Abstract
We previously reported that galectin-9 (Gal-9), a soluble lectin with immunomodulatory properties, is elevated in plasma during HIV infection and induces HIV transcription. The link between Gal-9 and compromised neuronal function is becoming increasingly evident, however, the association with neuroHIV remains unknown. We measured Gal-9 levels by ELISA in cerebrospinal fluid (CSF) and plasma of 70 HIV-infected (HIV+) adults stratified by age (older >40yr and younger <40yr) either ART suppressed or with detectable CSF HIV RNA, including a subgroup with cognitive assessments, and 18 HIV uninfected (HIV−) controls. Gal-9 tissue expression was compared in necropsy brain specimens from HIV− and HIV+ donors using gene datasets and immunohistochemistry. Among older HIV+ adults, CSF Gal-9 was elevated in the ART suppressed and CSF viremic groups compared to controls, whereas in the younger group Gal-9 levels were elevated only in the CSF viremic group (p<0.05). CSF Gal-9 positively correlated with age in all groups (p<0.05). CSF Gal-9 tracked with CSF HIV RNA irrespective of age (β=0.33;p<0.05). Higher CSF Gal-9 in the older viremic HIV+ group correlated with worse neuropsychological test performance scores independently of age and CSF HIV RNA (p<0.05). Furthermore, CSF Gal-9 directly correlated with myeloid activation (CSF soluble CD163 and neopterin) in both HIV+ older groups (p<0.05). Among HIV+ necropsy specimens, Gal-9 expression was increased in select brain regions compared to controls (p<0.05). Gal-9 may serve as a novel neuroimmuno-modulatory protein that is involved in driving cognitive deficits in those aging with HIV and may be valuable in tracking cognitive abnormalities.
Keywords: Galectin-9, HIV, Biomarkers, Neuroinflammation, Cognitive disorders
Introduction
Despite viral suppression through antiretroviral therapy (ART) more than 30% of HIV-infected individuals are estimated to have some degree of cognitive deficit that will significantly impact activities involved in their day-to-day functioning [1-3]. Moreover, HIV-associated cognitive impairments (CI) are twice as prevalent in older HIV-infected persons and particularly those greater than 40 years of age have a decennial 18% increase in odds of developing cognitive deficiencies compared to the uninfected population [4, 5]. This is of importance in the current era of HIV infection as more than half of the HIV population in the United States is over 50 years of age [6] and susceptible to a higher prevalence of age-related comorbidities [7, 8]. Furthermore, targeted therapies beyond ART to prevent or delay cognitive impairment are unavailable and will require better insight into the mechanisms driving HIV-associated CI [9-11].
The underlying neuropathogenesis of HIV-associated CI in the era of widespread ART use is complex, likely multifactorial, mainly involving viral factors and dysregulated immunity. Low-level viral replication in the CNS, as a result of insufficient penetrance of ART and/or concurrent HIV driven-peripheral immune activation may trigger a neuroinflammatory state leading to a cascade of neurotoxic events driving neuronal injury and detrimental cognitive outcomes [12, 13]. Additional factors such as neurotoxicity from ART, microbial translocation, substance abuse, mental health conditions (anxiety disorders, major depression, bipolar disorder), and other age-associated comorbidities contribute to the development of CI in HIV-infected individuals [14-16].
Cerebrospinal fluid (CSF) functions as a “sink” for brain extracellular solutes and can flow through the brain parenchyma [17]; therefore, alterations of solutes in the CSF could potentially reflect underlying CNS pathologies. CSF markers of monocyte/macrophage activation (neopterin, CD163) and CNS injury (neurofilament light [NFL] protein, t-tau and S100β) have been linked, to some degree, with CNS abnormalities and poor cognitive performance in HIV-infected populations [18-23]. Continued studies identifying novel biomarkers with greater sensitivity and higher specificity to monitor and reveal potential mechanisms involved in CI in those aging with HIV will ultimately lead to therapeutic options to prevent or ameliorate cognitive dysfunction in this population.
Galectins, a family of proteins that exhibit binding affinity for β-galactosides, are shown to modulate inflammatory responses and participate in homeostasis and regeneration of injured tissues in the CNS [24-26]. We recently determined that plasma levels of galectin-9 (Gal-9), an important pleiotropic modulator of both innate and adaptive immune responses, are elevated early after HIV acquisition [27-33]. Gal-9 also appears to play an important role in inducing HIV transcription and viral production during suppressive ART [34], and thereby implicated in HIV pathogenesis and persistence. Other galectins, such as galectin-1 and galectin-3, exhibit varying roles in the CNS, ranging from neuroprotective effects to promoting inflammatory processes [35-39], while Gal-9 has shown to be predominantly associated with CNS disease progression. Gal-9 expression is elevated in astrocytes residing in multiple sclerotic lesions and increases progressively in brain tissues in relation to glioma severity [40, 41]. Furthermore, an elevation of CSF Gal-9 levels was observed in individuals with secondary progressive multiple sclerosis, thus illustrating a link between Gal-9 and compromised neurological function [42].
The primary goal of this study is to establish a relationship between Gal-9 levels in the CSF and several virologic and immunologic measures, and clinical characteristics associated with HIV-associated CI. We hypothesized that higher CSF Gal-9 in HIV infection would associate with higher biomarker levels of HIV CNS involvement as well as with impaired cognitive performance. Secondarily, we explored the expression profile of Gal-9 in the brain in the context of HIV infection and neuroinflammation.
Materials and Methods
Study participants and specimens
This study utilized archived CSF and matched plasma samples from cohort studies that prospectively observed HIV-infected participants: the Hawai'i Aging with HIV Cohort (HAHC), University of California, San Francisco (UCSF) and University of Gothenburg (UGOT) cohorts. Study details of enrollment and clinical characterization were previously published [20, 43]. For inclusion in this study, we required that HIV-infected individuals be ART suppressed (virally suppressed in both plasma and CSF) or have detectable CSF VL (despite ART-exposure status and with or without plasma viremia). Individuals with a detectable CSF VL were from the HAHC (n=38), while virally suppressed participants were from the UCSF (n=16) and UGOT (n=16) cohorts. HIV-uninfected (HIV−) age-matched controls were volunteers from the UCSF cohort and confirmed by serological testing at study visit. Medication and demographic data, as well as measurements of HIV laboratory parameters (VL, CD4 T cell count, and nadir CD4) were available. All CSF and plasma samples were obtained and stored at time of visit at − 80°C until use. The institutional review board on each site approved respective studies.
HAHC evaluations also included a neurological examination and neuropsychological (NP) testing. HAHC participants were categorized with research-based cognitive diagnoses into normal, minor cognitive motor disorder (MCMD), and HIV-associated dementia (HAD) using the American Academy of Neurology (AAN) 1991 criteria [44]. Participants who were asymptomatic yet performed in an impaired range were also separated out in a group labeled “NP abnormal” in a manner that later became known as Asymptomatic Neurocognitive Impairment (ANI) in the revised 2007 nosology [45]. Neuropsychological composite z-scores (NPZ) were calculated from raw NP test scores that were normalized to z-scores using published norms adjusted for age, sex, and education, as described previously [46]. Major exclusions for the HAHC study included major psychiatric disorder, opportunistic brain infection, learning disability, and major neurologic disease [43].
Slides of formalin-fixed and parrafin-embedded brain tissue (frontal lobe cortex and white matter) were obtained through the National NeuroAIDS Tissue Consortium (NNTC). Necropsy specimens were from HIV-infected with or without encephalitis or HIV uninfected donors. Inclusion criteria: aged 30-60 years old with a post-mortem interval ≤ 24 hours; HIV+ without encephalitis were on cART with a VL ≤ 2000 copies consistent over 1 year before death and presented normal or minimal non-diagnostic brain abnormalities.
Soluble Marker Quantification
Detection of biomarkers in plasma and/or CSF was conducted according to the kit manufacturer’s instructions and all samples were analyzed in duplicate. Soluble markers were determined by the following quantitative kits: Human Galectin-9 Quantikine ELISA kit (R&D systems, MN, USA), Soluble CD163 ELISA (Trillium Diagnostics, LLC, ME, USA), Neopterin competitive enzyme immunoassay (ALPCO, NH, USA), and NF-Light (Neurofilament light) ELISA (UmanDiagnostics, Sweden). Optical density was read with a microplate spectrophotometer (Bio-Rad, Redmond, WA) and standard curve interpolation conducted on Prism version 7.0b (Graphpad Software Inc., CA, USA).
HIV DNA Quantification
DNA was extracted from peripheral blood mononuclear cells using the QIAamp DNA Micro Kit (Qiagen, CA, USA) as per manufacturer’s guidelines. Cell-associated HIV DNA was assessed as previously described [47]. Briefly, multiplex real-time polymerase chain reaction was performed using HIV gag and β-globin primers and probes with appropriate positive and negative controls. Copy numbers of each gene were generated using standard curves and HIV DNA copy number per 106 cells was calculated.
Gene expression analysis
Expression levels of Gal-9 (LGALS9) in post-mortem brain tissues were compared using a previously published dataset in the NCBI GEO database (http://www.ncbi.nlm.nih.gov/geo/).
Immunohistochemistry (IHC)
A Galectin-9 (LGalS9; clone 1G3) mouse IgG anti-human monoclonal antibody (mAb) (LSBio; Seattle, WA.) in conjunction with Vector Laboratories (Burlingame, Ca.) detection kit PK-6102 on the Dako Autostainer was utilized. Following deparaffinization in xylene and rehydration, tissue slides were subjected to antigen retrieval (10 mM citrate buffer; pH 6.0), followed by quenching of endogenous peroxide. Slides were then incubated with primary antibody (LGalS9 1:100) followed by incubation with secondary antibody, and detection with ABC reagent (Vector Laboratories, Burlingame, CA) and DAB substrate (3,3-diaminobenzidine; Sigma-Aldrich, St. Louis, MO). Counterstaining utilized hematoxylin. Stained slides were examined at 40x magnification by a pathologist for Gal-9 intensity and quantification of Gal-9 positive cells and were calculated at an average of 3 high-power fields.
Statistical analyses
Demographic and HIV-related characteristics were described using the median, first quartile (Q1), and third quartile (Q3) for continuous variables and frequency, and percent for categorical variables. Differences among continuous variables were evaluated by Mann-Whitney test, while categorical variable differences were evaluated via Chi-square tests. Correlations were analyzed by Spearman’s rank test or multiple linear regressions. All statistical tests were performed with Prism version 7.0b or SPSS version 24.0 (IBM SPSS Statistics, NY, USA). Statistical significance is indicated as an asterisk (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001) and P-values ≤0.10 and >0.05 are noted as statistical trends. Effect size was determined by Cohen’s d for Mann-Whitney tests .
Results
Demographic and Clinical Characteristics
We examined matched CSF and plasma samples from 70 HIV-infected participants either ART suppressed (n=32; virally suppressed on ART in both plasma and CSF) or CSF viral load (VL) detectable (n=38; despite ART-exposure status and with or without plasma viremia), as well as HIV uninfected controls (n=18) for comparison (Table 1). Given that age is an important factor linked to HIV-associated CI [14], and in particular CIs have increased prevalence in those 40 years or older [4], participants were stratified by age into younger (<40 years old) and older (40-70 years old) subgroups, which also followed the normal distribution of the entire sample. Clinical, laboratory, and demographic participant information for each participant group are summarized in Table 1. Current CD4 counts were significantly different in the HIV+ CSF VL detectable young and old groups compared to those that were virally suppressed or the uninfected controls. No differences were noted between HIV+ groups in terms of CD4 nadir or estimated duration of infection (EDI).
Table 1.
Clinical and demographic characteristics of participants (n=88)
| HIV-uninfected |
HIV-infected |
|||||
|---|---|---|---|---|---|---|
| Parameters | ART Suppressed |
CSF Viremic |
||||
| Younger | Older | Younger | Older | Younger | Older | |
| N | 6 | 12 | 18 | 14 | 23 | 15 |
| Gender (n, % male) | 6, 100% | 12, 100% | 7, 39% | 14, 100% | 19, 83% | 13, 87% |
| Age (years) | 27.7 (24.3, 32.3) | 52.6 (49.1, 58.1) | 34.1 (29.5, 38.0) | 54.9 (49.7, 59.40) | 35.4 (33.7, 38.3) | 53.8 (51.2, 55.0) |
| Ethnicity (n, % caucasian) | 4, 67% | 6, 50% | 7, 39% | 9, 56% | 10, 43% | 11, 73% |
| Drug use (n/available) | 6/6 | 5/9 | 2/18 | 11/14 | 5/23 | 3/15 |
| Depression (n/available) | 1/5 | 0/2 | 1/18 | 6/14 | 5/23 | 3/15 |
| CD4+ T-cell Count (cells/μL) | 778 (738, 946) | 820 (631, 864) | 783 (613, 915) | 585 (538, 687) | 315 (130, 522) | 344 (213, 522) |
| CD4+ T-cell nadir (cells-μL) | N/A | N/A | 267 (130, 301) | 154 (61, 207) | 81 (38, 407) | 253 (121, 388) |
| EDI (years) | N/A | N/A | 7.2 (4.3, 6.0) | 17.4 (11.3, 25.0) | 10.2 (2.9, 13.6) | 13.1 (9.4, 15.6) |
| CSF HIV VL Log10 (cps/mL) | N/A | N/A | <1.59 | <1.59 | 2.53 (2.20, 3.20) | 3.06 (2.54, 3.42) |
| Plasma HIV VL Log10 (cps/mL) | N/A | N/A | <1.59 | <1.59 | 4.70 (3.87, 5.13) | 4.66 (3.33, 5.20) |
| on ART (n, %) | N/A | N/A | 18, 100% | 14, 100% | 11, 48% | 6, 40% |
Data are shown as median (Q1, Q3) and frequency for continuous and categorical variables, respectively. P-values are determined by Kruskal-Wallis with Dunn’s multiple comparison correction or Mann-Whitney for continuous variables and by Chi-square test for categorical variables; significant differences among groups are bolded. Younger individuals aged <40; Older individuals aged 40-70. EDI=estimated duration of infection, VL=viral load. Drug use and depression is shown as number of individuals out of those with available information.
Soluble (CSF and plasma) Galectin-9 Levels According to HIV Status
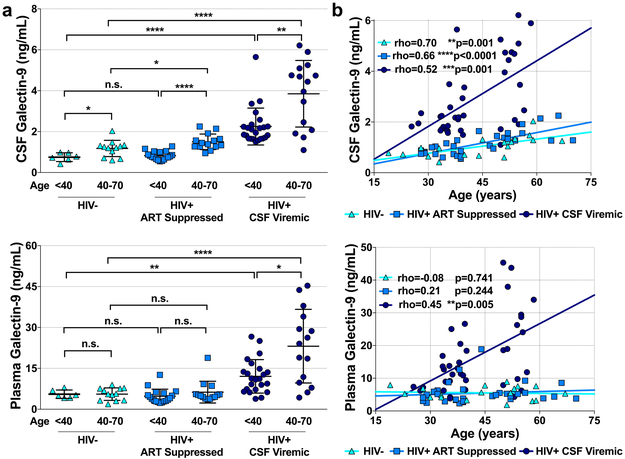
Differences in CSF Gal-9 levels among older and younger groups were observed in HIV+ CSF VL detectable (p<0.0001) and ART suppressed (p=0.003) subgroups as well as the HIV uninfected controls (p=0.032; Fig 1a). Among older individuals, CSF Gal-9 was higher in both the ART suppressed (1.50[1.27,1.62]ng/mL; p=0.014) and CSF VL detectable (4.44[2.27,4.92]ng/mL; p<0.0001) HIV+ groups compared to controls (1.15[1.08, 1.31]ng/mL). Differences in CSF Gal-9 levels among younger individuals were observed between the CSF viremic HIV+ group and uninfected controls (p<0.0001), but not with the ART suppressed (p=0.537) group. Plasma Gal-9 differences were only noted in CSF VL detectable individuals between the younger and older age groups (p=0.012) and with controls (younger, p=0.004; older, p<0.0001). We observed direct correlations between CSF Gal-9 and age in all three groups, however, an association between plasma Gal-9 and age was only observed among the HIV+ CSF VL detectable group (Fig 1b). Summary of CSF and plasma Gal-9 differences are presented in Supplementary Table 1.
Figure 1. Gal-9 associations with HIV status and age.
Differences in CSF and plasma levels of Gal-9 among younger (<40) and older (40-70) HIV negative (HIV−), HIV+ ART suppressed, and CSF HIV VL detectable (a). Correlations between CSF and plasma Gal-9 levels and age among participants HIV-, HIV+ ART suppressed, or HIV+ CSF VL detectable (b). Differences between groups were analyzed by Mann-Whitney tests. Associations between variables were analyzed by Spearman correlations. Statistical significance is indicated as *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Associations Between Soluble Gal-9 with Parameters of HIV Disease Progression and Viral Persistence.
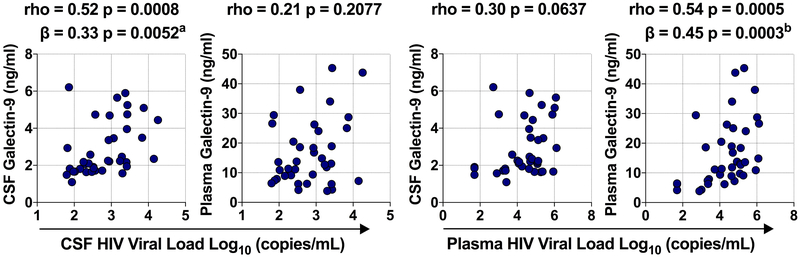
Among HIV+ individuals with measurable CSF VL, CSF Gal-9 levels significantly correlated with CSF VL and persisted when adjusting for age and plasma VL (Fig.2). Plasma Gal-9 correlated with plasma VL and remained significant with adjustments for age and CSF VL. However, correlations between CSF VL and plasma Gal-9 and between CSF Gal-9 and plasma VL were not observed. CD4 T-cell counts did not track with CSF Gal-9; however, plasma Gal-9 inversely correlated with CD4 T cell counts only in the CSF viremic group (Supplementary Fig.1a). Additionally, both CSF and plasma Gal-9 correlated with total peripheral blood mononuclear cell associated HIV DNA levels in the CSF viremic group (Supplementary Fig.1b).
Figure 2. Gal-9 and viral load associations in CSF and plasma.
CSF and plasma Gal-9 associations with CSF HIV VL (a) and plasma HIV VL (b). Associations between variables were analyzed by Spearman correlations and multivariable regression analysis. aAdjusted for age, plasma VL, and plasma Gal-9; badjusted for age, CSF VL, and CSF Gal-9.
Association Between Soluble Gal-9 and Cognitive Performance
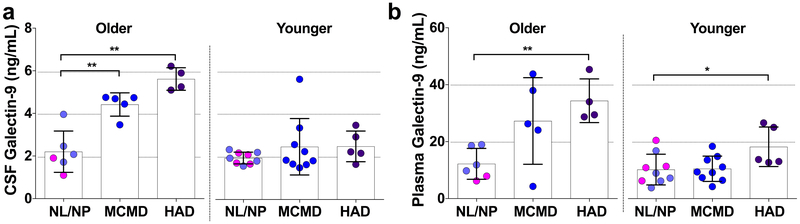
Next, we explored associations between Gal-9 levels and neuropsychological assessments that were available for individuals with a detectable CSF VL. No statistical differences in study participant characteristics and clinical parameters of age, education, EDI, CD4 count, CD4 nadir, plasma or CSF VL were observed among the categorized cognitive status subgroups in the older participants, while only a difference with current CD4 count (p=0.027) was observed among the younger group (Supplementary Table 2). Older HIV+ participants diagnosed with mild cognitive motor disorder (MCMD, n=5) or HIV-associated dementia (HAD, n=4) exhibited higher Gal-9 CSF levels compared to those with normal/NP abnormal cognition [(NL/NP, n=6); p=0.009 and p=0.010, respectively; Fig.3a]. In contrast, these CSF level differences with MCMD (n=10) and HAD (n=5) compared to NL/NP (n=9) were not observed among the younger participants (p=0.684 and p=0.190, respectively). Additionally, plasma Gal-9 was also elevated in both older and younger participants with HAD compared to individuals with normal/NP abnormal cognition (p=0.001 and p=0.029, respectively; Fig.3b). Summary of CSF and plasma Gal-9 differences among the younger and older CI status groups is presented in Supplementary Table 3.
Figure 3. Cognitive status and Gal-9 levels.
Gal-9 levels in the CSF and plasma were compared in younger and older HIV-infected CSF viremic individuals with normal cognition (NL)/neuropsychologic abnormal cognition (NP), minor cognitive motor disorder (MCMD), or HIV-associated dementia (HAD). Differences between groups were analyzed by Mann-Whitney tests. Statistical significance is indicated as *p<0.05, **p<0.01, ***p<0.001.
NPZ-global scores inversely correlated with CSF Gal-9 levels among older, but not younger, participants and this association persisted with adjustments in age and CSF VL (Table 2). Plasma Gal-9 did not correlate with NPZ-global scores in both the older and younger HIV+ groups. In addition, in the older CSF viremic HIV+ group none of the parameters of disease progression (CSF VL, CD4 count, CD4 nadir) were associated with NPZ-global scores (Supplementary Table 4). To determine whether specific cognitive domains drove the observed associations between Gal-9 and global cognitive performance, relationships between CSF and plasma Gal-9 and cognitive domain-specific summary NP z-scores were assessed. In older HIV+ individuals with CSF VL measures, higher CSF Gal-9 was associated with deficits in executive function, motor skills/motor speed, psychomotor speed, and working memory and attention (Table 2). CSF Gal-9 inversely trended with subdomain scores in language and verbal memory. A significant correlation between plasma Gal-9 and executive function in the older group was observed along with trends in memory, verbal memory, and visual memory. No associations were observed in the younger HIV+ group with the exception for a correlation between higher plasma Gal-9 and poorer recall memory.
Table 2.
Associations between soluble galectin-9 and cognitive performance
| Older CSF Viremic (n=15) |
Younger CSF Viremic (n=23) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cognitive Domain | CSF Galectin- 9* |
Plasma Galectin-9§ |
CSF Galectin-9* |
Plasma Galectin-9§ |
||||
| rho | Adjuste d β |
rho | Adjuste d β |
rho | Adjuste d β |
rho | Adjuste d β |
|
| Global Summary Score | −0.63c | −0.50d | −0.14 | −0.14 | 0.03 | 0.04 | 0.08 | 0.06 |
| Executive Function | 0.68b | −0.70b | −0.53c | −0.52c | 0.11 | 0.15 | 0.07 | 0.06 |
| Language | −0.45d | −0.38 | −0.04 | 0.06 | 0.19 | 0.23 | 0.19 | 0.10 |
| Learning | −0.36 | −0.36 | −0.25 | −0.13 | −0.08 | 0.25 | −0.23 | 0.03 |
| Memory | −0.33 | −0.36 | −0.51d | −0.34 | −0.16 | 0.12 | −0.19 | −0.01 |
| Motor Skills | −0.65c | −0.49d | −0.28 | −0.07 | −0.05 | −0.09 | −0.01 | 0.01 |
| Psychomotor Speed | −0.63c | −0.43 | −0.31 | −0.32 | −0.06 | 0.11 | 0.08 | 0.13 |
| Recall Memory | 0.04 | −0.32 | −0.34 | −0.63b | −0.29 | 0.14 | −0.51c | −0.21 |
| Verbal Memory | −0.49d | −0.39 | −0.49d | −0.32 | −0.05 | 0.17 | −0.14 | 0.01 |
| Visual Memory | −0.32 | −0.22 | −0.45d | −0.33 | −0.34 | −0.04 | −0.22 | −0.13 |
| Visuospatial | −0.45 | −0.17 | −0.28 | −0.07 | 0.12 | 0.16 | 0.02 | 0.02 |
| WMCA† | −0.67b | −0.53c | −0.38 | −0.02 | −0.29 | 0.03 | −0.14 | −0.03 |
β adjusted for age and CSF VL;
β adjusted for age and plasma VL.
P< .001,
P< .01,
P< .05,
P> .05 and P< .10
Working memory, concentration, and attention.
Associations between Soluble Gal-9 and Markers of Immune Activation and CNS Injury
To further evaluate the implications of elevated CSF Gal-9 in older HIV-infected individuals, correlations with CSF levels of neopterin, sCD163, and NFL were evaluated, as these markers are shown to associate with CNS pathogenesis and cognitive deficits [18, 48-51]. Correlations with neopterin and sCD163 were found among older HIV+ ART suppressed individuals and persisted with age adjustment (Table 3). In CSF VL detectable individuals, CSF Gal-9 correlated with CSF levels of neopterin and sCD163, as well as NFL and these correlations persisted with adjustments for age and CSF VL. Furthermore, while neopterin, sCD163, and NFL correlated with CSF Gal-9 in the HIV+ CSF VL detectable group, none of these markers correlated with NPZ-global (Supplementary Table 4).
Table 3.
CSF Gal-9 associations with CSF markers of CNS immune activation and neuronal injury
| Older ART suppressed (n=14) |
Older CSF Viremic (n=15) |
|||
|---|---|---|---|---|
| CSF Marker | rho | Adjusted β (age) |
rho | Adjusted β (age, CSF VL) |
| Neopterin | 0.68b | 0.54d | 0.72b | 0.66b |
| sCD163 | 0.55c | 0.87b | 0.58c | 0.48c |
| NFL | 0.41 | −0.23 | 0.72b | 0.66c |
Adjusted β values are from multivariable regression models with the indicated covariate. Values were Log-transformed prior to multivariable regression analysis
P< .001,
P< .01,
P< .05,
P> .05 and P< .10
Gal-9 Brain Tissue Expression in HIV Infection and Neuroinflammation
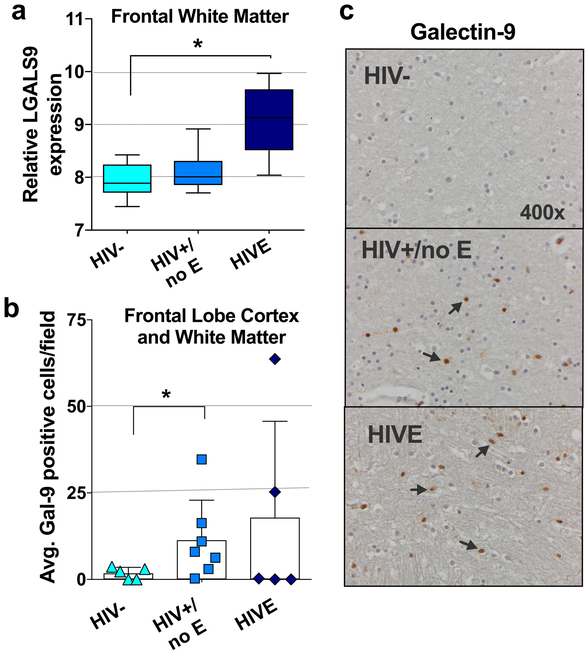
Finally, we determined whether Gal-9 levels were elevated specifically in the brain after HIV infection. Using datasets from the NCBI GEO database (accession number: GSE35864) we first compared Gal-9 (LGALS9) mRNA expression levels in post-mortem brain tissues (neocortex, white matter, and neostriatum) from either uninfected, HIV-infected without encephalitis (HIV+/no E), or HIV-infected with encephalitis (HIVE) donors. Gal-9 gene expression was significantly higher only in the frontal white matter brain region of HIVE donors compared to controls (p=0.016; Fig.4a). Given these findings, we next performed immunohistochemical (IHC) staining of Gal-9 expression in available autopsied frontal lobe cortex and white matter tissues from uninfected, HIV+/no E, and HIVE donors obtained from the National NeuroAIDS Tissue Consortium (NNTC); donor characteristics are summarized in Supplementary Table 5. Although the intensity of Gal-9 staining did not significantly differ among groups, an increase in the number of Gal-9 positive cells in HIV+/no E samples as compared to uninfected controls was observed (p=0.037; Fig.4b and 4c). Although slight age differences were observed among the groups, it was primarily driven by the HIVE group averaging at a younger age (Supplementary Table 5).
Figure 4. CNS expression of Gal-9 in HIV infection.
(a) Comparison of gene expression levels of Gal-9 between HIV negative (n=6; HIV−), HIV-infected without encephalitis (n=13; HIV+/no E) and HIV encephalitis (n=5; HIVE) CNS samples using microarray dataset available in the NCBI GEO database (accession number: GSE35864). (b) Graphical representation of Gal-9 positive cell counts in frontal lobe cortex and white matter tissues from HIV− (n=5), HIV+/no E (n=7), and HIVE (n=5) participants. (c) Representative immunohistochemical staining patterns and differences between groups were analyzed by Mann-Whitney tests. Statistical significance is indicated as *p<0.05.
Discussion
In this study we investigated CNS (CSF and brain) Gal-9 in the context of HIV, aging, immune activation, and cognition. Primary analysis encompassed quantifying Gal-9 in the CNS and examining the relationships between Gal-9 with indices of HIV CNS injury and cognitive dysfunction. We observed increased CSF Gal-9 levels in older HIV-infected individuals. Further analysis illustrated that elevated levels of CSF Gal-9 and brain tissue Gal-9 in HIV may partly be driven by viral burden and immune activation, while parameters of cognitive decline were associated with CSF Gal-9 among older HIV+ individuals.
Persons aging with HIV face higher rates and earlier onset of age-related co-morbidities [7, 8], exhibit accelerated aging effects [52], and have increased levels of several constituents in the blood and CSF, which may be reflective or influence these secondary complications observed in HIV infection [23]. Here, we observed CSF Gal-9 exhibiting an age-dependency as seen with several other proteins found in the CSF despite HIV status [51, 53, 54], however, the vast differences among younger and older HIV-infected individuals in contrast to the uninfected group may suggest that these age-related increases could be accelerated in this population. However, it is important to note that studies with well-matched controls do not show any accelerated aging of the brain itself in treated HIV infection [55].
Several circulating soluble mediators are being assessed to afford insight into how HIV infection affects the CNS and influences the development of cognitive impairment. However, in contrast to many markers being investigated, Gal-9 is unique due to its high functionality, particularly in that it has been shown to directly regulate immune responses [27, 29, 30] and exert effects on cells of the CNS [32]. Given the relationship between Gal-9 and myeloid activation in the CNS, particularly neopterin and sCD163, our findings suggest a link between Gal-9 and neuroinflammation. Gal-9 is shown to induce proinflammatory cytokines in microglia [32], and many resident cells of the CNS express ligands/receptors that Gal-9 could interact with [56-58]; thus, assessing Gal-9-induced responses and potential ligand profiles in the CNS during HIV infection may provide further mechanistic insight into the underlying interactions and drivers of CNS dysfunction in HIV. While Gal-9 has multifarious roles in immunity, regulating both adaptive and inflammatory responses, it remains to be determined whether these elevated levels could have a cause or effect relationship with CI development and immune activation in the CNS.
Cognitive impairment in the current HIV era of successful ART is problematic particularly in those that are aging given other age-related cognitive complications, such as Alzheimer’s disease. Biomarkers in NeuroHIV are of value to improve the diagnosis and monitoring of HIV-associated CI, as well as guide treatment decisions. However, many prospective biomarkers currently investigated are elevated only in those with more severe neurological complications and/or often normalized or fall rapidly upon ART initiation [59-63]. Although, plasma Gal-9 similarly decreases with the use of ART [64],. While plasma is more clinically relevant, CSF provides a snapshot of the CNS. For example, both NFL and S100β are associated with HIV-associated CI and their presence at high levels reflects ongoing neuronal damage and astrocytosis, respectively [65]. Based on our results, Gal-9 could potentially serve as an additional marker of CNS dysfunction in those aging with HIV.
Cognitive decline and continued CSF immune activation despite ART could indicate ongoing low-level viral replication in the CNS. Evidence suggests that the CNS compartment is an important tissue sanctuary site for HIV persistence during ART, possibly related to suboptimal drug penetration across the BBB and the immune privileged environment of the CNS [66]. Gal-9 modulates the expression of p21[67], a host determinant of HIV persistence [68], and is linked to modulating HIV transcription and reactivation [34]; therefore, the impact of endogenous Gal-9 on HIV CNS reservoirs warrants further investigation. Additionally, the association of Gal-9 with PMBC HIV DNA further distinguishes Gal-9 as an important factor in investigating CNS complications and reservoirs in HIV infection, as cell-associated HIV DNA is shown to be linked with cognitive decline in HIV-infected patients either virally suppressed or ART naive [69, 70].
To further determine the potential of Gal-9 as a mediator and/or marker of cognitive decline and HIV persistence, the predominant source needs to be addressed, as cells both in the periphery (myeloid dendritic cells, monocytes, fibroblasts, vascular endothelial cells) and CNS (astrocytes, Purkinje cells) can express and secrete Gal-9 [71-78]. Although CSF Gal-9 could originate from the CNS, diffusion between the plasma and CSF compartments can also occur due to BBB disruption, a condition that arises in untreated HIV infection and can persist after ART implementation [79]. Nonetheless the source of Gal-9, the effects of HIV infection, viral protein exposure, and inflammatory mediators on expression and secretion of Gal-9 in these potential cell sources need to be further explored as well as to understand how Gal-9 impacts anti-HIV immunity and HIV persistence in the CNS.
This study has several limitations. Individual groups were relatively small in size due to the availability of archived cohort samples fitting our inclusion criteria, which impeded our ability to detect minute differences among groups. Overall, the participants in our study were predominately white males, so it would be of value to replicate our findings in other populations. Cognitive status assessments were made with prior definitions under the AAN criteria rather than the modern Frascati criteria, but this is unlikely to have had a major impact on the grouping given tight overlap in diagnoses, our use of an NP abnormal phenotype, and similar exclusions for major comorbidities. Finally, we did not take in to account multiple co-morbid conditions that occur in older individuals (both HIV negative and infected), which could potentially affect the levels of plasma and/or CSF Gal-9.
The strength of this study is the ability to correlate Gal-9 levels in the CNS compartment with clinical HIV parameters of cognitive impairment, while also highlighting the association of Gal-9 with aging and to speculate on a mechanistic role for Gal-9 in the neuropathogenesis of HIV-associated CI. Gal-9 has not been extensively studied in other CNS diseases, which might reveal the sensitivity and specificity of Gal-9 for HIV-associated CI. CSF Gal-9 could also be explored as a potential valuable marker of CNS pathology or other chronic infections with CNS involvement. Given that our cognitive data is based on samples derived from individuals with productive viral replication (CSF viremic), it would be of further interest to evaluate these parameters with Gal-9 levels in those with HIV infection that are either virally suppressed or experience asymptomatic CSF viral escape to see if associations with HIV-associated CI persist.
Supplementary Material
Figure S1. Gal-9 correlates with indices of HIV disease progression. (a) Correlations between CSF and plasma Gal-9 levels and CD4 T-cell counts. (b) CSF and plasma Gal-9 correlations with PBMC-associated HIV DNA levels. Associations between variables were analyzed by Spearman correlations. Statistical significance is indicated as *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Acknowledgements:
We thank all study participants and study groups, Scott Bowler for his help with specimen management, and Vedbar Khadka, Daniel Laspisa, and Lindsay Kohorn for their guidance with data analysis.
Funding:
This work was supported in part by National Institutes of Health (NIH) grants 1R01MH112457-01 (LCN and SP), U54NS43049 (CS), MH098759 (VV), R01 NS094067 (RWP), P01 DA026134 (RWP, Project PI), and the Swedish State support for Clinical Research (ALFGBG-717531). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This publication was also made possible from NIH funding through the NIMH and NINDS Institutes by the following grants: Texas NeuroAIDS Research Center: U24MH100930, California NeuroAIDS Tissue Network: U24MH100928, National Neurological AIDS Bank: U24MH100929, Manhattan HIV Brain Bank: U24MH100931, Data Coordinating Center: U24MH100925. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NNTC or NIH.
Footnotes
Declarations
Ethics approval and consent to participate:
Informed consent was obtained from participants following procedures approved by the University of Hawai'i Human Studies Institutional Review Board, UCSF committee on Human Research, and the Regional Ethics Review Board in Gothenburg.
Availability of data and material:
The datasets generated and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests:
There is neither a relationship nor a support that might be perceived as constituting a conflict of interest of any of the authors.
References
- 1.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14): 1915–21. doi: 10.1097/QAD.0b013e32828e4e27. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 2.Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96. doi: 10.1212/WNL.0b013e318200d727. PubMed PMID: ; PubMed Central PMCID: PMCPMC2995535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10(3):317–31. doi: 10.1017/S1355617704102130. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 4.Coban H, Robertson K, Smurzynski M, Krishnan S, Wu K, Bosch RJ, et al. Impact of aging on neurocognitive performance in previously antiretroviral-naive HIV-infected individuals on their first suppressive regimen. AIDS. 2017;31(11):1565–71. doi: 10.1097/QAD.0000000000001523. PubMed PMID: ; PubMed Central PMCID: PMCPMC5509215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18 Suppl 1:S11–8. PubMed PMID: . [PubMed] [Google Scholar]
- 6.Mahy M, Autenrieth CS, Stanecki K, Wynd S. Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS. 2014;28 Suppl 4:S453–9. doi: 10.1097/QAD.0000000000000479. PubMed PMID: PubMed Central PMCID: PMCPMC4247270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schouten J, Wit FW, Stolte IG, Kootstra NA, van der Valk M, Geerlings SE, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59(12):1787–97. doi: 10.1093/cid/ciu701. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 8.Duffau P, Ozanne A, Bonnet F, Lazaro E, Cazanave C, Blanco P, et al. Multimorbidity, age-related comorbidities and mortality: association of activation, senescence and inflammation markers in HIV adults. AIDS. 2018. doi: 10.1097/QAD.0000000000001875. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 9.D'Antoni ML, Paul RH, Mitchell BI, Kohorn L, Fischer L, Lefebvre E, et al. Improved Cognitive Performance and Reduced Monocyte Activation in Virally Suppressed Chronic HIV Following Dual CCR2 and CCR5 Antagonism. J Acquir Immune Defic Syndr. 2018. doi: 10.1097/QAI.0000000000001752. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ndhlovu LC, Umaki T, Chew GM, Chow DC, Agsalda M, Kallianpur KJ, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND). J Neurovirol. 2014;20(6):571–82. doi: 10.1007/s13365-014-0279-x. PubMed PMID: ; PubMed Central PMCID: PMCPMC4268390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gates TM, Cysique LA, Siefried KJ, Chaganti J, Moffat KJ, Brew BJ. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS. 2016;30(4):591–600. doi: 10.1097/QAD.0000000000000951. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 12.Spudich S, Gonzalez-Scarano F. HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb Perspect Med. 2012;2(6):a007120. doi: 10.1101/cshperspect.a007120. PubMed PMID: ; PubMed Central PMCID: PMCPMC3367536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen MF, Gill AJ, Kolson DL. Neuropathogenesis of HIV-associated neurocognitive disorders: roles for immune activation, HIV blipping and viral tropism. Curr Opin HIV AIDS. 2014;9(6):559–64. doi: 10.1097/COH.0000000000000105. PubMed PMID: ; PubMed Central PMCID: PMCPMC4319532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen RA, Seider TR, Navia B. HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimers Res Ther. 2015;7(1):37. doi: 10.1186/s13195-015-0123-4. PubMed PMID: ; PubMed Central PMCID: PMCPMC4386102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright EJ, Grund B, Robertson K, Brew BJ, Roediger M, Bain MP, et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology. 2010;75(10):864–73. doi: 10.1212/WNL.0b013e3181f11bd8. PubMed PMID: ; PubMed Central PMCID: PMCPMC2938971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tedaldi EM, Minniti NL, Fischer T. HIV-associated neurocognitive disorders: the relationship of HIV infection with physical and social comorbidities. Biomed Res Int. 2015;2015:641913. doi: 10.1155/2015/641913. PubMed PMID: ; PubMed Central PMCID: PMCPMC4359826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147): 147ra11. doi: 10.1126/scitranslmed.3003748. PubMed PMID: ; PubMed Central PMCID: PMCPMC3551275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagberg L, Cinque P, Gisslen M, Brew BJ, Spudich S, Bestetti A, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. PubMed PMID: ; PubMed Central PMCID: PMCPMC2890504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Antoni ML, Byron MM, Chan P, Sailasuta N, Sacdalan C, Sithinamsuwan P, et al. Normalization of Soluble CD163 Levels After Institution of Antiretroviral Therapy During Acute HIV Infection Tracks with Fewer Neurological Abnormalities. J Infect Dis. 2018;218(9):1453–63. doi: 10.1093/infdis/jiy337. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gisslen M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis. 2007;195(12):1774–8. doi: 10.1086/518043. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 21.Peterson J, Gisslen M, Zetterberg H, Fuchs D, Shacklett BL, Hagberg L, et al. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One. 2014;9(12):e116081. doi: 10.1371/journal.pone.0116081. PubMed PMID: ; PubMed Central PMCID: PMCPMC4277428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eden A, Marcotte TD, Heaton RK, Nilsson S, Zetterberg H, Fuchs D, et al. Increased Intrathecal Immune Activation in Virally Suppressed HIV-1 Infected Patients with Neurocognitive Impairment. PLoS One. 2016;11(6):e0157160. doi: 10.1371/journal.pone.0157160. PubMed PMID: ; PubMed Central PMCID: PMCPMC4905676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Oliveira MF, Murrel B, Perez-Santiago J, Vargas M, Ellis RJ, Letendre S, et al. Circulating HIV DNA Correlates With Neurocognitive Impairment in Older HIV-infected Adults on Suppressive ART. Sci Rep. 2015;5:17094. doi: 10.1038/srep17094. PubMed PMID: ; PubMed Central PMCID: PMCPMC4658529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.John CM, Jarvis GA, Swanson KV, Leffler H, Cooper MD, Huflejt ME, et al. Galectin-3 binds lactosaminylated lipooligosaccharides from Neisseria gonorrhoeae and is selectively expressed by mucosal epithelial cells that are infected. Cell Microbiol. 2002;4(10):649–62. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 25.Shin T The pleiotropic effects of galectin-3 in neuroinflammation: a review. Acta Histochem. 2013;115(5):407–11. doi: 10.1016/j.acthis.2012.11.010. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 26.Chen HL, Liao F, Lin TN, Liu FT. Galectins and neuroinflammation. Adv Neurobiol. 2014;9:517–42. doi: 10.1007/978-1-4939-1154-7_24. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 27.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12): 1245–52. doi: 10.1038/ni1271. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 28.Sehrawat S, Reddy PB, Rajasagi N, Suryawanshi A, Hirashima M, Rouse BT. Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response. PLoS Pathog. 2010;6(5):e1000882. doi: 10.1371/journal.ppat.1000882. PubMed PMID: ; PubMed Central PMCID: PMCPMC2865527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seki M, Oomizu S, Sakata KM, Sakata A, Arikawa T, Watanabe K, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127(1):78–88. doi: 10.1016/j.clim.2008.01.006. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 30.Lv K, Zhang Y, Zhang M, Zhong M, Suo Q. Galectin-9 promotes TGF-beta1-dependent induction of regulatory T cells via the TGF-beta/Smad signaling pathway. Mol Med Rep. 2013;7(1):205–10. doi: 10.3892/mmr.2012.1125. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 31.Wada J, Kanwar YS. Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin. J Biol Chem. 1997;272(9):6078–86. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 32.Steelman AJ, Li J. Astrocyte galectin-9 potentiates microglial TNF secretion. J Neuroinflammation. 2014;11:144. doi: 10.1186/s12974-014-0144-0. PubMed PMID: ; PubMed Central PMCID: PMCPMC4158089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tandon R, Chew GM, Byron MM, Borrow P, Niki T, Hirashima M, et al. Galectin-9 is rapidly released during acute HIV-1 infection and remains sustained at high levels despite viral suppression even in elite controllers. AIDS Res Hum Retroviruses. 2014;30(7):654–64. doi: 10.1089/AID.2014.0004. PubMed PMID: ; PubMed Central PMCID: PMCPMC4077009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdel-Mohsen M, Chavez L, Tandon R, Chew GM, Deng X, Danesh A, et al. Human Galectin-9 Is a Potent Mediator of HIV Transcription and Reactivation. PLoS Pathog. 2016;12(6):e1005677. doi: 10.1371/journal.ppat.1005677. PubMed PMID: ; PubMed Central PMCID: PMCPMC4890776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horie H, Inagaki Y, Sohma Y, Nozawa R, Okawa K, Hasegawa M, et al. Galectin-1 regulates initial axonal growth in peripheral nerves after axotomy. J Neurosci. 1999;19(22):9964–74. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakaguchi M, Shingo T, Shimazaki T, Okano HJ, Shiwa M, Ishibashi S, et al. A carbohydrate-binding protein, Galectin-1, promotes proliferation of adult neural stem cells. Proc Natl Acad Sci U S A. 2006;103(18):7112–7. doi: 10.1073/pnas.0508793103. PubMed PMID: ; PubMed Central PMCID: PMCPMC1447526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parikh NU, Aalinkeel R, Reynolds JL, Nair BB, Sykes DE, Mammen MJ, et al. Galectin-1 suppresses methamphetamine induced neuroinflammation in human brain microvascular endothelial cells: Neuroprotective role in maintaining blood brain barrier integrity. Brain Res. 2015;1624:175–87. doi: 10.1016/j.brainres.2015.07.033. PubMed PMID: ; PubMed Central PMCID: PMCPMC4630155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang HR, Al Rasebi Z, Mensah-Brown E, Shahin A, Xu D, Goodyear CS, et al. Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J Immunol. 2009;182(2):1167–73. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 39.Lerman BJ, Hoffman EP, Sutherland ML, Bouri K, Hsu DK, Liu FT, et al. Deletion of galectin-3 exacerbates microglial activation and accelerates disease progression and demise in a SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Brain Behav. 2012;2(5):563–75. doi: 10.1002/brb3.75. PubMed PMID: ; PubMed Central PMCID: PMCPMC3489809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stancic M, van Horssen J, Thijssen VL, Gabius HJ, van der Valk P, Hoekstra D, et al. Increased expression of distinct galectins in multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2011;37(6):654–71. doi: 10.1111/j.1365-2990.2011.01184.x. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 41.Liu Z, Han H, He X, Li S, Wu C, Yu C, et al. Expression of the galectin-9-Tim-3 pathway in glioma tissues is associated with the clinical manifestations of glioma. Oncol Lett. 2016;11(3):1829–34. doi: 10.3892/ol.2016.4142. PubMed PMID: ; PubMed Central PMCID: PMCPMC4774531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burman J, Svenningsson A. Cerebrospinal fluid concentration of Galectin-9 is increased in secondary progressive multiple sclerosis. J Neuroimmunol. 2016;292:40–4. doi: 10.1016/j.jneuroim.2016.01.008. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 43.Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63(5):822–7. PubMed PMID: ; PubMed Central PMCID: PMCPMC1382180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bottiggi KA, Chang JJ, Schmitt FA, Avison MJ, Mootoor Y, Nath A, et al. The HIV Dementia Scale: predictive power in mild dementia and HAART. J Neurol Sci. 2007;260(1-2):11–5. doi: 10.1016/j.jns.2006.03.023. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 45.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. PubMed PMID: ; PubMed Central PMCID: PMCPMC4472366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamoto BK, Valcour VG, Kallianpur K, Liang CY, McMurtray A, Chow D, et al. Impact of cerebrovascular disease on cognitive function in HIV-infected patients. J Acquir Immune Defic Syndr. 2011;57(3):e66–8. doi: 10.1097/QAI.0b013e31821ff8bd. PubMed PMID: ; PubMed Central PMCID: PMCPMC3160604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kusao I, Shiramizu B, Liang CY, Grove J, Agsalda M, Troelstrup D, et al. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci. 2012;24(1):71–80. doi: 10.1176/appi.neuropsych.11050109. PubMed PMID: ; PubMed Central PMCID: PMCPMC3335340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, et al. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998; 12(11): 1327–32. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 49.Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis. 2013;207(11): 1703–12. doi: 10.1093/infdis/jit088. PubMed PMID: ; PubMed Central PMCID: PMCPMC3636785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63(11):2084–90. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 51.Yilmaz A, Blennow K, Hagberg L, Nilsson S, Price RW, Schouten J, et al. Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert Rev Mol Diagn. 2017;17(8):761–70. doi: 10.1080/14737159.2017.1341313. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 52.Horvath S, Levine AJ. HIV-1 Infection Accelerates Age According to the Epigenetic Clock. J Infect Dis. 2015;212(10):1563–73. doi: 10.1093/infdis/jiv277. PubMed PMID: ; PubMed Central PMCID: PMCPMC4621253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blomberg M, Jensen M, Basun H, Lannfelt L, Wahlund LO. Cerebrospinal fluid tau levels increase with age in healthy individuals. Dement Geriatr Cogn Disord. 2001;12(2):127–32. doi: 10.1159/000051246. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 54.Vagberg M, Norgren N, Dring A, Lindqvist T, Birgander R, Zetterberg H, et al. Levels and Age Dependency of Neurofilament Light and Glial Fibrillary Acidic Protein in Healthy Individuals and Their Relation to the Brain Parenchymal Fraction. PLoS One. 2015;10(8):e0135886. doi: 10.1371/journal.pone.0135886. PubMed PMID: ; PubMed Central PMCID: PMCPMC4552591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole JH, Caan MWA, Underwood J, De Francesco D, van Zoest RA, Wit F, et al. No Evidence for Accelerated Aging-Related Brain Pathology in Treated Human Immunodeficiency Virus: Longitudinal Neuroimaging Results From the Comorbidity in Relation to AIDS (COBRA) Project. Clin Infect Dis. 2018;66(12):1899–909. doi: 10.1093/cid/cix1124. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 56.Dzwonek J, Wilczynski GM. CD44: molecular interactions, signaling and functions in the nervous system. Front Cell Neurosci. 2015;9:175. doi: 10.3389/fncel.2015.00175. PubMed PMID: ; PubMed Central PMCID: PMCPMC4423434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang HW, Zhu XL, Qin LM, Qian HJ, Wang Y. Microglia activity modulated by T cell Ig and mucin domain protein 3 (Tim-3). Cell Immunol. 2015;293(1):49–58. doi: 10.1016/j.cellimm.2014.12.005. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 58.Yeo YA, Martinez Gomez JM, Croxford JL, Gasser S, Ling EA, Schwarz H. CD137 ligand activated microglia induces oligodendrocyte apoptosis via reactive oxygen species. J Neuroinflammation. 2012;9:173. doi: 10.1186/1742-2094-9-173. PubMed PMID: ; PubMed Central PMCID: PMCPMC3420242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ellis RJ, Seubert P, Motter R, Galasko D, Deutsch R, Heaton RK, et al. Cerebrospinal fluid tau protein is not elevated in HIV-associated neurologic disease in humans. HIV Neurobehavioral Research Center Group (HNRC). Neurosci Lett. 1998;254(1):1–4. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 60.Gisslen M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63. doi: 10.1186/1471-2377-9-63. PubMed PMID: ; PubMed Central PMCID: PMCPMC2807422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mellgren A, Price RW, Hagberg L, Rosengren L, Brew BJ, Gisslen M. Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology. 2007;69(15):1536–41. doi: 10.1212/01.wnl.0000277635.05973.55. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 62.Brew BJ, Bhalla RB, Paul M, Gallardo H, McArthur JC, Schwartz MK, et al. Cerebrospinal fluid neopterin in human immunodeficiency virus type 1 infection. Ann Neurol. 1990;28(4):556–60. doi: 10.1002/ana.410280413. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 63.Valle M, Price RW, Nilsson A, Heyes M, Verotta D. CSF quinolinic acid levels are determined by local HIV infection: cross-sectional analysis and modelling of dynamics following antiretroviral therapy. Brain. 2004;127(Pt 5):1047–60. doi: 10.1093/brain/awh130. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 64.Saitoh H, Ashino Y, Chagan-Yasutan H, Niki T, Hirashima M, Hattori T. Rapid decrease of plasma galectin-9 levels in patients with acute HIV infection after therapy. Tohoku J Exp Med. 2012;228(2):157–61. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 65.Hu J, Ferreira A, Van Eldik LJ. S100beta induces neuronal cell death through nitric oxide release from astrocytes. J Neurochem. 1997;69(6):2294–301. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 66.Letendre S Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Topics in antiviral medicine. 2011;19(4):137–42. PubMed PMID: ; PubMed Central PMCID: PMC4666587. [PMC free article] [PubMed] [Google Scholar]
- 67.Kurose Y, Wada J, Kanzaki M, Teshigawara S, Nakatsuka A, Murakami K, et al. Serum galectin-9 levels are elevated in the patients with type 2 diabetes and chronic kidney disease. BMC Nephrol. 2013;14:23. doi: 10.1186/1471-2369-14-23. PubMed PMID: ; PubMed Central PMCID: PMCPMC3556305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdel-Mohsen M, Wang C, Strain MC, Lada SM, Deng X, Cockerham LR, et al. Select host restriction factors are associated with HIV persistence during antiretroviral therapy. AIDS. 2015;29(4):411–20. doi: 10.1097/QAD.0000000000000572. PubMed PMID: ; PubMed Central PMCID: PMCPMC4385712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valcour VG, Ananworanich J, Agsalda M, Sailasuta N, Chalermchai T, Schuetz A, et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One. 2013;8(7):e70164. doi: 10.1371/journal.pone.0070164. PubMed PMID: ; PubMed Central PMCID: PMCPMC3729685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cysique LA, Hey-Cunningham WJ, Dermody N, Chan P, Brew BJ, Koelsch KK. Peripheral blood mononuclear cells HIV DNA levels impact intermittently on neurocognition. PLoS One. 2015;10(4):e0120488. doi: 10.1371/journal.pone.0120488. PubMed PMID: ; PubMed Central PMCID: PMCPMC4390276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Imaizumi T, Kumagai M, Sasaki N, Kurotaki H, Mori F, Seki M, et al. Interferon-gamma stimulates the expression of galectin-9 in cultured human endothelial cells. J Leukoc Biol. 2002;72(3):486–91. PubMed PMID: . [PubMed] [Google Scholar]
- 72.Ishikawa A, Imaizumi T, Yoshida H, Nishi N, Nakamura T, Hirashima M, et al. Double-stranded RNA enhances the expression of galectin-9 in vascular endothelial cells. Immunol Cell Biol. 2004;82(4):410–4. doi: 10.1111/j.0818-9641.2004.01248.x. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 73.Harwood NM, Golden-Mason L, Cheng L, Rosen HR, Mengshol JA. HCV-infected cells and differentiation increase monocyte immunoregulatory galectin-9 production. J Leukoc Biol. 2016;99(3):495–503. doi: 10.1189/jlb.5A1214-582R. PubMed PMID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asakura H, Kashio Y, Nakamura K, Seki M, Dai S, Shirato Y, et al. Selective eosinophil adhesion to fibroblast via IFN-gamma-induced galectin-9. J Immunol. 2002;169(10):5912–8. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 75.de la Fuente H, Perez-Gala S, Bonay P, Cruz-Adalia A, Cibrian D, Sanchez-Cuellar S, et al. Psoriasis in humans is associated with down-regulation of galectins in dendritic cells. J Pathol. 2012;228(2):193–203. doi: 10.1002/path.3996. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 76.Steelman AJ, Smith R, 3rd, Welsh CJ, Li J. Galectin-9 protein is up-regulated in astrocytes by tumor necrosis factor and promotes encephalitogenic T-cell apoptosis. J Biol Chem. 2013;288(33):23776–87. doi: 10.1074/jbc.M113.451658. PubMed PMID: ; PubMed Central PMCID: PMCPMC3745324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoshida H, Imaizumi T, Kumagai M, Kimura K, Satoh C, Hanada N, et al. Interleukin-1beta stimulates galectin-9 expression in human astrocytes. Neuroreport. 2001;12(17):3755–8. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 78.The Human Protein Atlas. http://www.proteinatlas.org/ENSG00000168961-LGALS9/tissue. Accessed 23 Aug 2016. [Google Scholar]
- 79.Rahimy E, Li FY, Hagberg L, Fuchs D, Robertson K, Meyerhoff DJ, et al. Blood-Brain Barrier Disruption Is Initiated During Primary HIV Infection and Not Rapidly Altered by Antiretroviral Therapy. J Infect Dis. 2017;215(7):1132–40. doi: 10.1093/infdis/jix013. PubMed PMID: ; PubMed Central PMCID: PMCPMC5426376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gal-9 correlates with indices of HIV disease progression. (a) Correlations between CSF and plasma Gal-9 levels and CD4 T-cell counts. (b) CSF and plasma Gal-9 correlations with PBMC-associated HIV DNA levels. Associations between variables were analyzed by Spearman correlations. Statistical significance is indicated as *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.