=61) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable (N, %) | Transitioned to SLE N=22 |
Did Not Transition N=353 |
p-valueb | Unadjusted Orc (95% CI) | Transitioned to SLE N=34 |
Did Not Transition N=27 |
p-valueb | Unadjusted ORc (95% CI) |
| Age at baseline in yearsd: mean (median) ± sd | 47.0 (48.5) ± 13.5 | 47.1 (48.0) ± 16.0 | (0.9) | 1.0 (0.9–1.1) | 47.6 (48.5) ± 11.4 | 48.2 (49.0) ± 12.4 | 0.83 | 1.0 (0.9–1.1) |
| Time between contact in yearsd: mean (median) ± sd | 7.7 (6.7) ± 4.4 | 6.5 (5.8) ± 3.9 | 0.18 | 1.1 (1.0–1.2) | 4.8 (4.6) ± 2.3 | 4.0 (3.1) ± 3.0 | 0.21 | 1.1 (0.9–1.4) |
| Sex: Female | 20 (90.9%) | 293 (83.0%) | 0.33 | 2.1 (0.5–9.1) | 29 (85.3%) | 23 (85.2%) | 0.99 | 0.9 (0.2–3.4) |
| Race: | 0.52 | 0.20 | ||||||
| European American | 17 (77.3%) | 264 (74.8%) | 1 | 26 (76.5%) | 15 (55.6%) | 1 | ||
| African American | 3 (13.6%) | 49 (13.9%) | 0.96 (0.3–3.5) | 6 (17.6%) | 6 (22.2%) | 0.8 (0.3–2.7) | ||
| Othere | 2 (9.1%) | 40 (11.3%) | 0.8 (0.3–2.7) | 2 (5.9%) | 6 (22.2%) | 0.3 (0.06–1.7) | ||
| Relationship to SLE | 0.18 | 0.45 | ||||||
| Proband: | ||||||||
| FDR: Sister | 10 (45.4%) | 166 (47.0%) | 1.3 (0.5–3.8) | 14 (38.2%) | 5 (18.5%) | 2.8 (0.3–25.5) | ||
| Brother | 2 (9.1%) | 27 (7.6%) | 1.7 (0.3–8.6) | 1 (0.3%) | 1 (3.7%) | 1.0 (0.03–29.8) | ||
| Child | 4 (18.2%) | 21 (5.9%) | 4.3 (1.1–16.3) | 3 (8.8%) | 4 (14.8%) | 0.8 (0.06–8.8) | ||
| Parent | 6 (27.3%) | 134 (38.0%) | 1 | 2 (5.9%) | 2 (7.4%) | 1 | ||
| SDR and more distant relative | 0 (0.0%) | 5 (1.4%) | NA | 14 (41.2%) | 15 (55.5%) | 0.9 (0.1–7.6) | ||
| Highest Level of Education Baseline: | 0.008 | 0.74 | ||||||
| > High School | 9 (40.9%) | 234 (66.3%) | 0.3 (0.1–0.7) | 24 (70.6%) | 18 (66.7%) | 1.2 (0.4–3.6) | ||
| ANA Positive Baselinef | 18 (81.8%) | 161 (45.6%) | 0.001 | 5.4 (1.8–16.2) | 25 (73.5%) | 22 (81.5%) | 0.46 | 0.6 (0.2–2.2) |
| SLE-CSQ Categories at Baseline: | <0.0001 | 0.55 | ||||||
| Unlikely SLE | 3 (13.6%) | 245 (69.4%) | 1 | 2 (5.9%) | 3 (11.1%) | 1 | ||
| Possible SLE | 3 (13.6%) | 36 (10.2%) | 6.8 (1.3–34.4) | 2 (5.9%) | 3 (11.1%) | 1.0 (0.1–12.6) | ||
| Probable SLE | 16 (72.7%) | 72 (20.4%) | 18.2 (5.2–63.7) | 30 (88.1%) | 21 (77.8%) | 1.8 (0.3–11.0) | ||
| Number of ACR Criteria at Baseline: | <0.0001 | 0.14 | ||||||
| 0–1g | 6 (27.3%) | 289 (77.1%) | 1 | 1 (2.9%) | 2 (7.4%) | 1 | ||
| 2 | 7 (31.8%) | 56 (15.9%) | 6.0 (2.0–18.4) | 16 (47.1%) | 18 (66.7%) | 0.8 (0.2–3.0) | ||
| 3 | 9 (40.9%) | 8 (2.3%) | 53.7 (16.1–178.6) | 17 (50.0%) | 7 (25.9%) | 2.2 (0.7–6.5) | ||
Significant differences noted in bold. ANA, anti-nuclear antibodies. CSQ, Connective Tissue Disease Screening Questionnaire. ACR, American College of Rheumatology. OR, odds ratio. CI, confidence interval.
For the first phase, we sent letters to 3544 individuals who reported a family member with SLE that was confirmed with medical records. For the second phase, we sent letters to 279 individuals for whom their family member’s SLE was not confirmed, and preferentially selecting individuals who had 2–3 confirmed ACR criteria at their baseline visit.
T-tests for continuous variables and chi-square tests for categorical variables were used to determine differences between individuals who transitioned to SLE and individuals who did not transition to SLE.
Odds ratios for transitioning to SLE were calculated accounting for correlation within families.
Age and time between contact: OR is presented for a 5-year increase
Other includes Native American, Hispanic, and Asian/Pacific Islander.
ANA positivity: ≥ 1:120 titer by indirect immunofluorescence (IIF) on HEp-2 cells.
The bottom 2 categories (0 and 1) for number of ACR criteria were combined as no individuals in the transitioned group had 0 ACR criteria at baseline.
We then asked whether limiting prospective follow-up assessment to family members with specific characteristics at baseline would allow us to identify the majority of cases of SLE while missing the fewest number of cases. We performed this analysis only in those in the first recruitment phase of our study, in order to avoid the potential bias created by the second phase wherein we preferentially recruited individuals with 2–3 ACR criteria at baseline. We examined baseline characteristics alone and in combination to determine the best characteristics on which to select at-risk individuals for follow-up in a prospective study for SLE transition (Table 3). Characteristics were separated according to the amount of effort required to collect the data, from data gathered by questionnaire alone, data gathered with questionnaire and blood tests, and data collected using blood draw and medical record review for ACR criteria confirmation. We first examined characteristics that could be identified using questionnaire data alone. Targeting family members who scored possible or probable SLE on the CSQ (SLE-CSQ+) for follow-up indicates a low PPV (15.0%), high NPV (98.8%) and sensitivity (86.4%), and moderate specificity (69.4%). If only female relatives were analyzed, PPV, NPV, sensitivity and specificity all decrease. Further restricting our analysis to only females that were SLE-CSQ+ did not improve the predictive accuracy over the SLE-CSQ+ alone.
Table 3.
Predictive Accuracy of Baseline Characteristics for Transitioning to SLE at Follow-up in Those Recruited In the First Phase of Recruitment.
| Baseline Characteristic(s) | N (%) with Characteristic(s) | Positive predictive value | Negative Predictive Value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Using Questionnaire Data Alone | |||||
| SLE-CSQ+a | 127 (33.9%) | 15.0% | 98.8% | 86.4% | 69.4% |
| Female | 313 (83.5%) | 6.4% | 96.8% | 90.9% | 17.0% |
| SLE-CSQ+a and Female | 118 (31.5%) | 16.1% | 98.8% | 86.4% | 72.0% |
| Using Questionnaire Data and Blood Draw for Measurement of ANA | |||||
| ANA+ | 179 (47.7%) | 10.1% | 98.0% | 81.8% | 54.4% |
| ANA+ and SLE− CSQ+a | 78 (20.8%) | 20.5% | 98.0% | 72.7% | 82.4% |
| ANA+ and Female | 159 (42.4%) | 10.7% | 97.7% | 77.3% | 59.8% |
| ANA+, SLE− CSQ+a, and Female | 75 (20.0%) | 21.3% | 98.0% | 72.7% | 83.3% |
| ANA+ and Positive Photosensitivity from CSQ | 46 (12.3%) | 17.4% | 95.7% | 36.4% | 89.2% |
| ANA+ and Positive Raynaud from CSQ | 76 (20.3%) | 14.5% | 96.5% | 50.0% | 81.6% |
| Using Blood Draw for Measurement of ANA and Medical Records to confirm ACR Criteria | |||||
| ANA+ and Immunologic Disorder by Confirmed ACR Criteria | 7 (1.9%) | 57.1% | 95.1% | 18.2% | 99.2% |
| ANA+ and Positive Photosensitivity by Confirmed ACR Criteria | 8 (2.1%) | 50.0% | 95.1% | 18.2% | 98.9% |
| ANA+ and Positive Clinicalb ACR Criteria | 20 (5.3%) | 55.0% | 96.9% | 50.0% | 97.5% |
ANA, anti-nuclear antibodies. CSQ, Connective Tissue Disease Screening Questionnaire.
SLE-CSQ+: either probable or possible SLE by CSQ score.
Clinical ACR Criteria includes malar or discoid rash, oral ulcers, photosensitivity, arthritis, renal disorder including proteinuria and cellular changes, neurologic disorder including seizures and psychosis, and serositis including pericarditis or pleuritis.
We then examined whether the addition of information from a blood draw (ANA positivity, ANA+) increased the predictive accuracy of these baseline characteristics (Table 3). ANA positivity alone had lower PPV, NPV, sensitivity and specificity than the SLE-CSQ+. If one further restricts those family members who are SLE-CSQ+ to only ANA+, PPV and specificity for transitioning to SLE increases, but NPV and sensitivity decrease compared to SLE-CSQ+ alone. Similar results were seen with the additional restriction of SLE-CSQ+, ANA+ and female relatives. Restricting ANA+ individuals to those who also meet photosensitivity ACR criterion or report positive Raynaud on the SLE-CSQ increased the specificity for transitioning to SLE. The addition of medical record confirmed ACR criteria to ANA positivity had the highest PPV and specificity for transitioning to SLE out of all characteristics examined. Individuals who were ANA+ and had confirmed immunologic disorder had the highest PPV and specificity, followed by those who were ANA positive with confirmed clinical features and those with confirmed photosensitivity (Table 3).
Discussion
Due to the relatively rare nature of SLE 34, 35, one must enrich for individuals with the highest risk of developing SLE, whether through presentation of early clinical signs and symptoms, early serological abnormalities, genetic risk, or some combination thereof, to enable the design of successful natural history or prevention studies. The primary goal of this study was to determine factors associated with transitioning to SLE in family members that could indicate easy tools for screening populations to identify at-risk individuals warranting further follow-up assessment by a rheumatologist, or inclusion in prospective observational or interventional prevention trials. The most informative factors included scoring as possible or probable SLE on the SLE-CSQ, being ANA positive, and having more than one medical-record confirmed ACR clinical criteria. Not surprisingly, all of these methods can detect early signs and symptoms of SLE, either directly as an ANA test or number of ACR criteria noted in the medical record, or indirectly by asking about symptoms specific to SLE with the CSQ questionnaire.
While increasing number of baseline ACR criteria resulted in a larger proportion of individuals who transitioned to SLE, obtaining medical records and verifying ACR classification criteria is labor intensive, requires rheumatology training for accurate medical record review, and is impacted by the amount of documentation provided by the treating clinician who may have been seeing the patient for other clinical reasons. Baseline CSQ score was nearly as good as ACR criteria at predicting transition to classified SLE, likely due to the correlation between SLE-CSQ score and number of ACR criteria (rho=0.43). Indeed, scoring unlikely SLE on the CSQ had the highest negative predictive value, indicating those least likely to transition, further supporting the CSQ as a possible screening tool. Interestingly, only a few of the family members who were ANA positive only with no other confirmed ACR classification criteria transitioned. Both ANA testing and administering the SLE-CSQ are relatively simple tasks which could be accomplished in a single visit. Administering and scoring the CSQ may be the most efficient way to identify specific relatives who warrant further clinical and serological assessment. However, limiting follow-up assessment to individuals who are SLE-CSQ+ or ANA+ may result in a higher proportion of the cohort who transition to SLE at the expense of capturing early pathogenic events. In addition, in natural history studies inclusion of individuals at low risk of transitioning is important to understand the complete range of disease. Therefore, one should weigh the benefits of increasing the chances for transitioning to SLE versus understanding the full etiology of the disease in determining who to follow in prospective observational or interventional clinical trials.
While it is clear that family members with early clinical signs of SLE were more likely to participate in our study, this is positive as these are the individuals who are most likely to transition to SLE during prospective follow-up. We were relatively successful in enrolling individuals who were most likely to transition, such as those who were ANA positive and had other medical record confirmed ACR criteria such as photosensitivity (30.7%), other clinical criteria (26.4%), and immunologic disorder (26.1%). Targeting individuals with a larger number of confirmed ACR criteria as we did in phase two is a very productive way to recruit by comparing the percent transitioned in each recruitment phase. However, it does require medical record review to ascertain ACR criteria, which places this method into the highest effort for screening. In addition, we successfully enrolled those who scored possible or probable SLE on the CSQ (16.3%), as well as individuals who scored possible or probable on the CSQ, were female, and were ANA positive (21.0%). These were the individuals who were most likely to transition to SLE without examining medical records, with approximately 33% transitioning over follow-up.
Remarkably, we did not see a large difference in the odds of transitioning to classified SLE between men and women. This could be due to the low numbers of men in our cohort, or to the higher risk incurred from being a genetic relative of a SLE patient. We also did not see statistically significant differences in disease transition by age or race. The majority of the family members who transitioned were over 40 years old, with 48% who transitioned aged 50 years or older. This could be a reflection of the older SLE incidence rate seen in European Americans 36 and we had fewer African American and other minority populations who participated in this follow-up study.
We increased our chances of identifying individuals who may transition to classified SLE by targeting genetically related relatives of SLE patients who themselves have a higher risk for disease due to family history; 5–10% of SLE patients have a second family member with SLE 37. We were relatively successful in enrolling a large proportion of first degree relatives (11.1%), which are at the highest risk of developing SLE themselves. Contrary to other studies that report higher rates of SLE in siblings 28, 29, we did not see a statistically significant difference in transitioning between being a sister compared to other FDRs. It is possible as the cohort ages, we could see the increased risk in sisters as sisters who transitioned were on average older than the sisters who did not transition (47.2 vs. 42.6 years). It is also possible that we did not see increased risk in sisters as they may have transitioned earlier and were therefore not eligible for this study. Nonetheless, along with number of family members with SLE, this did not appear to affect who transitioned. In addition, we had several second degree and more distantly related relatives enrolled in the study, and they were more likely to transition to SLE. These relatives may have been more likely to participate and transition as they were further along the pathway to SLE. Indeed, after controlling for number of ACR criteria at baseline, being a second degree or more distantly related relative was no longer significantly associated with transitioning to SLE.
One limitation of the current study is that we do not know the exact time when the study participants transitioned to classified SLE (i.e. the exact date it was determined that the individual met ≥ 4 ACR classification criteria), as this information was not extracted from the medical record or the medical record was incomplete. We know that family members transitioned to SLE in between baseline and follow-up; exactly when one transitions to SLE is difficult to ascertain from medical record abstraction, particularly when records need to be requested from multiple health care providers. Factors elucidated in the current study that can help identify at-risk individuals for recruitment to prospective observational cohorts will allow for repeated, protocolized, longitudinal clinical assessment to better establish time and mechanisms of SLE disease transition. However, these results apply only to family members of individuals with SLE, and therefore cannot be generalized to all individuals at risk for SLE.
This cohort of family members of individuals with SLE has allowed us to begin examining additional biomarkers and pathogenic mechanisms important to the development of SLE 38. In addition, we can examine known genetic and environmental risk factors, and elucidate whether there are important interactions between genetic and environmental risk factors important to transitioning to SLE39. We can also examine the biologic samples for novel genetic, metabolic, and other molecular risk factors associated with SLE and its different phenotypes. It is also important to study those individuals who do not progress to SLE, who could provide insight into possible protective factors, along with helping us to understand the biological mechanism behind potential genetic and environmental risk factors. This cohort could help further understand SLE and potential subtypes by identifying new biomarkers and by defining SLE by mechanisms and pathways rather than more subjective outcomes.
Acknowledgements
The authors would like to thank Lupus Family Registry and Repository and Systemic Lupus Erythematosus in Gullah Health personnel, participants, and the referring physicians for making this study possible.
Funding
Research reported in this publication was supported by the National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institute of Allergy, Immunology and Infectious Diseases, National Institute of General Medical Sciences, National Human Genome Research Institute, and US Department of Veterans Affairs under award numbers under award numbers P30 AR053483, P30 GM103510, U54 GM104938, U01 AI101934, U19 AI082714, R01 AI024717, U01 HG008666, and R01 AI130830.
Footnotes
Ethics approval and consent to participate
All protocols were approved by the Institutional Review Boards at the Oklahoma Medical Research Foundation, the Medical University of South Carolina, and the University of Colorado Denver (COMIRB).
Declaration of conflicting interests
The authors declare that they have no conflicting interests.
References
- 1.Bentham J, Morris DL, Graham DSC, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nature genetics 2015; 47: 1457–1464. 2015/10/27. DOI: 10.1038/ng.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niewold TB. Advances in lupus genetics. Current opinion in rheumatology 2015; 27: 440–447. 2015/07/29. DOI: 10.1097/bor.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards CJ, Syddall H, Goswami R, et al. Infections in infancy and the presence of antinuclear antibodies in adult life. Lupus 2006; 15: 213–217. 2006/05/12. [DOI] [PubMed] [Google Scholar]
- 4.James JA, Neas BR, Moser KL, et al. Systemic lupus erythematosus in adults is associated with previous Epstein-Barr virus exposure. Arthritis and rheumatism 2001; 44: 1122–1126. 2001/05/16. DOI: . [DOI] [PubMed] [Google Scholar]
- 5.Harley JB and James JA. Epstein-Barr virus infection may be an environmental risk factor for systemic lupus erythematosus in children and teenagers. Arthritis and rheumatism 1999; 42: 1782–1783. 1999/08/14. DOI: . [DOI] [PubMed] [Google Scholar]
- 6.Parks CG, Cooper GS, Nylander-French LA, et al. Occupational exposure to crystalline silica and risk of systemic lupus erythematosus: a population-based, case-control study in the southeastern United States. Arthritis and rheumatism 2002; 46: 1840–1850. 2002/07/19. DOI: 10.1002/art.10368. [DOI] [PubMed] [Google Scholar]
- 7.Young KA, Terrell DR, Guthridge JM, et al. Smoking is not associated with autoantibody production in systemic lupus erythematosus patients, unaffected first-degree relatives, nor healthy controls. Lupus 2014; 23: 360–369. 2014/01/23. DOI: 10.1177/0961203314520838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. The New England journal of medicine 2003; 349: 1526–1533. 2003/10/17. DOI: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism 1997; 40: 1725 1997/10/27. DOI: . [DOI] [PubMed] [Google Scholar]
- 10.Aberle T, Bourn RL, Munroe ME, et al. Clinical and serological features distinguish patients with incomplete lupus classification from systemic lupus erythematosus patients and controls. Arthritis care & research 2017. 2017/01/25. DOI: 10.1002/acr.23201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinlen LD, McClain MT, Merrill J, et al. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis and rheumatism 2007; 56: 2344–2351. 2007/06/30. DOI: 10.1002/art.22665. [DOI] [PubMed] [Google Scholar]
- 12.Olsen NJ, Yousif M, Mutwally A, et al. Organ damage in high-risk patients with systemic and incomplete lupus syndromes. Rheumatology international 2013; 33: 2585–2590. 2013/05/30. DOI: 10.1007/s00296-013-2783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson C, Kokkonen H, Johansson M, et al. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis research & therapy 2011; 13: R30 2011/02/24. DOI: 10.1186/ar3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosca M, Tavoni A, Neri R, et al. Undifferentiated connective tissue diseases: the clinical and serological profiles of 91 patients followed for at least 1 year. Lupus 1998; 7: 95–100. 1998/04/16. [DOI] [PubMed] [Google Scholar]
- 15.Bodolay E, Csiki Z, Szekanecz Z, et al. Five-year follow-up of 665 Hungarian patients with undifferentiated connective tissue disease (UCTD). Clinical and experimental rheumatology 2003; 21: 313–320. 2003/07/09. [PubMed] [Google Scholar]
- 16.Calvo-Alen J, Alarcon GS, Burgard SL, et al. Systemic lupus erythematosus: predictors of its occurrence among a cohort of patients with early undifferentiated connective tissue disease: multivariate analyses and identification of risk factors. The Journal of rheumatology 1996; 23: 469–475. 1996/03/01. [PubMed] [Google Scholar]
- 17.Cavazzana I, Franceschini F, Belfiore N, et al. Undifferentiated connective tissue disease with antibodies to Ro/SSa: clinical features and follow-up of 148 patients. Clinical and experimental rheumatology 2001; 19: 403–409. 2001/08/09. [PubMed] [Google Scholar]
- 18.Danieli MG, Fraticelli P, Salvi A, et al. Undifferentiated connective tissue disease: natural history and evolution into definite CTD assessed in 84 patients initially diagnosed as early UCTD. Clinical rheumatology 1998; 17: 195–201. 1998/08/07. [DOI] [PubMed] [Google Scholar]
- 19.Mosca M, Neri R, Bencivelli W, et al. Undifferentiated connective tissue disease: analysis of 83 patients with a minimum followup of 5 years. The Journal of rheumatology 2002; 29: 2345–2349. 2002/11/05. [PubMed] [Google Scholar]
- 20.Szodoray P, Nakken B, Barath S, et al. Progressive divergent shifts in natural and induced T-regulatory cells signify the transition from undifferentiated to definitive connective tissue disease. International immunology 2008; 20: 971–979. 2008/06/14. DOI: 10.1093/intimm/dxn056. [DOI] [PubMed] [Google Scholar]
- 21.Zold E, Szodoray P, Kappelmayer J, et al. Impaired regulatory T-cell homeostasis due to vitamin D deficiency in undifferentiated connective tissue disease. Scandinavian journal of rheumatology 2010; 39: 490–497. 2010/07/10. DOI: 10.3109/03009741003781951. [DOI] [PubMed] [Google Scholar]
- 22.Greer JM and Panush RS. Incomplete lupus erythematosus. Archives of internal medicine 1989; 149: 2473–2476. 1989/11/01. [PubMed] [Google Scholar]
- 23.Stahl Hallengren C, Nived O and Sturfelt G. Outcome of incomplete systemic lupus erythematosus after 10 years. Lupus 2004; 13: 85–88. 2004/03/05. [DOI] [PubMed] [Google Scholar]
- 24.Vila LM, Mayor AM, Valentin AH, et al. Clinical outcome and predictors of disease evolution in patients with incomplete lupus erythematosus. Lupus 2000; 9: 110–115. 2000/04/29. [DOI] [PubMed] [Google Scholar]
- 25.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia 1996; 39: 807–812. 1996/07/01. [DOI] [PubMed] [Google Scholar]
- 26.The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Annals of the New York Academy of Sciences 2008; 1150: 1–13. 2009/01/06. DOI: 10.1196/annals.1447.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolfenbach JR, Deane KD, Derber LA, et al. A prospective approach to investigating the natural history of preclinical rheumatoid arthritis (RA) using first-degree relatives of probands with RA. Arthritis and rheumatism 2009; 61: 1735–1742. 2009/12/02. DOI: 10.1002/art.24833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo CF, Grainge MJ, Valdes AM, et al. Familial Aggregation of Systemic Lupus Erythematosus and Coaggregation of Autoimmune Diseases in Affected Families. JAMA internal medicine 2015; 175: 1518–1526. 2015/07/21. DOI: 10.1001/jamainternmed.2015.3528. [DOI] [PubMed] [Google Scholar]
- 29.Alarcon-Segovia D, Alarcon-Riquelme ME, Cardiel MH, et al. Familial aggregation of systemic lupus erythematosus, rheumatoid arthritis, and other autoimmune diseases in 1,177 lupus patients from the GLADEL cohort. Arthritis and rheumatism 2005; 52: 1138–1147. 2005/04/09. DOI: 10.1002/art.20999. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen A, Sevier S, Kelly JA, et al. The lupus family registry and repository. Rheumatology (Oxford, England) 2011; 50: 47–59. 2010/09/25. DOI: 10.1093/rheumatology/keq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamen DL, Barron M, Parker TM, et al. Autoantibody prevalence and lupus characteristics in a unique African American population. Arthritis and rheumatism 2008; 58: 1237–1247. 2008/04/29. DOI: 10.1002/art.23416. [DOI] [PubMed] [Google Scholar]
- 32.Karlson EW, Sanchez-Guerrero J, Wright EA, et al. A connective tissue disease screening questionnaire for population studies. Annals of epidemiology 1995; 5: 297–302. 1995/07/01. [DOI] [PubMed] [Google Scholar]
- 33.Bruner BF, Guthridge JM, Lu R, et al. Comparison of autoantibody specificities between traditional and bead-based assays in a large, diverse collection of patients with systemic lupus erythematosus and family members. Arthritis and rheumatism 2012; 64: 3677–3686. 2012/11/01. DOI: 10.1002/art.34651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim SS, Bayakly AR, Helmick CG, et al. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: the georgia lupus registry. Arthritis & rheumatology (Hoboken, NJ) 2014; 66: 357–368. 2014/02/08. DOI: 10.1002/art.38239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somers EC, Marder W, Cagnoli P, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the michigan lupus epidemiology and surveillance program. Arthritis & rheumatology (Hoboken, NJ) 2014; 66: 369–378. 2014/02/08. DOI: 10.1002/art.38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pons-Estel GJ, Alarcon GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Seminars in arthritis and rheumatism 2010; 39: 257–268. 2009/01/13. DOI: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence JS, Martins CL and Drake GL. A family survey of lupus erythematosus. 1. Heritability. The Journal of rheumatology 1987; 14: 913–921. 1987/10/01. [PubMed] [Google Scholar]
- 38.Munroe ME, Young KA, Kamen DL, et al. Discerning Risk of Disease Transition in Relatives of Systemic Lupus Erythematosus Patients Utilizing Soluble Mediators and Clinical Features. Arthritis & rheumatology (Hoboken, NJ) 2017; 69: 630–642. 2016/11/20. DOI: 10.1002/art.40004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young KA, Munroe ME, Guthridge JM, et al. Combined role of vitamin D status and CYP24A1 in the transition to systemic lupus erythematosus. Annals of the rheumatic diseases 2017; 76: 153–158. 2016/06/11. DOI: 10.1136/annrheumdis-2016-209157. [DOI] [PMC free article] [PubMed] [Google Scholar]


