Abstract
Chronic activation of the brain renin-angiotensin system contributes to the development of hypertension by altering autonomic balance. Beyond the essential role of Angiotensin-II type 1 receptors, A Disintegrin And Metalloprotease 17 (ADAM17) is also found to promote brain renin-angiotensin system over-activation. ADAM17 is robustly expressed in various cell types within the central nervous system. The aim of this study was to determine whether ADAM17 modulates pre-sympathetic neuronal activity to promote autonomic dysregulation in salt-sensitive hypertension. To test our hypothesis, ADAM17 was selectively knocked down in glutamatergic neurons using Cre-loxP technology. In mice lacking ADAM17 in glutamatergic neurons, the blood pressure increase induced by deoxycorticosterone acetate-salt treatment was blunted. Deoxycorticosterone acetate-salt significantly elevated cardiac and vascular sympathetic drive in control mice, while such effects were reduced in mice with ADAM17 knockdown. This blunted sympatho-excitation was extended to the spleen, with a lesser activation of the peripheral immune system, translating into a sequestration of circulating T cells within this organ, compared to controls. Within the paraventricular nucleus, Angiotensin-II-induced activation of kidney-related pre-sympathetic glutamatergic neurons was reduced in ADAM17 knockdown mice, with the majority of cells no longer responding to Angiotensin-II stimulation, confirming the supportive role of ADAM17 in increasing pre-sympathetic neuronal activity. Overall, our data highlight the pivotal role of neuronal ADAM17 in regulating sympathetic activity, and demonstrate that activation of ADAM17 in glutamatergic neurons leads to a selective increase of sympathetic output, but not vagal tone, to specific organs, ultimately contributing to dysautonomia and salt-sensitive hypertension.
Keywords: renin-angiotensin system, sympatho-excitation, immune system, central nervous system
Introduction:
The central nervous system (CNS) plays a critical role in blood pressure (BP) regulation by modulating sympathetic drive to individual organs, leading to changes in blood volume, cardiac output and vascular resistance.1 Within the forebrain, regions like the subfornical organ are capable of sensing changes in sodium and osmolality, which relay to the paraventricular nucleus (PVN) of the hypothalamus and further down to the rostral ventrolateral medulla (RVLM). Altered plasticity of these neuronal networks can lead to an enhanced sympathetic drive and/or compromised vagal tone to various organs and trigger neurogenic hypertension.
The brain renin-angiotensin system (RAS) plays an essential role in the development of neurogenic hypertension. Angiotensin (Ang)-II, the main peptide in this system, increases sympathetic outflow and BP, while decreasing baroreflex gain and vagal tone, by acting on brain Ang-II type 1 receptors (AT1R).2 In addition to the classic axis, compensatory mechanisms exist within the RAS. As a major component of the rescue mechanisms, angiotensin-converting enzyme type 2 (ACE2) plays a critical role in modulating the balance of RAS, by transforming Ang-II into Ang-(1–7), a vasodilator peptide.3 However, CNS expression and activity of ACE2 are found to be reduced during hypertension.4,5 We previously identified that A Disintegrin And Metalloprotease 17 (ADAM17)-mediated shedding is responsible for the reduction of brain ACE2 during salt-sensitive hypertension, and this process was then demonstrated to be mediated via neuronal AT1R.6,7
ADAM17 is a type of sheddase, which cleaves membrane-anchored proteins.8 Upon brain RAS activation, ADAM17 can be up-regulated and activated by AT1R-mediated signaling, such as ROS/ERK and ROS/p38MAPK, ultimately leading to a decrease in ACE2 activity.7 We previously reported that targeting brain ADAM17 using siRNA was able to blunt hypertension, confirming its contribution in BP dysregulation. However, ADAM17 is robustly expressed in various cell types within the CNS,7,9 with a large array of target proteins that could support (e.g. ACE2, TNFα, IL-6) or prevent (e.g. TNFα receptor, NRG1) the development of hypertension.10 Therefore, it is important to understand how activation of ADAM17 in specific neuronal populations could contribute to autonomic regulation.
The action of glutamate accounts for >90% of the excitatory synaptic connections in the vertebrate CNS.11 We recently reported that optogenetic stimulation of the PVN glutamatergic neurons can lead to an immediate increase in BP, showing their critical role in regulating autonomic nervous system.12 Glutamatergic activity is found to be enhanced in neurons projecting from PVN to brainstem or spinal cord in several hypertensive models, including spontaneous hypertensive rats, salt-sensitive hypertension and Ang-II-induced hypertension.13–15 Moreover, activation of AT1R induces both decrease of GABAergic inhibition and increase of glutamatergic activity in neurons projecting from PVN to RVLM.16–18 ACE2/Ang-(1–7) signaling has been suggested to contribute to GABA release,19,20 in which case, ADAM17-mediated ACE2 shedding on GABAergic neurons could potentially promote sympatho-excitation. Previous work performed in the cortex and hippocampus showed that glutamate induces ADAM17-mediated shedding of NRG1, which is thought to modulate excitatory synapse maturation.21 However, there is a lack of knowledge regarding the potential role of ADAM17, located on glutamatergic neurons, in autonomic regulation. In this study, we hypothesized that ADAM17 up-regulation in glutamatergic pre-autonomic neurons would lead to enhanced sympathetic activity during deoxycorticosterone acetate (DOCA)-salt treatment. To test this hypothesis, we generated mice with ADAM17, or AT1R, selectively knocked down from these cells, and investigated the impact of this deletion on sympatho-excitation during the development of salt-sensitive hypertension.
Methods:
A detailed Methods section is available in the Online Data Supplement.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Transgenic mice and animal husbandry
Experiments were performed on adult male mice (14–16 weeks old). ADAM17 knockdown mice (A17G), AT1R knockdown mice (AT1G), and controls carrying one copy of Cre recombinase (heterozygous, Cre+/0) under the control of the vGluT2 (vesicular glutamate transporter 2) promoter (Fig. 1A). All three strains have a C57BL/6J genetic background. All animals were housed in a temperature- and humidity-controlled facility under a 12-hour dark/light cycle, fed standard mouse chow (Envigo, Teklad) and water ad libitum. All procedures were approved by the LSU Health Sciences Center-NO (#3418), Tulane University (#387) and University of Iowa (#5111549) Animal Care and Use Committees and are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
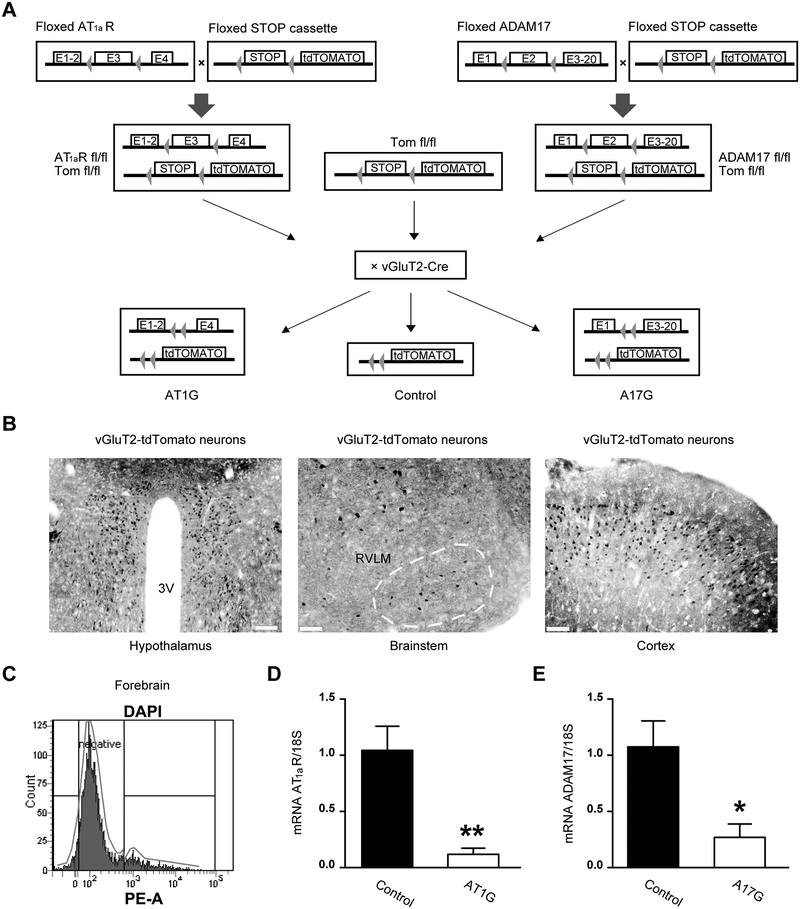
Figure 1. Validation and phenotyping of AT1aR and ADAM17 conditional knockout models.
(A) Schematic of the breeding strategy. Following Cre-mediated (vGluT2-cre) excision of the STOP cassette, tdTomato reporter is expressed specifically in glutamatergic neurons. vGluT2-cre excision of exon 3 from the AT1aR gene and exon 2 of the ADAM17 gene, specifically in glutamatergic neurons, allows for the generation of selective AT1aR (AT1G) and ADAM17 (A17G) knockdown mice. (B) Representative images for tdTomato-positive glutamatergic neurons (black) within the paraventricular nucleus (PVN) of hypothalamus (left, scale bar represents 100 μm), the rostral ventrolateral medulla (RVLM, middle, scale bar represents 50 μm) and cortex (right, scale bar represents 50 μm). (C) Representative sorting strategy for fluorescence-activated cell sorting (FACS) in brain cells. The tdTomato-positive gate (Tomato+ cells) was created based on the PE areas of the sorted cells (DAPI events). The Tomato+ cells were then collected and processed for quantitative reverse transcription polymerase chain reaction (qRT-PCR). (D) AT1aR gene expression (qRT-PCR) performed in tdTomato-positive cells from control and AT1G mice (n=4 mice/group), confirming that AT1aR expression is knocked down in glutamatergic neurons from AT1G mice. (E) qRT-PCR result confirms ADAM17 expression is knocked down in glutamatergic neurons from A17G mice (n=4 mice/group). Data are shown as mean ±SEM. Statistical significance: Student’s t-test: *P<0.05, **P<0.01 vs. control.
To test our hypothesis, conditional knockdown and control mice were subjected to salt-sensitive hypertension while sympathetic drive to various end organs (i.e., heart, vasculature, and spleen) was evaluated using molecular, cellular and pharmacological approaches.
Statistics
Data are presented as means ±SEM. Unless specified otherwise, data were analyzed by repeated measures ANOVA, or two-way ANOVA, followed by Bonferroni post-hoc test for multiple comparisons between means, when appropriate. Statistical analyses were performed using Prism5 (GraphPad Software). Differences were considered statistically significant at P<0.05.
Results:
ADAM17 in glutamatergic neurons mediates salt-sensitive hypertension and impaired baroreflex gain.
To test our hypothesis, we generated two transgenic mouse models, in which ADAM17 (A17G) or AT1R (AT1G) were selectively knocked down from glutamatergic neurons, using Cre-recombinase driven by the vGluT2 promoter. Cre-targeted glutamatergic neurons were visualized using a tdTomato reporter (Tomato+, Fig. 1A–B). Using a FACS approach, Tomato+ cells were collected from the forebrain of AT1G, A17G and controls (Fig. 1C). Validation of the gene knockdown was assessed by qRT-PCR, and significant reduction of AT1aR mRNA in AT1G (Fig. 1D) and ADAM17 mRNA in A17G (Fig. 1E) were confirmed. Mice with ADAM17 or AT1R knockdown from glutamatergic neurons developed normally and there was no significant difference in body, organs or tissues weight compared to controls (Table. S1). Basal renal sympathetic nerve activity (RSNA, Fig. S1A) trended toward a reduction in both A17G and AT1G mice. Surprisingly, sympathetic nerve activity to the brown adipose tissue (BAT-SNA) was found to be markedly higher in A17G mice (Fig. S1B), indicating that the impact of ADAM17 on neuronal activity is not homogenous throughout glutamatergic neurons.
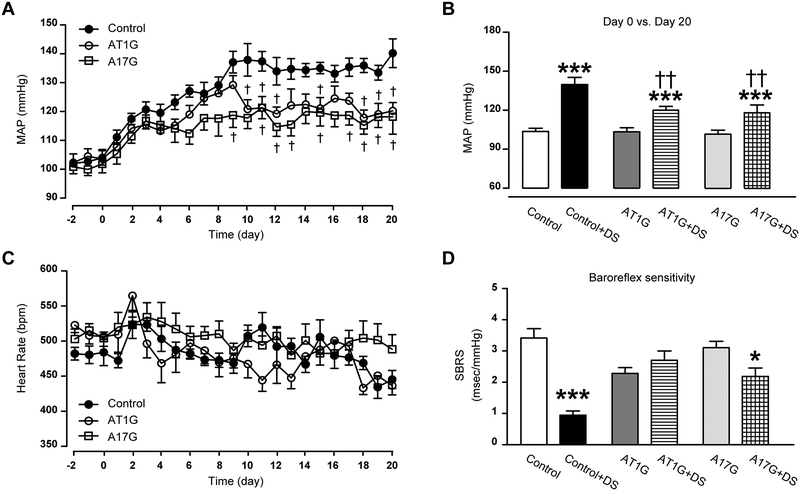
Baseline BP showed no difference among A17G, AT1G and control mice, suggesting that neither ADAM17 nor AT1R in glutamatergic neurons significantly contribute to the maintenance of BP in basal conditions (Fig. 2A–B). Similar to AT1G, daily BP recording following DOCA-salt treatment showed a significantly-blunted hypertension in A17G mice (Fig. 2A), with a plateau shown as early as the end of the first week, when BP is still rising in controls. Despite this blunted hypertension, reflex bradycardia was similar among the 3 groups (Fig. 2C). Further analysis (Fig. 2D) showed that the baroreflex gain of DOCA-salt-treated mice ranged from improved in A17G to preserved in AT1G, while it was dramatically reduced in control mice.
Figure 2. Knockdown of ADAM17 in glutamatergic neurons attenuates salt-sensitive hypertension.
(A) DOCA implanted subcutaneously (1 mg/g of body weight) and combined with 1% saline in the drinking water, induced a progressive increase in mean arterial pressure (MAP) in uninephrectomized control mice. This DOCA-salt-induced hypertension was blunted in both A17G and AT1G mice (n=10 mice/group). (B) Summary data for the MAP values, before and after 20 days of DOCA-salt treatment. (C) Changes in heart rate for control, AT1G and A17G mice over time. (D) Summary data for the spontaneous baroreceptor reflex sensitivity (SBRS), before and after 20 days of DOCA-salt treatment (n=10 mice/group). SBRS was calculated using the sequence method. Data are shown as mean ±SEM. Statistical significance: Two-way ANOVA, Bonferroni post hoc test: *P<0.05, ***P<0.001 vs. respective baselines; †P<0.05, ††P<0.01 vs. control+DS. DS: DOCA-salt.
ADAM17 in glutamatergic neurons contributes to cardiac and vascular sympatho-excitation.
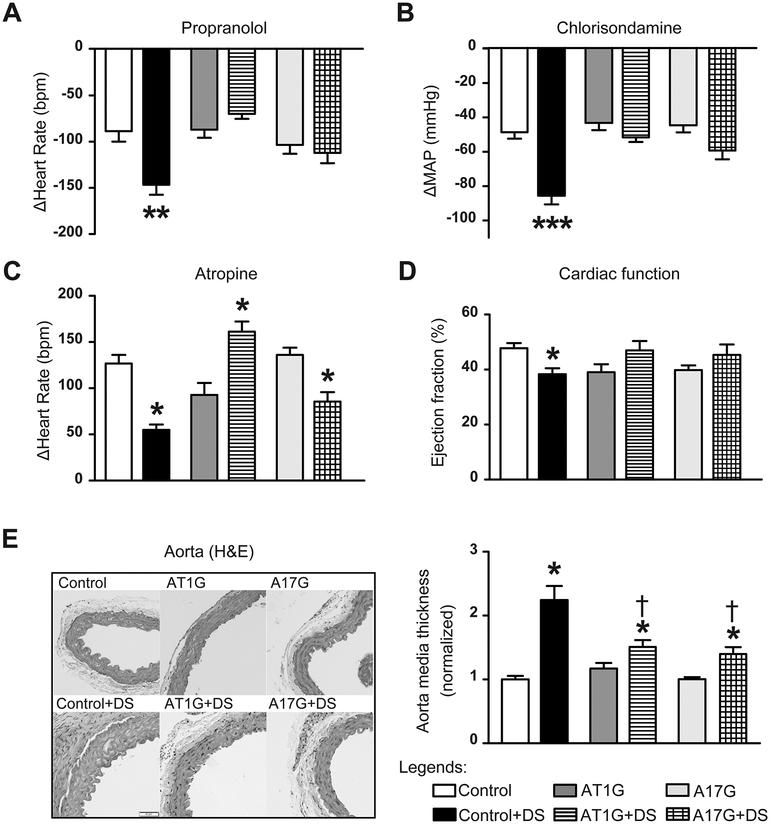
As expected, analysis of autonomic function in control mice showed a typical increase in both cardiac (Fig. 3A) and vascular (Fig. 3B) sympathetic activities, as well as a reduction of vagal tone (Fig. 3C), which altogether contribute to the maintenance of hypertension during DOCA-salt treatment. Knockdown of ADAM17 in glutamatergic neurons did not induce any significant change in autonomic function at baseline. Similar to AT1G, the DOCA-salt-induced increase in sympathetic drive was prevented in A17G mice (Fig. 3A–B), as neither cardiac nor vascular sympathetic drives were increased following DOCA-salt treatment in these mice. Interestingly, cardiac vagal tone was still impaired by DOCA-salt challenge in A17G (*P<0.05 vs. A17G sham, Fig. 3C).
Figure 3. DOCA-salt-induced dysautonomia is attenuated in mice with ADAM17 knockdown in glutamatergic neurons.
(A-C) Autonomic function was assessed pharmacologically by determining the changes in MAP (ΔMAP) and heart rate (ΔHeart Rate) after intraperitoneal injections of a β-blocker (A, propranolol, 4 mg/kg), a ganglionic blocker (B, chlorisondamine, 2.5 mg/kg), and a muscarinic antagonist (C, atropine, 1 mg/kg), n=10 mice/group. (D) Ejection fraction, representing cardiac systolic function, was measured in DOCA-salt or sham-treated mice (n=8 mice/group), by echocardiography. (E) Aorta sections were stained with H&E and the media thickness was quantified using Image J (n=6 mice/group). Data are shown as mean ±SEM. Statistical significance: Two-way ANOVA: *P<0.05, **P<0.01, ***P<0.001 vs. respective baselines or shams; †P<0.05 vs. control+DS. DS: DOCA-salt.
To further confirm the contribution of ADAM17 activation to the increase of sympathetic outflow, cardiac function was evaluated via echocardiography in these mice. DOCA-salt treatment-induced cardiac hypertrophy (Fig. S2A) and early signs of heart failure in control mice, including decreased systolic function (Fig. 3D) and enlarged left ventricle (LV) chamber size (Fig. S2B). This hypertension-driven LV hypertrophy is often considered as end-organ damage resulting from sympathetic activation.22 The early signs of heart failure were completely prevented in A17G, confirming a normalized cardiac sympathetic drive in these mice. Hypertension-induced vascular hypertrophy was also attenuated in A17G mice (†P<0.05 vs. Control+DS, Fig. 3E), as evidenced by the smaller media increase following DOCA-salt treatment. As a global index of sympathetic activity, urinary NE levels were measured at the end of DOCA-salt treatment. Although still elevated, compared to baseline, NE excretion was reduced by ~50% in A17G mice post DOCA-salt treatment (Fig. S2C).
ADAM17 in glutamatergic neurons mediates peripheral immune system activation in salt-sensitive hypertension.
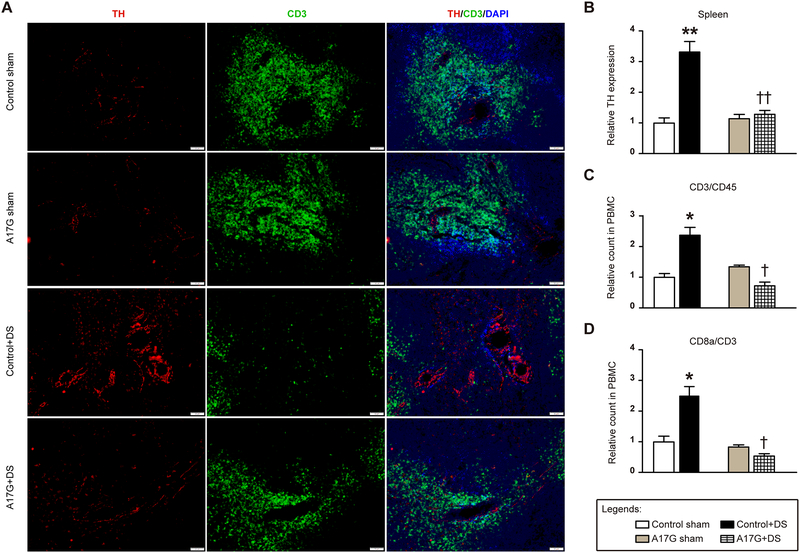
Sympathetic activation is thought to be an important drive for activation of the peripheral immune system, which in turn contributes to hypertension. Our data (Fig. 4A–B) show that 3 weeks of DOCA-salt treatment increases splenic sympathetic activity, as evidenced by tyrosine hydroxylase (TH) levels - a rate-limiting enzyme in the formation of norepinephrine - in a splenic region (white pulp) known for T cells storage. TH expression in the spleen was dramatically elevated in DOCA-salt-treated control mice (**P<0.01 vs. control sham), while it was significantly attenuated in mice lacking ADAM17 in glutamatergic neurons. DOCA-salt treatment is known to induce a significant reduction of splenic T cells (i.e. CD3 expression) and this deployment of T cells appears to be critical for downstream infiltration of target organs and peripheral inflammation.23,24 Indeed, analysis of peripheral blood mononuclear cells (PBMC), isolated from the whole blood, confirmed that specific inflammatory cell populations, including CD3+ and CD8+ T cells, were increased in the circulation of control mice following DOCA-salt challenge (Fig. 4C–D). However, in A17G mice, DOCA-salt hypertension was not able to recruit CD3+ and CD8+ T cells to the circulation and they remained sequestered to the spleen.
Figure 4. Normalized sympathetic outflow attenuates T cells activation in mice with ADAM17 knockdown in glutamatergic neurons.
(A) Immunofluorescence triple-labeling of spleen sections from A17G and control mice, showing tyrosine hydroxylase (TH, red), cluster of differentiation 3 (CD3, green) and nuclei (DAPI, blue). The scale bar represents 50 μm. (B) Summary data for TH expression in mouse spleens (n=4–6 sections/mouse, 4 mice/group). (C) Blood panel analysis on T cells (CD3+/CD45+) in isolated peripheral blood mononuclear cells (PBMC) via flow cytometry (n=10 mice/group). (D) Blood panel analysis on CD8+ T cells in isolated PBMC (n=10 mice/group). Statistical significance: Two-way ANOVA: *P<0.05, **P<0.01 vs. respective shams; †P<0.05, ††P<0.01 vs. control+DS. DS: DOCA-salt.
ADAM17 supports Ang-II-induced neuronal excitation in the PVN.
ADAM17 is robustly expressed in glutamatergic PVN neurons (Fig. 5A). To assess whether ADAM17 modulates neuronal activity, whole-cell patch-clamp recordings were performed on retrogradely-labeled pre-sympathetic glutamatergic PVN neurons (Fig. S3), which project to sympathetic ganglia that innervate the kidney.
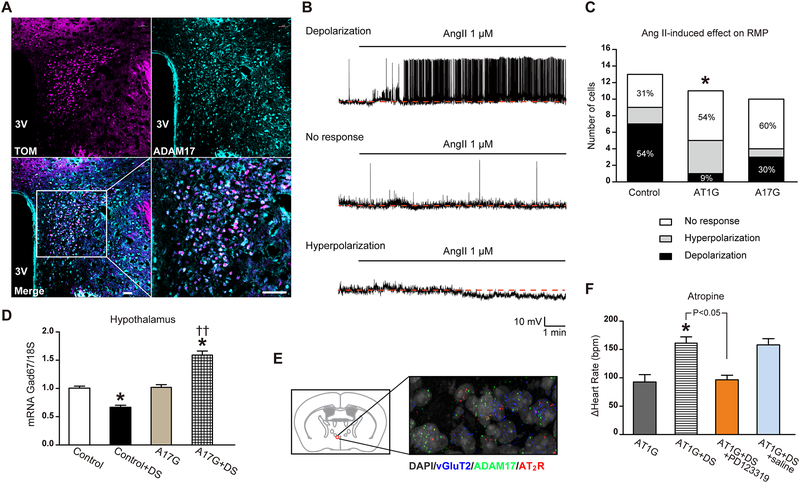
Figure 5. Ang-II-induced sympatho-excitation was reduced in mice with ADAM17 knockdown in glutamatergic neurons.
(A) Within the PVN, tdTomato-expressing glutamatergic neurons (magenta) show robust expression of ADAM17 (cyan), as indicated by the merged panel (white). The scale bar represents 38 μm. (B) Representative traces demonstrating depolarization, no response and hyperpolarization of PVN kidney-related glutamatergic neurons from control mice following Ang-II application. All recorded neurons were visualized through tdTomato and PRV-152 labeling (see Fig. S3). (C) Distribution of the various responses to Ang-II application on resting membrane potential of kidney-related glutamatergic PVN neurons for control (n=13 cells from 6 mice), AT1G (n=11 cells from 5 mice) and A17G (n=10 cells from 4 mice) groups. (D) Glutamate decarboxylase 1 (Gad67) gene expression (qRT-PCR) in hypothalamus of control and A17G mice (n=6 mice/group). (E) Fluorescent in situ hybridization (FISH) on a PVN brain section for visualization of ADAM17 (green) and AT2R (red) in glutamatergic neurons (vGluT2-labeled, blue); the nuclei are labeled with DAPI (grey). Magnification: 400X. (F) Cardiac vagal tone was studied through intraperitoneal injections of a muscarinic antagonist (atropine, 1 mg/kg) in AT1G mice (n=10 mice/group). Saline or PD123319 (5 μg/mouse) was given to the DOCA-salt-treated AT1G mice through intracerebroventricular injection, 20 min before atropine injection (n=6 mice/group). Statistical significance: Chi-square test: *P<0.05 vs. control; Two-way ANOVA: *P<0.05 vs. respective sham or baseline, ††P<0.01 vs. control+DS. Student’s t-test: AT1G+DS vs. AT1G+DS+PD123319, P<0.05. DS: DOCA-salt.
At baseline, the resting membrane potential (RMP) of kidney-related PVN neurons was similar in control: −48.88 ±2.50 mV (n=13 cells from 6 mice); AT1G: −47.41 ±2.37 mV (n=11 cells from 5 mice) and A17G: −44.73 ±1.26 mV (n=10 cells from 4 mice) (Fig. S4A–C). In control mice, bath application of Ang-II did not have a significant effect on the overall RMP, but analysis of individual neurons revealed that 7 out of 13 kidney-related PVN neurons depolarized (−50.96 ±4.40 mV vs. −44.93 ±4.74 mV, P=0.015), 2 hyperpolarized and 4 were non-responsive to Ang-II (Fig. 5B–C and S4D). In contrast, in AT1G only 1 out of 11 pre-sympathetic PVN neurons depolarized following Ang-II, confirming that this Ang-II-induced response was mainly mediated by AT1R on glutamatergic neurons (*P<0.05, AT1G vs. control). In A17G mice, only 3 out of 10 kidney-related PVN neurons depolarized following Ang-II application, supporting a reduced excitability of glutamatergic neurons in the shortage of ADAM17. In addition, while it showed no difference in control neurons (Fig. S4F), the input resistance (Rin) was significantly lower in responder neurons of A17G, compared to their non-responder counterparts (Fig. S4G). This suggests that depolarization of A17G neurons might require more ion channels to open.
We assessed the expression of Gad67, a critical enzyme that catalyzes the formation of GABA, to possibly explain the blunted sympatho-excitation in A17G mice. As shown in Figure 5D, DOCA-salt treatment significantly reduced Gad67 mRNA level in the hypothalamus, implying an impaired GABAergic inhibitory activity in DOCA-salt hypertension. On the other hand, Gad67 expression was significantly higher in the hypothalamus of DOCA-salt-treated A17G mice, suggesting a negative impact of ADAM17 activation on GABAergic signaling. Meanwhile, mRNA levels for NMDA receptors (Grin1), AMPA receptors (GluR1) and GABAa receptors in the hypothalamus of A17G mice were unaffected compared to controls (Fig. S5A). Assessment of ADAM17’s presence in other neuronal populations and brain regions revealed its expression in GABAergic neurons within the MnPO (median preoptic nucleus), PVN and BNST (bed nucleus of the stria terminalis) (Fig. S5B). As to the brainstem, ADAM17 is also distributed in glutamatergic neurons, including the RVLM and NTS (Fig. S5C–D).
Finally, to clarify the paradoxical increase of vagal tone during hypertension in mice lacking AT1R on glutamatergic neurons (Fig. 3C), we speculated that AT2R might be involved. In situ hybridization revealed that AT2R are also expressed on glutamatergic neurons within the PVN (Fig. 5E), possibly opposing the AT1R-mediated sympatho-excitation. Icv pre-treatment with PD123319, a specific AT2R antagonist, completely abolished the enhanced cardiac vagal tone in DOCA-salt-treated AT1G mice (Fig. 5F).
Discussion:
Although ADAM17 was originally discovered as a key sheddase for cytokines release, with a wide spectrum of substrates,8 our previous reports highlighted its important role, beyond inflammation, in the development of hypertension.6,7 We previously reported that during salt-sensitive hypertension, up-regulation of ADAM17 takes place selectively in neurons, following activation of AT1R-mediated signaling pathways.7 Here we aimed at clarifying the contribution of neuronal ADAM17 to autonomic dysregulation in salt-sensitive hypertension.
Among the new findings of our study in mice lacking glutamatergic ADAM17, are: (i) a preserved cardiovascular function (Fig. 3), (ii) a blocked activation of the immune system (Fig. 4) and (iii) persistence of an impaired vagal tone following DOCA-salt treatment. Specifically, these mice exhibited a blunted surge of sympathetic activity, which resulted in a lesser BP increase, reduced cardiac and vascular remodeling, and impaired deployment of T cells to target organs.
Recently, activation of splenic sympathetic nerves was shown to play an essential role in the development of peripheral inflammation and hypertension.23 The spleen is mainly innervated by sympathetic nerve terminals, and splenic sympathetic nerve activity was found to be increased at the early phase of DOCA-salt treatment.24 In mice with ADAM17 knockdown, DOCA-salt-induced increases in TH expression and circulating T cells were effectively blocked, while their BP was still significantly higher than baseline, supporting a critical role of the sympathetic drive, rather than BP itself, in activating the peripheral immune system. Although global deletion of ADAM17 has been associated with impaired T cell development and B cell maturation,25 persistence of ADAM17 activity outside of glutamatergic neurons is sufficient to maintain a normal physiological function,8 so our observation is not related to developmental defects.
According to our previous study, the activity of glutamatergic neurons, in regions like the PVN, is directly linked to autonomic regulation.12 Indeed, photo-activation of these PVN glutamatergic neurons resulted in an immediate BP rise, while their destruction prevented the development of salt-sensitive hypertension. Therefore, we speculated that ADAM17 could take part in sympatho-excitation by modulating the excitability of these glutamatergic neurons. Our patch clamp experiments confirmed this assumption as the proportion of depolarized cells, in response to Ang-II, was reduced in kidney-projecting pre-sympathetic glutamatergic neurons from ADAM17 knockdown mice. This supportive role of ADAM17 towards neuronal excitability of pre-sympathetic glutamatergic neurons has not been reported previously but is consistent with an early study in ADAM17 global KO mice where the authors speculated an increase in sympathetic activity to the brown adipose tissue,26 a feature that was confirmed in our animals (Fig. S1B).
Interestingly, targeting ADAM17 did not produce the same effects as knocking down AT1R, with ADAM17 knockdown solely impacting sympathetic activity while AT1R removal affected both sympathetic and parasympathetic outputs. Unlike AT1G and other models we reported previously,6,7 cardiac vagal tone was not protected in DOCA-salt-treated A17G mice (Fig. 3C), suggesting that activation of ADAM17 in glutamatergic neurons has no or fairly limited role in reducing vagal tone, but exclusively contributes to sympathetic output. Accordingly, Ang-II/AT1R signaling is thought to impair vagal activity through an ADAM17-independent manner, which would support the reduction of baroreflex sensitivity observed in DOCA-salt treated A17G mice (Fig. 2D).
The involvement of brain AT1R in autonomic dysfunction has been well established in hypertension and heart failure.2 Recent studies revealed a critical role of astrocyte AT1R in modulating the excitability of glutamatergic neurons and sympatho-excitation.27,28 In our study, strong evidence was provided to support the indispensable role of neuronal AT1R in Ang-II-induced increase of glutamatergic activity and sympatho-excitation. In addition to in vivo data (Fig. 2–3), the Ang-II-induced depolarization of glutamatergic neurons was found to be altered towards a non-responding state or hyperpolarization, when AT1R were knocked down from those neurons (Fig. 5C). Interestingly, DOCA-salt treatment produced an unexpected rise in vagal tone in AT1G mice (Fig. 3C). This was demonstrated to be mediated by Ang-II/AT2R signaling (Fig. 5E–F), which was unmasked when AT1R were knocked down. A similar role for AT2R has previously been reported in the brainstem.29
AT1R-mediated ADAM17 activation could support sympatho-excitation by decreasing the compensatory activity of ACE2 and increasing soluble TNFα release. Indeed, ACE2 cleaves a phenylalanine from the carboxyl terminal end of Ang-II to form Ang-(1–7).30 Through activation of a nitric oxide (NO)-mediated pathway, Ang-(1–7) can significantly increase extracellular GABA level, without affecting glutamate release,19 supporting its sympatho-inhibitory role. However, the limited expression of ACE2 in PVN glutamatergic neurons (Fig. S6) suggests that glutamatergic ADAM17 might promote sympatho-excitation in a manner independent of ACE2 shedding. Unlike Ang-(1–7), TNFα plays a contributory role in glutamate-induced excitotoxicity, by inhibiting glutamate transporters on astrocytes both directly and indirectly.31,32 On one hand, TNFα has direct effects on glutamate transmission, for example: stimulating the activity of NMDA receptors and up-regulating the expression of synaptic AMPA receptors.33,34 On the other, acute application of TNFα can induce a rapid and persistent decrease of inhibitory synaptic strength and down-regulation of GABAa receptors.35 Moreover, downstream of the ADAM17/TNFα pathway, activation of NF-κb is also found to be involved in the reduction of Gad67 and GABA in the PVN.36–38
Despite the novel finding of ADAM17 selective contribution to sympathetic activity, there are some limitations to our study. First, in A17G mice, knockdown of ADAM17 is taking place in a vast network of glutamatergic neurons beyond those located in BP regulation-related nuclei (e.g. PVN, NTS and RVLM) and the impact of this additional deletion is difficult to estimate. We recognize the possible contribution from sensory neurons, such as DRG neurons, which also express vGluT2. Although further work is needed to address those contributions, we consider our approach necessary to support further, more discrete, deletion in specific regions.12 Second, although the percentage of Ang-II-depolarized neurons was convincingly decreased in A17G mice, it did not reach statistical significance, due to the small sample size. However, we are confident that together our in vivo and ex vivo data support the important role of ADAM17 in sympatho-excitation.
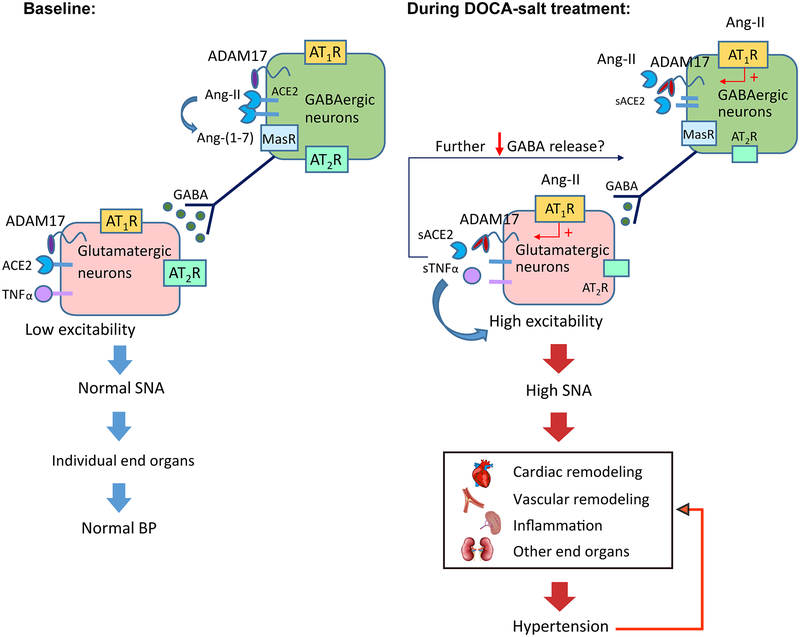
Overall, our study suggests that activation of ADAM17 in glutamatergic neurons promotes the development of DOCA-salt hypertension by selectively increasing sympathetic outflow. As illustrated in our working model (Fig. 6), activation of ADAM17 in glutamatergic neurons could increase the excitability of these neurons by releasing TNFα and compromising the compensatory activity of ACE2. Although the mechanism still needs to be verified, ADAM17-mediated shedding in glutamatergic neurons seems to have a negative impact on GABAergic inhibitory activity in DOCA-salt hypertension. Altogether, ADAM17 activation supports the increase of glutamatergic neuronal excitability and sympatho-excitation, thus contributing to the development of neurogenic hypertension.
Figure 6. A working model explaining how neuronal ADAM17 support the development of salt-sensitive hypertension.
At baseline, sympathetic outflow and BP is controlled, due to the low excitability of pre-sympathetic glutamatergic neurons. Upon DOCA-salt treatment, up-regulated ADAM17 could increase the excitability of these neurons by releasing TNFα and compromising the compensatory activity of ACE2, therefore promoting sympatho-excitation. As result, cardiac output, vascular capacitance and resistance, and deployment of T cells from spleen are increased, inducing uncontrolled elevation of BP. With prolonged dysregulation of autonomic nervous system and BP, cardiovascular remodeling and peripheral inflammation are developed, further supporting the maintenance of hypertension.
Perspective:
Using a salt-sensitive hypertension model, we uncovered that activation of ADAM17 in glutamatergic neurons leads to a selective increase of sympathetic output. ADAM17-mediated shedding is also taking place in the CNS of hypertensive patients. Exploring the role of ADAM17 in a specific population of brain cells might help us better understand drug-resistant hypertension, and identify more effective treatments. Despite our findings, questions still remain. Further investigation is required to map the precise neuronal networks and mechanisms, through which ADAM17 participates to neuronal activation and sympatho-excitation.
Supplementary Material
Novelty and Significance:
1). What is new?
We demonstrated that activation of ADAM17 (A Disintegrin And Metalloprotease 17) in glutamatergic neurons supports the increase of excitability on pre-sympathetic neurons and sympatho-excitation in a salt-sensitive hypertension model.
We found that activation of ADAM17 in glutamatergic neurons has no role in reducing vagal tone, but exclusively contributes to the increase of sympathetic drive.
Salt-induced activation of peripheral immune system depends on the activation of ADAM17 in glutamatergic neurons.
2). What is relevant?
The autonomic nervous system is dysregulated in salt-sensitive hypertension.
During salt-sensitive hypertension, ADAM17 is upregulated and activated in neurons, leading to sympatho-excitation and neuro-inflammation.
3). Summary:
Our data highlight the pivotal role of ADAM17 in glutamatergic neurons in regulating sympathetic activity, and demonstrate that its activation leads to a selective increase of sympathetic output to specific organs, such as heart, vasculature and spleen, ultimately contributing to salt-sensitive hypertension through altering cardiovascular function and promoting peripheral inflammation.
Acknowledgements:
The authors would like to thank Ms. Constance Porretta for technical assistance with cell sorting and PBMC analysis, Dr. Luis Marrero and the Morphology and Imaging Core for technical support with HE staining and confocal microscopy, and Dr. Deng-Fu Guo (Univ. of Iowa) for technical assistance in RNAscope.
Sources of Funding:
This study was supported in part by research grants from the National Institutes of Health (HL093178 to E.L. and COBRE P30GM106392), the department of Veterans Affairs (BX004294) and the Research Enhancement Program at LSUHSC-NO. K.R. is supported by the NIH (HL084207), the American Heart Association (14 EIA18860041) and University of Iowa Fraternal Order of Eagles Diabetes Research Center.
Footnotes
Conflict of interest/Disclosure:
None.
References:
- 1.Averina VA, Othmer HG, Fink GD, Osborn JW. A mathematical model of salt-sensitive hypertension: The neurogenic hypothesis. The Journal of physiology. 2015;593:3065–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zucker IH, Schultz HD, Patel KP, Wang W, Gao L. Regulation of central angiotensin type 1 receptors and sympathetic outflow in heart failure. Am J Physiol Heart Circ Physiol. 2009;297:H1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia H, Lazartigues E. Angiotensin-converting enzyme 2: Central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension. 2007;49:926–931. [DOI] [PubMed] [Google Scholar]
- 5.Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin ii type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension. 2009;53:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia H, Sriramula S, Chhabra KH, Lazartigues E. Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ Res. 2013;113:1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Sriramula S, Xia H, Moreno-Walton L, Culicchia F, Domenig O, Poglitsch M, Lazartigues E. Clinical relevance and role of neuronal at1 receptors in adam17-mediated ace2 shedding in neurogenic hypertension. Circ Res. 2017;121:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Mukerjee S, Silva-Alves C, Carvalho-Galvão A, Cruz J, Balarini C, Braga V, Lazartigues E, França-Silva MdS. A disintegrin and metalloprotease 17 in the cardiovascular and central nervous systems. Front Physiol. 2016;7:doi: 10.3389/fphys.2016.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palazuelos J, Crawford HC, Klingener M, Sun B, Karelis J, Raines EW, Aguirre A. Tace/adam17 is essential for oligodendrocyte development and cns myelination. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:11884–11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song XA, Jia LL, Cui W, Zhang M, Chen W, Yuan ZY, Guo J, Li HH, Zhu GQ, Liu H, Kang YM. Inhibition of tnf-alpha in hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy by inhibiting neurohormonal excitation in spontaneously hypertensive rats. Toxicology and applied pharmacology. 2014;281:101–108. [DOI] [PubMed] [Google Scholar]
- 11.Meldrum BS. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. The Journal of nutrition. 2000;130:1007S–1015S. [DOI] [PubMed] [Google Scholar]
- 12.Basting T, Xu J, Mukerjee S, Epling J, Fuchs R, Sriramula S, Lazartigues E. Glutamatergic neurons of the paraventricular nucleus are critical contributors to the development of neurogenic hypertension. The Journal of physiology. 2018;596:6235–6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi J, Zhang DM, Suo YP, Song XA, Yu XJ, Elks C, Lin YX, Xu YY, Zang WJ, Zhu Z, Kang YM. Renin-angiotensin system modulates neurotransmitters in the paraventricular nucleus and contributes to angiotensin ii-induced hypertensive response. Cardiovascular toxicology. 2013;13:48–54. [DOI] [PubMed] [Google Scholar]
- 14.Gabor A, Leenen FH. Cardiovascular effects of angiotensin ii and glutamate in the pvn of dahl salt-sensitive rats. Brain research. 2012;1447:28–37. [DOI] [PubMed] [Google Scholar]
- 15.Qiao X, Zhou JJ, Li DP, Pan HL. Src kinases regulate glutamatergic input to hypothalamic presympathetic neurons and sympathetic outflow in hypertension. Hypertension. 2017;69:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q, Pan HL. Signaling mechanisms of angiotensin ii-induced attenuation of gabaergic input to hypothalamic presympathetic neurons. Journal of neurophysiology. 2007;97:3279–3287. [DOI] [PubMed] [Google Scholar]
- 17.Li DP, Pan HL. Angiotensin ii attenuates synaptic gaba release and excites paraventricular-rostral ventrolateral medulla output neurons. The Journal of pharmacology and experimental therapeutics. 2005;313:1035–1045. [DOI] [PubMed] [Google Scholar]
- 18.Freeman KL, Brooks VL. At(1) and glutamatergic receptors in paraventricular nucleus support blood pressure during water deprivation. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;292:R1675–1682. [DOI] [PubMed] [Google Scholar]
- 19.Stragier B, Hristova I, Sarre S, Ebinger G, Michotte Y. In vivo characterization of the angiotensin-(1–7)-induced dopamine and gamma-aminobutyric acid release in the striatum of the rat. The European journal of neuroscience. 2005;22:658–664. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, de Kloet AD, Pati D, Hiller H, Smith JA, Pioquinto DJ, Ludin JA, Oh SP, Katovich MJ, Frazier CJ, Raizada MK, Krause EG. Increasing brain angiotensin converting enzyme 2 activity decreases anxiety-like behavior in male mice by activating central mas receptors. Neuropharmacology. 2016;105:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwakura Y, Wang R, Inamura N, Araki K, Higashiyama S, Takei N, Nawa H. Glutamate-dependent ectodomain shedding of neuregulin-1 type ii precursors in rat forebrain neurons. PLoS One. 2017;12:e0174780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenwood JP, Scott EM, Stoker JB, Mary DA. Hypertensive left ventricular hypertrophy: Relation to peripheral sympathetic drive. J Am Coll Cardiol. 2001;38:1711–1717. [DOI] [PubMed] [Google Scholar]
- 23.Carnevale D, Perrotta M, Pallante F, Fardella V, Iacobucci R, Fardella S, Carnevale L, Carnevale R, De Lucia M, Cifelli G, Lembo G. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nature communications. 2016;7:13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrotta M, Lori A, Carnevale L, Fardella S, Cifelli G, Iacobucci R, Mastroiacovo F, Iodice D, Pallante F, Storto M, Lembo G, Carnevale D. Deoxycorticosterone acetate-salt hypertension activates placental growth factor in the spleen to couple sympathetic drive and immune system activation. Cardiovascular research. 2018;114:456–467. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Boyd K, Dempsey PJ, Vignali DA. Non-cell autonomous expression of tnf-alpha-converting enzyme adam17 is required for normal lymphocyte development. Journal of immunology. 2007;178:4214–4221. [DOI] [PubMed] [Google Scholar]
- 26.Gelling RW, Yan W, Al-Noori S, Pardini A, Morton GJ, Ogimoto K, Schwartz MW, Dempsey PJ. Deficiency of tnfalpha converting enzyme (tace/adam17) causes a lean, hypermetabolic phenotype in mice. Endocrinology. 2008;149:6053–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stern JE, Son S, Biancardi VC, Zheng H, Sharma N, Patel KP. Astrocytes contribute to angiotensin ii stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension. 2016;68:1483–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isegawa K, Hirooka Y, Katsuki M, Kishi T, Sunagawa K. Angiotensin ii type 1 receptor expression in astrocytes is upregulated leading to increased mortality in mice with myocardial infarction-induced heart failure. Am J Physiol Heart Circ Physiol. 2014;307:H1448–1455. [DOI] [PubMed] [Google Scholar]
- 29.Gao L, Zucker IH. At2 receptor signaling and sympathetic regulation. Current opinion in pharmacology. 2011;11:124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gironacci MM, Longo Carbajosa NA, Goldstein J, Cerrato BD. Neuromodulatory role of angiotensin-(1–7) in the central nervous system. Clin Sci (Lond). 2013;125:57–65. [DOI] [PubMed] [Google Scholar]
- 31.Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB. Insights into glutamate transport regulation in human astrocytes: Cloning of the promoter for excitatory amino acid transporter 2 (eaat2). Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickering M, Cumiskey D, O’Connor JJ. Actions of tnf-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–670. [DOI] [PubMed] [Google Scholar]
- 33.Jara JH, Singh BB, Floden AM, Combs CK. Tumor necrosis factor alpha stimulates nmda receptor activity in mouse cortical neurons resulting in erk-dependent death. Journal of neurochemistry. 2007;100:1407–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial tnfalpha. Science. 2002;295:2282–2285. [DOI] [PubMed] [Google Scholar]
- 35.Pribiag H, Stellwagen D. Tnf-alpha downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of gaba(a) receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:15879–15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurtado O, Lizasoain I, Fernandez-Tome P, Alvarez-Barrientos A, Leza JC, Lorenzo P, Moro MA. Tace/adam17-tnf-alpha pathway in rat cortical cultures after exposure to oxygen-glucose deprivation or glutamate. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22:576–585. [DOI] [PubMed] [Google Scholar]
- 37.Madrigal JL, Hurtado O, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, Leza JC. The increase in tnf-alpha levels is implicated in nf-kappab activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2002;26:155–163. [DOI] [PubMed] [Google Scholar]
- 38.Kang YM, Gao F, Li HH, Cardinale JP, Elks C, Zang WJ, Yu XJ, Xu YY, Qi J, Yang Q, Francis J. Nf-kappab in the paraventricular nucleus modulates neurotransmitters and contributes to sympathoexcitation in heart failure. Basic research in cardiology. 2011;106:1087–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.