Abstract
Introduction:
Liquid biopsy platforms are being actively developed in the biomarker field. Extracellular vesicles (EVs), especially the tumor-derived exosome subsets of EVs, represent a platform that allows for molecular and genetic profiling of parent tumor cells. Tumor-derived exosomes (TEX) are ubiquitous in body fluids of cancer patients and are promising clinically-relevant surrogates of cancer cells.
Areas covered:
Isolation from body fluids of cancer patients and subsetting of exosomes based on immunoaffinity capture offers a means of evaluating proteins, lipids, nucleic acids and other molecular contents that are a characteristic of TEX and exosomes produced by reprogrammed normal cells. The same liquid biopsy can inform about the status of a tumor and simultaneously evaluate the competency of immune cells to mediate anti-tumor activities.
Expert Commentary:
TEX and reprogrammed non-TEX isolated from plasma of cancer patients have the potential to become non-invasive biomarkers of cancer diagnosis, prognosis and response to therapies.
Keywords: extracellular vesicles, tumor-derived exosomes, TEX, cancer, non-invasive biomarkers, immune capture, molecular profiling, protein cargo
1. Introduction
The concept of “liquid biopsy” is an attractive alternative to a solid tumor biopsy, largely because repeated sampling of tumor tissue is usually not possible and because the detection of residual tumor tissue or of early relapse following therapy is seldom successful. The use of liquid biopsy for early cancer diagnosis or monitoring responses to therapy and predicting outcome has been pursued for a long time employing various approaches. Liquid biopsy platforms evaluated to date include assessments of plasma proteins [1], immune complexes [2], cytokine profiling [3], circulating tumor cells [4], circulating tumor (CT) DNA [5], cell-free (CF) RNA [6,7] and, more recently, extracellular vesicles [8]. Each of these platforms has advantages as well as disadvantages that have been broadly discussed in the literature and to date, a few have been validated for clinical monitoring in cancer. The major barrier has been the failure to identify a unique profile of biomarkers that robustly correlates with clinical endpoints and outcome. The currently favored approach relies on the system approach in which combinations of various platforms are used to come up with a combination biomarker profile that also includes mutational analyses [9]. In this ongoing global search for cancer biomarkers, extracellular vesicles (EVs) have emerged as a promising liquid biopsy platform. In the recent 3-4 years, EVs have been attracting increasing attention as future biomarkers of response to cancer therapies and clinical outcome.
EVs are produced by all cells and are present in all body fluids. They represent a heterogenous mix of membrane-bound nanovesicles of various sizes and different cellular origins [10,11]. Among them, EVs produced by tumor cells are of special interest, because they appear to play an active role in tumor progression, including transfer of bioactive molecules from the tumor to normal cells that leads to reprogramming of the tumor microenvironment (TME) [12]. EVs produced by the tumor carry messages from the tumor to non-tumor cells and are responsible for converting the TME into a tumor-promoting milieu, a process referred to as “reprogramming.” Tumors release a variety of EVs which, based on their smallest to largest size, can be divided into exomeres, exosomes, microvesicles, oncosomes and large apoptotic bodies [13]. These EV subpopulations differ not only in size but also in the molecular cargo, displaying diverse protein, lipid, glycosylation profiles and different nucleic acid contents [14]. Specifically, oncosomes differ from other EVs by the cargo of oncogenic proteins acquired from cancer cells[15]. Currently, the nomenclature of EVs remains unsettled, and the field waits for more formal guidelines to be formulated.
Tumor-derived exosomes (TEX), which consist of vesicles ranging in size from 30-150nm, have a well-defined biogenesis and are of greatest interest as potential cancer biomarkers [16]. Exosomes are formed in multivesicular bodies (MVBs) of the parent cell, and the presence in the exosome cargo of the endocytic markers, such as Tsg101 or Alix, confirms their endocytic origin [11]. TEX also carry tetraspanins, although their content varies depending on the tumor type: for example, lung cancer-derived exosomes are rich in CD38, while melanoma-derived exosomes carry high levels of CD63 (TLW personal observations). The exosome molecular content mimics that of the parent cell, although it may be that this similarity is partial rather than complete. Nevertheless, TEX have been looked upon as potential liquid biopsies of parent cancer cells, and numerous recent studies have shown that protein, lipid or nucleic acid content of exosomes resembles that found in the parent tumor cells [17]. Further, TEX have been shown to carry and deliver to recipient cells biologically active molecules which upon gaining entry into the recipient cell interior proceed to reprogram its functions [18]. Thus, TEX serve not only carriers of molecular contents from the parent to recipient cell, but are functionally active nanoparticles conveying distinct messages from the tumor to local or distantly situated normal cells. As biomarkers, TEX have an advantage of circulating freely in all body fluids, while carrying molecular information as well as functional signals necessary for reprogramming of recipient cells [19]. It is because of these attributes that TEX are considered especially promising as a future liquid biopsy platform.
TEX carry and deliver protein cargos as well as RNA and micro (mi)-RNAs to recipient cells. Both protein and nucleic acid components of the TEX cargo have received much attention, and their respective roles in the TME reprogramming have been much debated [20]. The current perception is that miRNAs delivered to and internalized by recipient cells in a protective, membrane-bound form, represent the major mechanism of transcriptional alterations resulting in reprogramming of the TME. Accordingly, much recent effort has been devoted to mi-RNA-mediated signaling and to studies of mi-RNA profiles TEX carry and deliver to recipient cells [21]. These studies have been recently reviewed [22]. The objective of this review is to consider recent new developments in TEX isolation from cancer plasma and to take advantage of new insights into TEX signaling initiated by the surface proteins these vesicles deliver to recipient cells. Mainly, this review will attempt to illustrate how the molecular signals delivered by TEX to immune cells can be explored and channeled into a search for new cancer biomarkers.
2. Exosome isolation from body fluids
Most of the studies profiling TEX molecular or genetic content have utilized tumor cell lines and exosomes isolated from their supernatants. While this approach provides an excellent opportunity for comparative analysis of TEX and their parent cells without interference from non-tumor exosomes, it is a model culture system that does not approximate the complexity of exosomes present in human body fluids. Plasma or serum of a patient with cancer is a complex heterogenous mixture of millions of vesicles derived from tumor and normal cells, all carrying their unique cargos as well as plasma proteins that invariably coat exosome surface membranes. Human tumors produce large quantities of exosomes, and the total exosome levels in plasma of cancer patients are elevated (Figures 1 and 3). It appears that total plasma levels of exosomes in patients with cancer reflects disease activity, tumor stage, response to therapy and even outcome [23]. Total plasma exosomes can be isolated by a wide variety of methods as recently described [8,24]. Ultracentrifugation has been most frequently used for exosome isolation, but since it is not a rapid high throughput procedure, it is being gradually replaced by more clinically- applicable isolation methods such as, e.g., size exclusion chromatography (SEC). Figure 2 illustrates the SEC method and the characteristics of exosomes isolated by SEC. The major advantage of SEC is that it yields exosomes that are morphologically intact, partly depleted of “contaminating” plasma proteins and functionally competent, as determined in co-incubation assays of exosomes with immune cells [25]. SEC commonly recovers 1×1010-1×1012 exosomes from 1 mL of pre-cleared cancer plasma [25].
Figure 1.
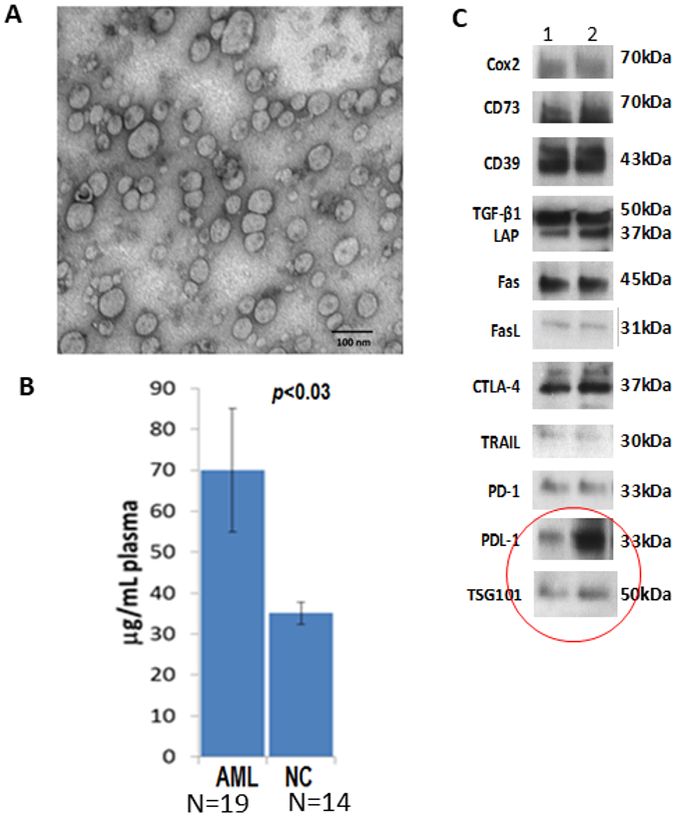
Characteristics of blast-derived exosomes isolated from plasma of patients with acute myeloid leukemia (AML). In A, transmission electron microscopy (TME) image of exosomes isolated by size exclusion chromatography (SEC) from plasma of an AML patient at diagnosis. Note the size heterogeneity of these exosomes. In B, protein levels (calculated per 1mL of plasma) of exosome fractions isolated by SEC from plasma of AML patients at the time of diagnosis and from normal controls. In C, representative Western blots of exosomes from plasma of two AML patients showing the presence of several immunoinhibitory proteins. The red circle calls attention to the presence of PD-L1 in the exosome cargo and of TSG101, an endocytic marker.
Figure 3.
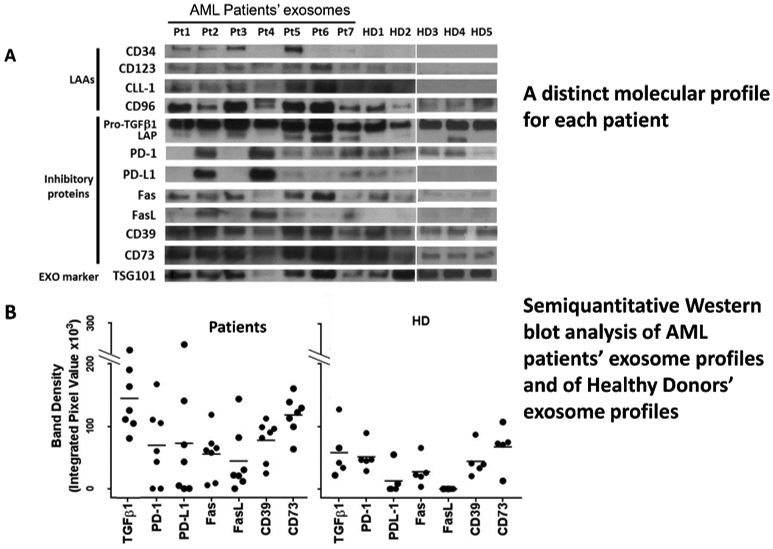
Western blots of exosomes isolated from plasma of 7 AML patients studied at the time of diagnosis and of 5 healthy donors. In A, note the prominent presence of leukemia-associated antigens (LAA) and numerous immunoinhibitory proteins in AML exosomes. In B, semiquantitation of the bands for the immunosuppressive exosome cargo indicates quantitative rather than qualitative differences in exosome proteins. Reproduced from Hong CS et al, ref. 26 under the terms of Creative Commons Attribution 4.0 International License: http://creativecommons.org/licenses/by/4.0/.
Figure 2.
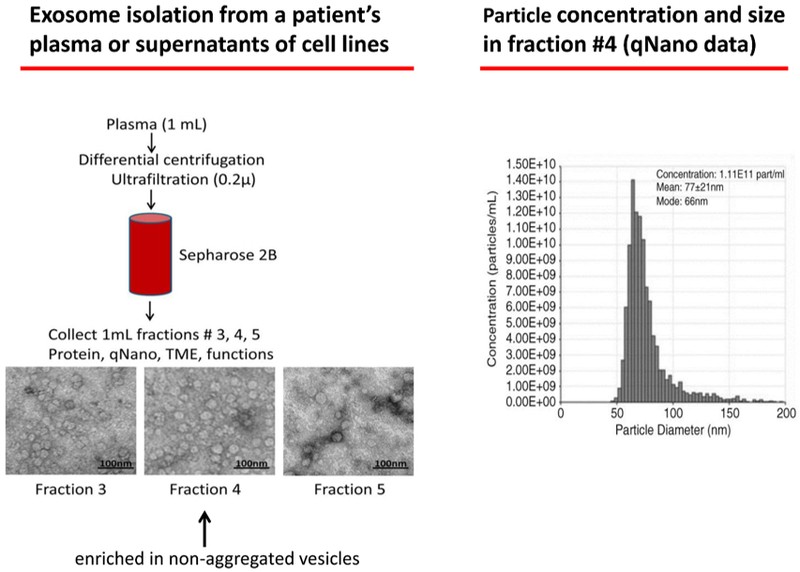
A schema for exosome isolation from plasma using miniSEC on a Sepharose 2B column and PBS as eluent. 1 mL fractions are collected and precleared from cell fragments and large protein aggregates by centrifugations, as previously described (ref. 24) and placed on the column. Under the experimental conditions used, fraction #4 is enriched in morphologically intact, non-aggregated vesicles that are partly depleted of “contaminating” plasma proteins. qNano measurements confirm the particle size and calculate their concentration at 1.1×1011 per mL.
The total exosome fractions isolated by SEC from plasma of cancer patients and studied for their molecular content by Western blots are found to be enriched in tumor-associated antigens and numerous immunoinhibitory proteins, as illustrated in Figure 1. Further, Western blots of total exosomes isolated from plasma of different donors have unique profiles, and the protein profiles of exosomes from cancer patients’ plasma are different from those of exosomes from healthy donors’ plasma (Figure 3A). Interestingly, the differences in protein cargos between exosomes from cancer vs healthy plasma are quantitative rather than qualitative (Figure 3B), indicating that quantitative profiling of the exosome molecular content is necessary to distinguish cancer patients from healthy donors. Another important and consistent feature of plasma exosomes in patients with cancer is their enrichment in immunosuppressive proteins, including PD-L1 or CTLA-4, and a relative paucity of immunostimulatory proteins [26]. This phenotypic profile of total exosome fractions in cancer patients’ plasma translates into their functional potential. We and others have been long cognizant of the fact that exosomes in plasma of cancer patients mediate strong immunosuppression that leads to a loss of anti-tumor functions in immune effector cells in vitro and in vivo [27,28].
3. Exosomes reprogram functions of immune cells
Exosomes from plasma of patients with cancer, but not exosomes from plasma of healthy donors, co-incubated briefly with human primary immune cells induce profound phenotypic and functional changes in these cells [27]. We have reported that exosome from cancer patients’ plasma interacting directly with immune cells can induce downregulation in expression of the TCR-associated zeta chain and of activation markers such as CD69 in T cells; they downregulate expression of NKG2D in natural killer (NK) cells and inhibit their cytolytic functions; they interfere with T-cell proliferation or rapidly induce apoptosis of activated CD8+ effector T cells [29]. These exosomes can also inhibit immune responses indirectly by skewing T-cell differentiation to regulatory T cells (Treg), promoting Treg expansion and their suppressive activity by, e.g., enhancing production of immunosuppressive adenosine or inhibitory cytokines (IL-10, TGF-β) by these Treg [29-31]. More recently, we have observed that tumor-derived exosomes altered expression of surface markers on maturing dendritic cells (DCs) and of antigen presenting machinery (APM) components in matured DCs [32]. These various effects were observed using either tumor-derived exosomes isolated from supernatants of tumor cell lines [32] or exosomes isolated from plasma of patients with cancer [33]. Interestingly, this reprograming of immune cells correlated to plasma levels of exosomes in patients with head and neck squamous cell carcinoma (HNSCC) and to levels of inhibitory proteins carried by these exosomes [33]. Thus, plasma exosomes of HNSCC patients with active disease, stage III/IV tumors and nodal involvement carried higher levels of immunosuppressive markers and mediated significantly greater immune suppression than exosomes from plasma of patients with no evident disease (NED) after therapy or patients with stage I/II tumors and no nodal mets [33]. These correlative data indicate that in vitro measured immune reprogramming mediated by plasma exosomes of patients with cancer provide information about disease progression and response to therapy in HNSCC patients.
4. Correlation of exosome molecular profiles with clinical endpoints
Our studies and those of others and those of others indicate that the examination of protein cargos in total exosome fractions from plasma of cancer patients can be used to differentiate cancer patients from healthy donors or patients with early- vs late-stage disease [23,33,34]. While Western blots have been useful in semi-quantitative analysis of protein contents in exosomes, the ability to measure levels of individual immunosuppressive proteins in exosomes could provide a more precise definition of their functional significance. Using on-bead flow cytometry for exosome capture from plasma and fluorochrome-labeled monoclonal antibodies specific for a target protein, e.g., PD-L1, carried by exosomes for detection, it is possible to quantitate PD-L1 levels in relative flow intensity (RFI) values, as recently described [35]. We showed that exosomes with elevated levels of PD-L1 were highly effective in down-regulating expression of CD69, an activation protein on CD8+ T cells. In contrast, exosomes with low levels of PD-L1 were ineffective in down-regulating T-cell activity [35]. Further, anti-PD-1 antibodies inhibited CD69 down-regulation, suggesting that binding of exosomal PD-L1 to PD-1 on activated CD8+ T cells was responsible for CD69 downregulation [35]. Importantly, high PD-levels on exosomes correlated with disease activity, advanced disease stage and the presence of nodal metastases in patients with HNC. No such correlation of disease progression with PD-1 levels on the same exosomes was observed. Further, only PD-L1 levels on exosomes, but not the levels of soluble PD-L1 in patients’ plasma, correlated with clinical endpoints in this study [35]. The conclusion that emerges from these experiments is that suppressive ligands, like PD-L1, presented to T cells in a membrane-bound form on the exosome surface are highly efficient as checkpoint inhibitors, mediating strong suppression of anti-tumor functions in these T cells. This example emphasizes the biological significance of plasma exosome levels and the exosome cargo as measures of disease progression in cancer.
The exosome levels and content in plasma is also critical for monitoring of cancer patients’ responses to therapy. In a small cohort of patients with metastatic recurrent HNC who received palliative treatment with photodynamic therapy (PDT), we monitored exosome protein levels in plasma and the content of epithelial and mesenchymal markers, E-cadherin vs N-cadherin, respectively, in plasma-derived exosomes. All patients responded positively to PDT. As shown in Figure 4, the patients’ serum exosome protein levels decreased dramatically in the course of therapy in concert with clinical improvement [36]. Remarkably, E-cadherin levels in plasma exosomes increased, while N-cadherin levels decreased, suggesting that a mesenchymal to epithelial transition was on going in the tumor in response to PDT. These results as well as our more recent data monitoring responses of HNSCC patients to immune therapy [37] suggest that total plasma exosomes can serve as reliable biomarkers of response to therapy and that exosome molecular content can be used as a measure of molecular pathways that are up- or down-regulated in response to therapy.
Figure 4.
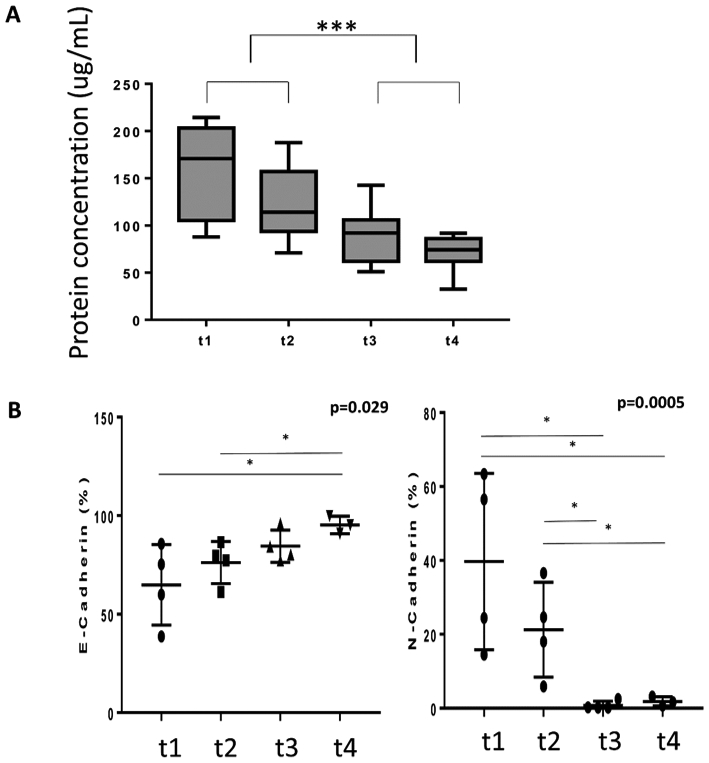
Changes in total exosome protein levels (A) in plasma of HNSCC patients treated with photodynamic therapy (PDT). Plasma exosomes were isolated by SEC and protein levels were measured prior to PDT (t1); 24h after PDT (t2); 7days after PDT (t3) and 4-6 weeks after PDT (t4). All 9 patients responded to PDT. In B, changes in E-cadherin and N-cadherin levels in exosomes isolated from plasma of HNSCC patients treated with and responding to PDT. Reproduced from Theodoraki M-N et al., ref. 36 under the terms of Creative Commons Attribution License 3.0 (CCBY 3.0): https://creativecommons.org/licenses/by/3.0/.
5. Isolation from plasma and monitoring of exosome subsets
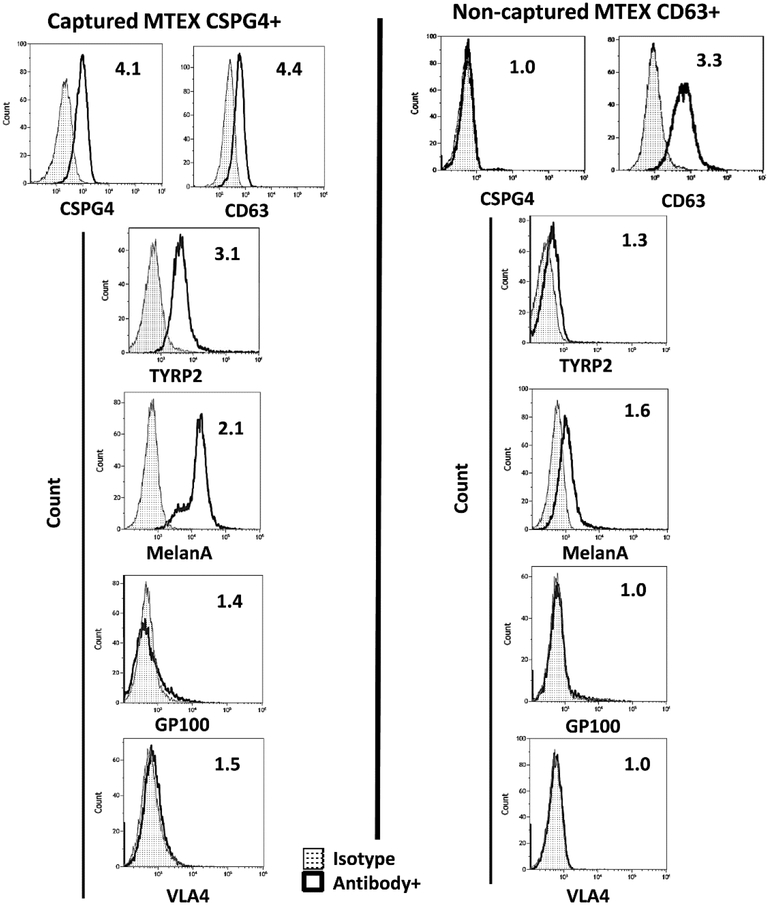
Exosomes isolated from cancer patients’ plasma by SEC are a mix of vesicles derived from tumor as well as normal cells [25]. The ratios of TEX to non-TEX are likely to vary broadly between patients, depending on the disease activity, tumor stage and the presence or absence of metastasis. Thus, to determine the role of TEX as markers of the tumor activity and of immune reprogramming, it becomes necessary to separate TEX from non-TEX in plasma obtained from cancer patients. An approach we developed to separate TEX from non-TEX depends on the immunoaffinity-based capture of TEX using antibodies specific for an antigen present only in the tumor and tumor-derived exosomes but not on normal cells or non-TEX. Monoclonal antibodies (mAbs) to an epitope of Chondroitin Sulfate Proteoglycan-4 (CSPG4) expressed on a large majority (85%) of melanoma cells but not on normal tissue cells were available from Dr. Soldano Ferrone [38]. As shown in Figure 5, melanoma cells express high levels of CSPG4, and the exosomes these cells produce also carry high levels of CSPG4 (Figure 5). The mAbs to CSPG4 were used for immunocapture of melanoma cell-derived exosomes (MTEX) from melanoma patients’ plasma [39]. As shown in Figure 6, exosomes isolated by SEC were co-incubated with biotin-labeled anti-CSPG4 Ab and avidin-labeled beads. MTEX positive for CSPG4 were immunocaptured on beads, while non-MTEX remained in solution and were later recovered using biotinylated anti-CD63 Abs, although it is possible that some exosomes not positive for CD63[40] were not recovered.
Figure 5.
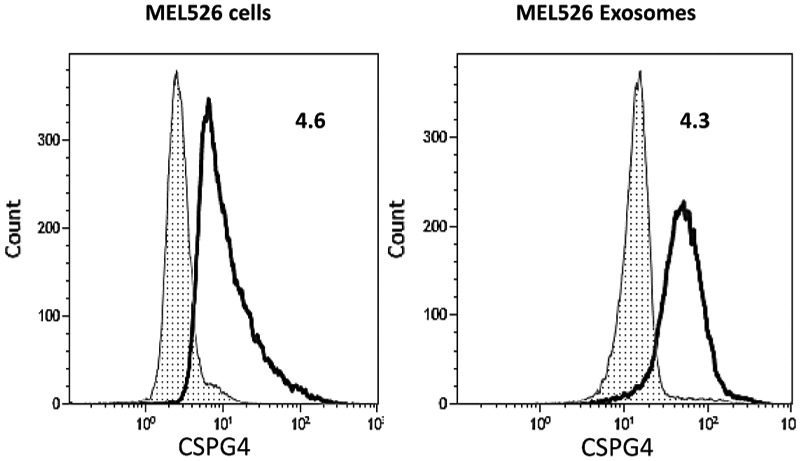
Expression of CSPG4 on MEL526 cells and on exosomes produced by these cells (i.e., on TEX). Note that TEX mimic the CSPG4 content of the parent cell. The data were acquired by flow cytometry using anti-CSPG4 mAbs produced by Dr. Soldano Ferrone. On-bead flow cytometry was used for detection of CSPG4 on exosomes. The data are presented as Relative Flow Intensity (RFI) values. Reproduced from Sharma P. et al., ref. 39 under the terms of Creative Commons Attribution 4.0 International License: http://creativecommons.org/licenses/by/4.0/.
Figure 6.
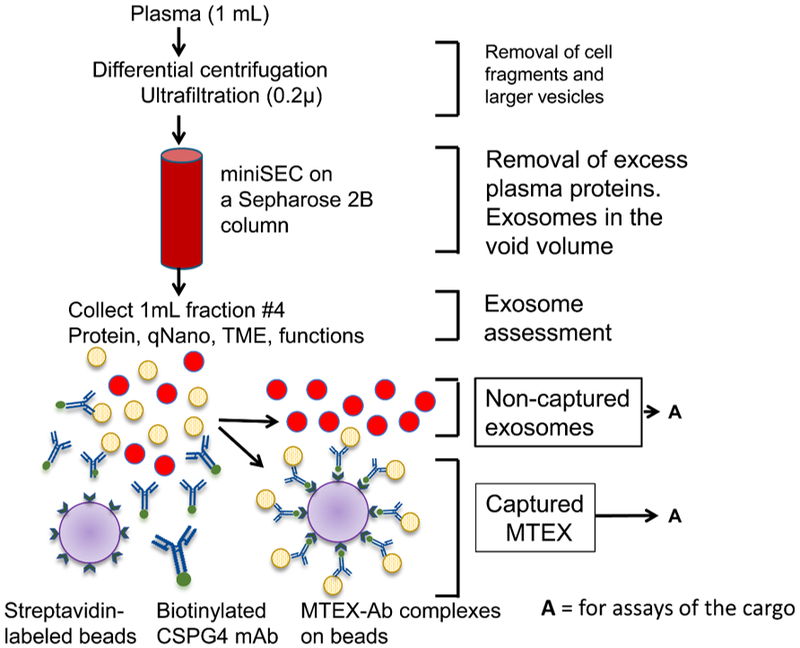
Immune capture with biotinylated anti-CSPG4 Abs of melanoma-derived exosomes (MTEX) on avidin-labeled beads from plasma of patients with melanoma. Non-captured exosomes were re-captured with anti-CD63 Abs for detection by on-bead flow cytometry. Two different anti-CSPG4 Abs (mAb 763.74 and mAb 225.28) specific for distinct epitopes of CSPG4 were used for capture and detection, respectively). Reproduced from Sharma P. et al., ref. 39 under the terms of Creative Commons Attribution 4.0 International License: http://creativecommons.org/licenses/by/4.0/.
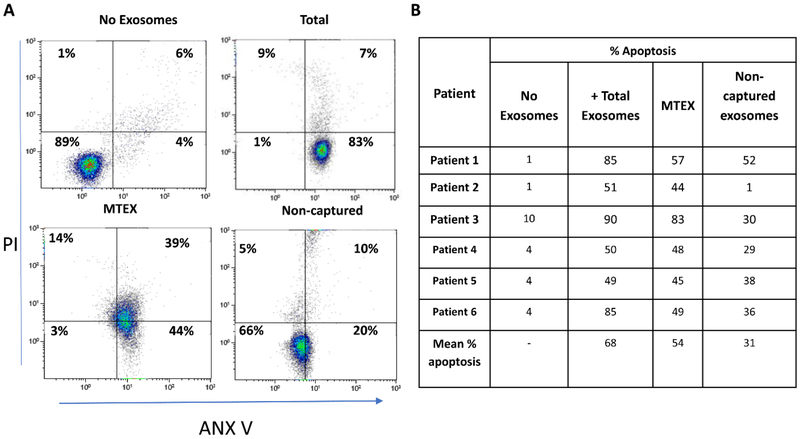
. Both fractions were evaluated by flow cytometry with the labeled detection Abs (distinct anti-CSPG4 mAbs, mAb 763.74 and mAb 225.28 were used for exosome capture and detection, respectively) to show the presence of melanoma-associated antigens (MAA), TYRP2 and MelanA, in MTEX only (Figure 7) as well the enrichment in immunosuppressive proteins (not shown). The key message derived from these studies was that MTEX in plasma of patients with melanoma were almost entirely responsible for suppression of effector T cell functions and induced apoptosis in a large fraction of these cells (Figure 8A). In contrast, non-MTEX, enriched in immunostimulatory proteins, were significantly less suppressive, induced low levels of early apoptosis (ANXV+PI-), as shown in Figure 8A, and tended to promote immune responses. Thus, the ratio of MTEX/non-MTEX in cancer plasma determined the degree of tumor-induced suppression in patients with melanoma. The correlative data linking MTEX induced immune suppression with clinical endpoints suggest that that functional rather than phenotypic features of MTEX are the key determinants of that response [41]. Future studies with larger patient cohorts will be necessary to confirm these preliminary correlations of MTEX functions to disease progression.
Figure 7.
Representative flow cytometry results for detection of melanoma-associated antigens (MAAs) on the immunocaptured MTEX and on non-captured exosomes from plasma of a melanoma patient. Note that MAAs are carried by CSPG4+ MTEX but not by non-captured exosomes. Reproduced from Sharma P. et al., ref. 39 under the terms of Creative Commons Attribution 4.0 International License: http://creativecommons.org/licenses/by/4.0/.
Figure 8.
Representative data for apoptosis of activated primary CD8+ T cells after 6h coincubation with isolated MTEX are shown in A. In B, comparative apoptosis data for CD8+ T cells co-incubated with total exosomes, MTEX and non-MTEX from 6 different patients with melanoma (10μg exosome protein/assay). Note that MTEX are largely responsible for apoptosis induced by the total exosome fractions.
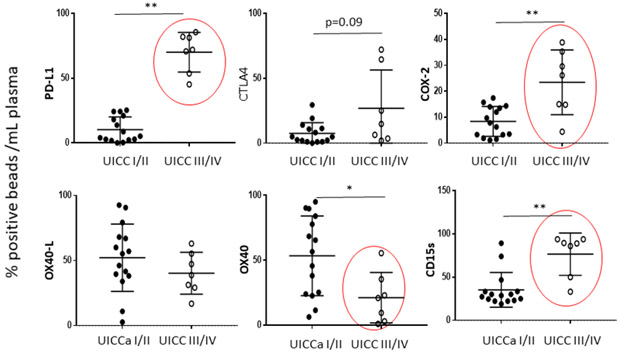
A similar approach based on immunoaffinity capture was used to subset plasma-derived exosomes in patients with HNSCC into CD3+ and CD3(neg) fractions using anti-CD3 Abs [42]. Since only T cells are CD3+ and carry T-cell receptor (TCR), it follows that CD3+ exosomes in plasma are derived from T cells. The near complete separation of the two fractions allowed for characterization of their cargo by on-bead flow cytometry. In HNC patients’ plasma, the CD3(neg) fraction was enriched in tumor- derived CD44v3+ exosomes (Figure 9). Surprisingly, we found that CD3+ exosomes represented more than 50% of total plasma exosomes in many HNSCC patients. Further, both exosome fractions were enriched in immunosuppressive proteins such as PD-L1, CTLA-4, COX-2 or CD15s, with mean levels that were similar in CD3+ and CD3(neg) exosomes. However, levels of these immunosuppressive exosome proteins varied broadly among patients, and the HNSCC patients with CD3+ exosomes carrying high levels (above the mean for the entire cohort) of these proteins had advanced disease, stage III/IV tumors and positive lymph nodes (Figure 10). CD3+ exosomes of these patients carried low levels of immunostimulatory proteins (OX40 and OX40L), and in functional in vitro assays, these exosomes induced apoptosis of activated CD8+ cells and promoted expansion of Treg [42]. Interestingly, the CD3(neg) exosome fractions, which were enriched in tumor-associated proteins (CD44v3) and were presumably tumor-derived, showed weaker association of the high suppressive cargo with late stage, N+ advanced disease than did CD3+ exosomes. However, in CD3(neg) exosomes, elevated levels of OX40 and OX40L associated with early-stage, N0 disease [42]. Comparisons of CD3+ or CD3(neg)exosomes from plasma of HNC patients (n=22) with CD3+ or CD3(neg) exosomes of healthy donors (n=6) indicated significantly higher levels of suppressive proteins and somewhat lower OX40 or OX40L levels in HNC patients’ exosomes.
Figure 9.
On bead-flow cytometry illustrating capture from plasma and separation of CD3+ and CD3(neg) exosomes. In A, representative data from two patients show significant enrichment in CD3+ exosomes in the immunocaptured fractions. The data are acquired as RFI values and are translated into percentages of positive exosomes for convenience only. In B and C, evidence that CD3(neg) exosomes are enriched in CD44v3+ exosomes, an indication that they are tumor-cell derived. Reproduced with permission from Theodoraki M-N et al., ref. 42.
Figure 10.
Correlations of the protein cargo in CD3+ exosomes from plasma of HNSCC patients with the tumor stages. The red circles indicate significant differences. The unpublished data were provided by Dr. M-N. Theodoraki.
The Ab-based capture of TEX vs non-TEX is a simple method, but it requires (a) the use of Abs specific for a tumor associated antigen(s) or antigens that are overexpressed on tumor cells and (b) prior isolation of exosomes and partial removal of plasma “contaminants” to allow for successful immunoaffinity capture. The advantage is that only small volumes of body fluids are needed for separation and recovery of TEX and non-TEX in a single procedure allowing for a simultaneous evaluation of the tumor and T-cell profiles. The evaluation can involve either the protein or miRNA detection or both, and the profiling can be targeted to specific cargo components or can be as extensive as desired.
6. Expert Commentary
The results of studies discussed above are provocative and potentially transformative. They showed that both CD3+ and CD3(neg) exosome fractions in HNSCC patients’ plasma were immunoinhibitory, reflecting the ability of the tumor as well as reprogrammed T cells to inhibit immune responses. We found that in HNSCC patients with stage III/IV disease and positive nodes, CD3+ and CD3(neg) exosomes had higher levels of immunosuppressive markers relative to exosomes in patients with stage I/II tumors and no nodal disease. Concomitantly, the exosomes in stage III/IV HNSCC had lower levels of stimulatory markers such as OX40 or OX40L [42]. The advantage of separating CD3+ from CD3(−) exosomes is that while the latter inform about the immunosuppressive activity of the tumor, CD3+ exosomes, derived from T cells reprogrammed in vivo by TEX, indicate how competently these T cells can mediate anti-tumor functions. The phenotypic and functional data obtained with subsets of tumor-derived and T-cell derived exosomes are encouraging. They suggest that the separation of plasma exosomes into TEX and T cell-derived fractions provides us with liquid biopsies of the tumor as well as with T-cell biomarkers, respectively. Both fractions carry molecular cargos of inhibitory and stimulatory proteins that reflect clinical events such as disease activity, stage or the presence of metastasis. In this scenario, TEX serve as tumor biomarkers and T cell-derived exosomes serve as biomarkers of immune competence in cancer. The combination, incorporating tumor and immune biomarkers, increases the value of liquid biopsy in monitoring cancer diagnosis, prognosis and responses to therapy. Our data also suggest that functional rather than phenotypic assessments of the two exosome fractions may be especially useful for establishing correlations with clinical endpoints. For the start, a simple apoptosis assay measuring the ability of isolated plasma TEX and non-TEX to mediate death of primary CD8+ cells might be sufficient. The liquid biopsy consisting of the phenotypic and functional profiles of the two exosome fractions might prove to be highly effective in diagnosis or prognostication in cancer.
A long and extensive debate has been on going considering the role of the immune system in cancer progression, therapy and outcome. The recent success of immune therapies with checkpoint inhibitors in a substantial proportion of cancer patients has brought home the realization that the host immune system is a major target of the tumor that hopes to grow and that restoration of anti-tumor immunity may be therapeutic [43]. It is well documented that advanced metastatic disease is associated with strong immune dysfunction. We have now learned that advanced cancers produce large quantities of highly immunosuppressive TEX, which carry a message informing about the tumor activity, stage, grade and ability to migrate. Hence both the quantitative and qualitative aspects of the TEX cargo are important as potential biomarkers. Further, TEX which are the tumor messengers, have the propensity to reprogram immune cells, converting them into cells that now promote tumor growth. With the tumor progression, these reprogrammed T cells produce exosomes that mirror suppressive features of the tumor and effectively block anti-tumor functions of other immune cells. As billions of exosomes are present in plasma, the reprogramming process goes on at a very large scale in patients with cancer. This creates an opportunity for capturing TEX and CD3+ exosomes from body fluids, as indicated above, and for reading the messages exosomes carry. However, the isolation and characterization technologies for TEX are not yet in place, and many technical challenges exist. While immune capture of TEX represents a feasible approach, it is limited by the availability of tumor antigen-specific Abs. To overcome this limitation, arrays of several TAA-reactive Abs have been used for immune capture[44]. The advantage of working with TEX is that the messages they carry are protected from harm within an exosome membrane. These messages appear to be accurate and are the basis for a liquid biopsy-based analysis of their cargos and functions in relation to clinical parameters or therapies given. In addition to the TEX isolation from bodily fluids, it is the encoding of the messages that TEX and/or CD3+ exosomes carry and fitting them together with clinical endpoints that represents the current challenge. Decoding of the exosome cargo has to be done at the nanoparticle level, creating the need for novel technologies and highly sensitive detection tools. This technical challenge is being rapidly and effectively met, judging by numbers of new platforms for exosome isolation and decoding that appear almost daily on the market.
7. Five-year view
The rapidly growing new industry of exosome-based liquid nano-biopsy for cancer diagnosis, prognosis and evaluating responses to therapy gives a sense that the new genre of cancer biomarkers is about to be made available for clinical consumption. However, this is not so, and it may not be so in five years from now. First, there is reluctance on the part of many to acknowledge physiological importance of exosomes and their role as cancer biomarkers. The biology of exosomes and their physical and functional heterogeneity are not yet understood, although rapid progress is being made in that arena. More attention is currently given to the cfRNA or ctDNA than to exosomes as liquid biopsy platforms. Studies with exosomes have barely advanced from the cell line supernatant stage to body fluids, and the technologies being developed are facing challenges related to sensitivity and specificity in the detection of femtomolar quantities of biologically active components in the exosome cargo. The validation of accumulating pre-clinical data with exosomes that is essential before any translation to the clinic can be contemplated is likely to occupy us in the next years to come. There is a need for well-defined clinical trials in which the role of TEX in diagnosis and treatment of cancer can be validated, using detection and validation patient cohorts. As exosome isolation from body fluids by new technologies is a relatively simple and inexpensive step, there is a good reason to expect that exosome characterization, both at the protein and miRNA levels, will soon be a routine procedure, allowing for reliable correlations with relevant clinical data. Validation of promising exosome diagnostic and prognostic profiles identified in the pre-clinical studies will then proceed in earnest on a large scale. While the validation of exosome clinical utility may take more than five years to complete, it is almost certain that TEX and CD3+ exosomes in plasma of cancer patients will emerge as clinically significant biomarkers, allowing for their translation to the bedside.
Key issues.
EVs represent a novel and attractive liquid biopsy nano-platform for discovery of cancer biomarkers.
The biology of EVs is incompletely understood, but it is known that EVs are heterogenous in terms of vesicle sizes and cargos these vesicles carry.
Tumor-derived exosomes (TEX), a subset of EVs sized at 30-150nm in diameter, are massively released by tumor cells and are present in all body fluids of cancer patients.
Preclinical studies indicate that TEX are mediate intercellular communication and reprogram the tumor microenvironment, converting it into a tumor promoting milieu
Using immunoaffinity capture it is now possible to isolate TEX and separate them from non-TEX in patients’ plasma
Protein and miRNA profiles of TEX and non-TEX can be evaluated using newly developing nanotechnologies and associated with clinical endpoints in cancer patients
Accumulating pre-clinical evidence suggests that TEX as well as immune cell profiles inform about the clinical status of the cancer and seem to be promising markers of response to therapy
The promise of plasma exosome subsets as cancer biomarkers is likely to be intensively explored in hope of their validation for cancer diagnosis, prognosis, response to therapy and outcome in the future.
Acknowledgments
Funding
The author acknowledges partial support from NIH grants RO-1 CA168 628 and R-21 CA205644.
Footnotes
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers Disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Pavlou MP, Diamandis EP. The cancer cell secretome: a good source for discovering biomarkers? J Proteomics, 73(10), 1896–1906 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Cramer DW, O’Rourke DJ, Vitonis AF et al. CA125 immune complexes in ovarian cancer patients with low CA125 concentrations. Clin Chem, 56(12), 1889–1892 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oppenheim JJ. The Future of the Cytokine Discipline. Cold Spring Harb Perspect Biol, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burz C, Pop VV, Buiga R et al. Circulating tumor cells in clinical research and monitoring patients with colorectal cancer. Oncotarget, 9(36), 24561–24571 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem, 61(1), 112–123 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Ferracin M, Lupini L, Mangolini A, et al. Circulating Non-coding RNA as Biomarkers in Colorectal Cancer. Advances in experimental medicine and biology, 937, 171–181 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Ishiba T, Hoffmann AC, Usher J et al. Frequencies and expression levels of programmed death ligand 1 (PD-L1) in circulating tumor RNA (ctRNA) in various cancer types. Biochem Biophys Res Commun, 500(3), 621–625 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. **.Lucchetti D, Fattorossi A, Sgambato A Extracellular Vesicles in Oncology: Progress and Pitfalls in the Methods of Isolation and Analysis. Biotechnol J, e1700716 (2018).In this review the authors analyze the methods to collect biological fluid samples (urine, plasma/serum, and cell supernatant), and to isolate and quantify extracellular vesicles highlighting advantages and drawbacks.
- 9.Hanash S The Wide World of Molecular Profiling for Tumor Classification. Clin Chem, 64(4), 743–744 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol, 200(4), 373–383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. **.Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol, 36(3), 301–312 (2016).This review provides an excellent and comprehensive view of EVs characteristics and their biology.
- 12. **.Whiteside TL. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol, (2017).This paper provides an overview of cellular reprogramming driven in the TME by tumor-derived exosomes.
- 13.Zhang H, Freitas D, Kim HS et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nature cell biology, 20(3), 332–343 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zijlstra A, Di Vizio D. Size matters in nanoscale communication. Nature cell biology, 20(3), 228–230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meehan B, Rak J, Di Vizio D. Oncosomes - large and small: what are they, where they came from? J Extracell Vesicles, 5, 33109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiteside TL. The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol, 13(28), 2583–2592 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang HG, Cao P, Teng Y et al. Isolation, identification, and characterization of novel nanovesicles. Oncotarget, 7(27), 41346–41362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles, 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyiadzis M, Whiteside TL. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia, 31(6), 1259–1268 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Atay S, Godwin AK. Tumor-derived exosomes: A message delivery system for tumor progression. Commun. Integr. Biol, 7(1), e28231 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanini F, Fabbri M. Cancer-derived exosomic microRNAs shape the immune system within the tumor microenvironment: State of the art. Seminars in cell & developmental biology, 67, 23–28 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frediani JN, Fabbri M. Essential role of miRNAs in orchestrating the biology of the tumor microenvironment. Molecular cancer, 15(1), 42 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. **.Peinado H, Aleckovic M, Lavotshkin S et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med, 18(6), 883–891 (2012).A landmark paper that is a must for all investigators working with exosomes.
- 24.Li P, Kaslan M, Lee SH, et al. Progress in Exosome Isolation Techniques. Theranostics, 7(3), 789–804 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong CS, Funk S, Muller L, et al. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles, 5, 29289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong CS, Sharma P, Yerneni SS et al. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Scientific reports, 7(1), 14684 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiteside TL. Exosomes and tumor-mediated immune suppression. J. Clin. Invest, 126(4), 1216–1223 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iero M, Valenti R, Huber V et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ, 15(1), 80–88 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Wieckowski EU, Visus C, Szajnik M, et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol, 183(6), 3720–3730 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. *.Szajnik M, Czystowska M, Szczepanski MJ, et al. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS One, 5(7), e11469 (2010).The first report of stimulatory effects of tumor-derived exosomes on human regulatory T cells (Treg).
- 31.Mrizak D, Martin N, Barjon C et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J. Natl. Cancer Inst, 107(1), 363 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Ludwig S, Sharma P, Theodoraki MN et al. Molecular and functional profiles of exosomes from HPV+ and HPV- head and neck cancer cell lines. submitted (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludwig S, Floros T, Theodoraki MN et al. Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research, 23(16), 4843–4854 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melo SA, Luecke LB, Kahlert C et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature, 523(7559), 177–182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. **.Theodoraki MN, Yerneni SS, Hoffmann TK, et al. Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients. Clinical cancer research : an official journal of the American Association for Cancer Research, 24(4), 896–905 (2018).The first report on PD-L1 on exosomes in plasma of patients with head and neck cancer that coorelates levels of PD-L1 on exosomes with clinicopathological endpoints and disease activity.
- 36.Theodoraki MN, Yerneni SS, Brunnner C, et al. Plasma-derived exosomes reverse epithelial-to-mesenchymal transition after photodynamic therapy of patients with head and neck cancer. Oncoscience, in press (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. **.Theodoraki MN, Gooding WE, Ferris RL, et al. Plasma‐derived exosomes carrying CTLA‐4, PD‐1 and PD‐L1 in head and neck squamous cell carcinoma patients treated with immunotherapy is associated with disease outcome. Journal for ImmunoTherapy of Cancer, 5(5(Suppl 2)), P89 (2017).An abstract reporting that monitoring of patients with HNC treated with biotherapy using exosomes dicrimated responders from non-responders to therapy.
- 38.Wang Y, Sabbatino F, Wang X, et al. Detection of chondroitin sulfate proteoglycan 4 (CSPG4) in melanoma. Methods Mol. Biol, 1102, 523–535 (2014). [DOI] [PubMed] [Google Scholar]
- 39. **.Sharma P, Ludwig S, Muller L et al. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. Journal of extracellular vesicles, 7(1), 1435138 (2018). Immunoaffinity-based isolation of melanoma cell-derived exosomes (MTEX) using anti-CSPG4 Abs for immune capture is presented.
- 40.Kowal J, Arras G, Colombo M et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. U. S. A, 113(8), E968–977 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma P, Gooding WE, Ferrone S, et al. Functional analysis and potential clinical significance of melanoma cell-derived exosomes in melanoma patients’ plasma. Journal of Clinical Oncology Insights, submitted. (2018). [Google Scholar]
- 42. **.Theodoraki MN, Hoffmann TK, Whiteside TL. Separation of plasma-derived exosomes into CD3((+)) and CD3((−)) fractions allows for association of immune cell and tumour cell markers with disease activity in HNSCC patients. Clinical and experimental immunology, 192(3), 271–283 (2018).The paper details how to use anti-CD3 Abs to separate T cell-derived from tumor-enriched exosome fractions and analyze their cargos by on-bead flow cytometry.
- 43.Whiteside TL, Demaria S, Rodriguez-Ruiz ME, et al. Emerging Opportunities and Challenges in Cancer Immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research, 22(8), 1845–1855 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang KS, Im H, Hong S et al. Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Sci. Transl. Med, 9(391) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]