Abstract
Objective:
Disturbances in self-regulatory control are involved in the initiation and maintenance of addiction, including cannabis use disorder (CUD). In adults, chronic cannabis use is associated with disturbances in fronto-striatal circuits during tasks that require the engagement of self-regulatory control, including the resolution of cognitive conflict. Understudied are the behavioral and neural correlates of these processes earlier in the course of cannabis use, disentangled from effects of long-term use. The present study investigates the functioning of fronto-striatal circuits during the resolution of cognitive conflict in cannabis-using youth.
Method:
Functional magnetic resonance imaging data was acquired from 28 cannabis-using (CU) youth and 32 age-matched healthy participants (HC) during the performance of a Simon task. General linear modeling was used to compare patterns of brain activation during correct responses to conflict stimuli across groups. Psychophysiological interaction analyses were used to examine conflict-related fronto-striatal connectivity across groups. Associations of fronto-striatal activation and connectivity with cannabis use measures were explored.
Results:
Reduced conflict-related activity was detected in CU relative to HC youth in fronto-striatal regions, including ventromedial prefrontal cortex (vmPFC), striatum, pallidum and thalamus. Fronto-striatal connectivity did not differ across groups, but negative connectivity between vmPFC and striatum was detected in both groups.
Conclusion:
These findings are consistent with previous reports of cannabis-associated disturbances in fronto-striatal circuits in adults and point to the specific influence of cannabis on neurodevelopmental changes in youth. Future studies should examine whether fronto-striatal functioning is a reliable marker of CUD severity and potential target for circuit-based interventions.
Keywords: cannabis use, Functional magnetic resonance imaging, Fronto-striatal circuits, Resolution of cognitive conflict, Youth
INTRODUCTION
A hallmark feature of substance use disorder (SUD) is compulsive drug-seeking long after the drug is no longer experienced as pleasurable and despite the associated adverse consequences of this behavior.1 Disturbances in self-regulatory control are believed to be involved in the initiation and maintenance of the compulsive drug-seeking that characterizes SUDs.1–3 Most adults with SUD began having problems with drugs and alcohol in adolescence,4–6 a developmental period during which the neural circuits underlying control processes continue to mature.7 As such, the adolescent brain may be particularly vulnerable to the effects of substance use.8–10 Cannabis is the most widely used illicit substance across ages.6, 11 Thus, a better understanding of the functioning of the fronto-striatal circuits that support regulatory capacities in cannabis-using youth could have great clinical and public health implications and may shed light on the emergence and maintenance of compulsive drug-seeking during this sensitive developmental period.
Acute12–14 and chronic15–19 exposure to cannabis have been associated with neurocognitive deficits in executive function, including inhibitory control processes. Neuroimaging findings from chronic cannabis-using adults show altered fronto-striatal functioning associated with resolution of cognitive conflict,15, 18, 20–23 selective attention,24 and behavioral inhibition.25 After a period of abstinence, abnormalities in control processes have been reported in some16, 26, 27 but not other28 studies. These discrepant findings may be attributed to differences in the age at which participants initiated or escalated cannabis use, consistent with the hypothesis that disturbances in fronto-striatal control processes may be more pronounced and persistent in earlier initiates due to a possibly greater impact of cannabis exposure (and related drug-seeking behaviors) at earlier stages of brain development.29 An alternative, but not mutually exclusive, explanation is that fronto-striatal disturbances may be present early in development and contribute to the early initiation of cannabis use.
In sum, the extant data point to altered fronto-striatal control processes in cannabis-using adults, particularly in those who became frequent users at an earlier age.16, 18, 19, 23, 30 This literature on neural functioning in adults is inherently confounded by chronic effects of cannabis exposure on brain and limited information on early history of use (only categorical early vs. late onset) and other important aspects of developmental history.16, 18, 19, 23, 30
In the present study, we used functional magnetic resonance imaging (fMRI) with the Simon Spatial Incompatibility Task31, 32 to investigate the functioning of the neural circuits that support the engagement of control required to resolve cognitive interference (i.e., conflict) in cannabis-using youth. Fronto-striatal regions are known to be involved in the resolution of cognitive conflict on this task,32–34 and alterations in structure and function of fronto-striatal circuits (specifically, the ventromedial prefrontal cortex [vmPFC]) have been associated with cannabis use in adults.20, 23, 35–38 We therefore predicted that, relative to healthy controls (HC), cannabis-using youth (CU) would show reduced conflict-related activation and altered connectivity within the vmPFC fronto-striatal circuit. Such deficient functioning of this circuit may contribute to the initiation and maintenance of problematic cannabis use. Thus, we further hypothesized that these alterations would be less severe with longer abstinence. We also examined whether frequency of use and age of first regular use were associated with functional disturbances in this circuit. Unlike previous studies in adults that treated age of onset as a categorical measure (i.e. early vs. late),16, 18, 19, 23, 30 we treated it as a continuous variable, allowing us to test whether variations in age of initiation linearly predicted variations in conflict-related activation and connectivity.
METHOD
Participants
Participants were 28 adolescents and emerging adults with significant cannabis use (CU) and 32 healthy controls (HC), aged 14–23 years, group matched on age, gender, socioeconomic status (SES), race and ethnicity. Demographics for all participants and clinical ratings for the CU group are shown in Table 1. Participants were recruited through flyers, Internet advertisements, word-of-mouth, and local mental health clinics and schools.
Table 1.
Demographic and Clinical Characteristics of Participants with Cannabis use Disorder (CU), and Age-Matched Healthy Controls (HCs)
| Characteristic | CU (n = 28) |
HC (n = 32) |
Analysis | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | t(58) | p | |
| Age (years) | 19.3 (2.0) | 18.9(2.7) | −0.719 | .48 |
| Socioeconomic status | 43.9(12.0) | 45.7 (14.7) | 0.608 | .55 |
| WAIS IQ score (Full-4) | 104.9(21.5) | 109.1 (14.4) | 0.879 | .38 |
| Days of use (past 28 days) | 20.7 (10.7) | - | - | - |
| Days abstinent prior to scan | 3.71 (5.8) | - | - | - |
| Age first marijuana use (years) | 15.4(2.1) | - | - | - |
| Age first regular marijuana use (years) | 16.6 (2.3) | - | - | - |
| Duration of marijuana use (years) | 3.6(1.7) | - | - | - |
| n (%) | n (%) | X2 (1,N=60) | p | |
| DSM-IV diagnoses | ||||
| Cannabis abuse | 17(60.7) | - | - | - |
| Cannabis dependence | 12 (42.9) | - | - | - |
| Alcohol abuse | 1 (3.6) | - | - | - |
| Attention-deficit/hyperactivity disorder | 4(14.9) | - | - | - |
| Major depressive disorder | 2 (7.0) | - | - | - |
| Anxiety disorder | 1 (3.6) | - | - | - |
| Gender | 0.350 | .55 | ||
| Male | 17 | 17 | ||
| Female | 11 | 15 | ||
| Race/Ethnicity | ||||
| Asian | 0(0) | 1 (3) | - | - |
| African-American | 12 (43) | 8 (25) | 2.143 | .14 |
| Caucasian | 8 (29) | 13 (41) | 0.954 | .33 |
| Hispanic | 7 (25) | 9 (28) | 0.075 | .78 |
| Other | 1 (4) | 1 (3) | 0.009 | .92 |
Note: WAIS IQ = Wechsler Adult Intelligence Scale
This study aimed to assess the functioning of fronto-striatal circuits early in the course of a problematic cannabis use trajectory. DSM diagnostic criteria poorly capture the patterns, consequences, and severity of cannabis use in youth39, 40 and teens often experience impairment without meeting criteria for DSM-IV substance abuse/dependence or a DSM-5 SUD. Thus, inclusion in the CU group required meeting at least one DSM-IV diagnostic criterion. Because it is possible that infrequent cannabis use may not be associated with neural changes, inclusion in the CU group required “moderate” use defined as at least twice weekly. CU participants were excluded if they had a lifetime diagnosis of SUD (dependence or abuse) other than cannabis or if any substance other than cannabis (THC) was detected by a urine drug test (Construction 12-Drug Screen Test, Innovacon, Inc., San Diego, CA) administered on the day of the scan. In addition, CU participants were asked to abstain at least 12 hours prior to assessment/scanning. It should be noted, however, that urine drug testing was not used to determine cannabis use abstinence prior to scanning because THC can be detected in urine beyond 12 hours after use. HC participants did not meet DSM-IV criteria for any SUD and had no history of cannabis use. Cannabis use and DSM criteria for cannabis-related disorders were assessed with the Comprehensive Adolescent Severity Inventory (CASI-A),41 the Timeline Follow-Back (TLFB),42, 43 the Structured Clinical Interview for DSM-IV Axis-I Disorders (SCID-I)44 for participants 18 years and older, and the Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL)45 for those under 18 years.
Participants with a history of neurological illness, past seizures, head trauma with loss of consciousness, mental retardation, developmental disorder, or Axis I psychiatric disorders were excluded. Depressive, anxiety and attention deficit hyperactivity (ADHD) disorders were permitted in the CU group, given their high co-morbidity with moderate to heavy cannabis use in youth. The presence of these and other Axis 1 disorders was determined based on the SCID-I or K-SADS-PL. The DuPaul-Barkley Attention-Deficit Hyperactivity Disorder Rating Scale46 quantified symptoms of inattention and hyperactivity. Full-scale IQs were estimated using the Wechsler Abbreviated Scale of Intelligence (WASI),47 and SES was calculated based on parental academic and occupational achievement using the Modified Hollingshead 4-Factor Index.48 All clinical data were reviewed and confirmed by a psychiatrist who also conducted clinical interviews for all participants. Adult participants provided informed consent. Minor participants provided assent, and their caregivers provided consent. The research protocol was approved by the Institutional Review Board of the New York State Psychiatric Institute.
FMRI Paradigm
Participants completed the Simon task (previously described).49–51 Briefly, participants were presented with a leftward or rightward pointing arrow on each trial that was either congruent or incongruent with its position (left or right) on the screen. They were instructed to respond as quickly and accurately as possible to the direction of the arrow by pressing a button on a response box using the index finger for left and the middle finger for right. Stimulus duration was 1300 ms, with jittered intervals ranging from 4160 to 6960 ms (M=5350, SD=1159.98) between each trial. Each of 3 runs consisted of 55 trials, with 11 blank, 22 congruent, and 22 incongruent stimuli. E-prime software (Psychology Software Tools, Inc., Sharpsburg, Pa.) was used to program and run the experiment and to record participants’ responses and reaction times (RTs).
Behavioral Analyses
RTs on correct trials were entered as dependent variables in a repeated measures, mixed-model ANOVA in IBM SPSS Statistics 22.0 (Armonk, NY: IBM Corp.) with Stimulus (post-congruent Incongruent vs. post-congruent Congruent) as the within-subject variable and group (CU vs. HC) as the between-subject variable.
Image Acquisition and Processing
Images were collected using a GE Signa 3 Tesla LC scanner (Milwaukee, WI). Functional images were acquired using a T2* sensitive, gradient-recalled, single shot, echo-planar pulse sequence (repetition time = 2200 ms, echo time = 30 ms, 90 degree flip angle, single excitation per image, 24*24 cm field of view, 64*64 matrix, 34 slices 3.5mm thick, no gap, covering the entire brain). We collected 140 echo-planar imaging (EPI) volumes for each run.
Image preprocessing and first-level analyses were carried out using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) and MATLAB 9.0 (Mathworks, Natick, MA). Functional images were corrected for differences in slice timing using sinc-interpolation, and head movement was corrected using a least-squares approach and a 6-parameter rigid body spatial transformation. Structural data were coregistered to the functional data and segmented into tissue probability maps, bias corrected and spatially normalized to the Montreal Neurological Institute (MNI) space of 1 × 1 × 1 mm3 voxels. Using the deformation fields of these segmented images, the functional images were subsequently spatially normalized to MNI space of and 3 × 3 × 3 mm3 voxels. An 8-mm full-width/half-maximum isotropic Gaussian smoothing kernel was applied to all normalized functional images. All analyses included a temporal high-pass filter (128 s) and correction for temporal autocorrelation using an autoregressive AR(1) model. Each image was scaled to have a global mean intensity of 100.
For each participant, preprocessed time series data from all three Simon task runs (420 volumes) were modeled using a General Linear Model (GLM) with six conditions: 1) Incongruent correct trials preceded by congruent trials (cI), 2) Congruent correct trials preceded by incongruent trials (iC), 3) Incongruent correct trials preceded by incongruent trials (iI), 4) Congruent correct trials preceded by congruent trials (cC), 5) fixation trials, and 6) incorrect trials (incongruent or congruent), including those trials with RTs below the minimum RT of 200 ms required for stimulus detection and processing. Because of the limited number of incorrect trials, contrasts involving these trials were not assessed. All of the task regressors were convolved with the canonical HRF, and least-squares regression was used to estimate parameters for each independent variable for each participant. To ensure that the non-significant motion differences could not account for our results, we included 24 nuisance motion regressors as covariates of non-interest to capture linear, quadratic, differential, and quadratic differential motion52. Runs in which a participant had more than 30% error rate (ER) on the task or more than 3mm of total displacement in any of the 6 standard motion parameters, following conversion of rotational parameters to distances by computing the arc length displacement on the surface of a sphere with radius 50 mm,53, 54 were considered poor quality data and excluded from our analyses.
Conflict-related Activation
Parameter estimates averaged across the 3 runs were used to produce an incongruent (cI and cC) versus congruent (cC and iC) contrast (I-C) for each participant to isolate brain activation associated with the engagement of self-regulatory control and resolution of cognitive conflict (i.e., interference effect). The I-C parameter estimates maps were compared across groups in a second-level GLM adjusting for age and gender, using the two-sample t-test function with covariates implemented in SPM12. A voxel-wise cluster-defining threshold (CDT) of p<.001 was applied whole-brain, and minimum cluster extent for significance at a Family Wise Error (FWE) rate of p<.05 was determined by the FWEc cluster-level correction for multiple comparison function implemented in SPM12.
Conflict-related Functional Connectivity
To further assess the functioning of fronto-striatal circuits involved in the resolution of cognitive conflict in CU relative to HC, we investigated group differences in conflict-related functional connectivity. To do so, we computed a psychophysiological interaction (PPI)55 of the trial-by-trial psychometric estimate of conflict (I-C) by the physiological activation during I-C trials in a functionally defined frontal seed. The seed was defined as a 6-mm-radius sphere centered on the coordinates where maximal group differences in I-C activation were found within the vmPFC. We then regressed this PPI term against whole-brain activation maps to identify regions in which coupling with the vmPFC varied as a function of conflict (I-C). Positive and negative connectivity are respectively defined as significant positive and negative correlations between regions’ timeseries. Regions in which timeseries are uncorrelated are considered unconnected.
A second-level analysis was conducted to test group differences in conflict-related functional connectivity, adjusting for age and gender. Because of our a priori hypotheses of fronto-striatal disturbances, these group-level analyses of connectivity with vmPFC were restricted to the striatal voxels within the caudate and putamen as defined by the AAL atlas. A voxel-wise CDT of p<.001 was applied to the second-level t-maps, and minimum cluster extent for significance at a FWE rate of p<.05 was determined by the FWEc cluster-level correction for multiple comparison function implemented in SPM12. Findings that did not survive FWEc correction are reported as uncorrected.
Clinical Correlates
In the CU group, correlation analyses were conducted to examine the relationships of conflict-related activation and connectivity with cannabis use. Each participant’s average activation and connectivity estimates were extracted from each cluster that was determined to be significant in our second-level group analyses. Pearson’s coefficients were then computed between these estimates and the 1) age of first regular cannabis use, 2) duration of use (in years),3) days of use in the past 30 days, and 4) days of abstinence prior to scanning.
Potential Confounding Effects
To assess potential confounding effects of comorbidity in the CU group, I-C activation maps were compared across groups in additional second-level two-sample t-tests (FWEc) after excluding CU participants with comorbid 1) attention-deficit/hyperactivity disorder (ADHD), 2) major depressive disorder (MDD), 3) anxiety disorders, and 4) significant alcohol use. We additionally explored and controlled for the effects of IQ and SES on our group differences in conflict-related activation and connectivity by conducting ROI-based analyses using the extracted parameter estimates from each significant cluster detected in our main analyses.
RESULTS
Participants
Demographics are shown in Table 1, along with clinical ratings for the CU group. Twenty-eight CU participants (17 males) and 32 HCs (17 males), aged 14–23 years, group matched on age, gender, SES, race and ethnicity were included in this study. Six additional participants (three CUs and three HCs) were scanned but excluded from analyses because of poor data quality. Seven scan runs from four CU participants were also excluded because of severe head motion. Mean framewise displacement did not differ across groups (HC: M=0.07, SD=0.04; CU: M=0.07, SD=0.06; t(58)=‒ 0.05, p-.959). In the CU group, 12 participants met DSM-IV criteria for cannabis dependence, and 17 for cannabis abuse. In addition, four CU participants had comorbid ADHD, two had comorbid MDD, one had a comorbid anxiety disorder (i.e., specific phobia), and one reported significant alcohol use. Twenty-four CU participants screened positive for THC, but no other substances. No HC participants screened positive for any substances.
Behavioral Results
Table S1 (available online) presents descriptive statistics and group comparisons on task performance (RT and ER) for each condition (cC, cI, iC, iI). On average, participants responded correctly to 94% (SD = 3%) of the trials. As a result, only 6% of the trials were excluded from our image analyses. The repeated measures ANOVA revealed a significant main effect of stimulus indicating that all participants responded faster to congruent compared to incongruent stimuli (F(1,58)=98.02, p<.001). No significant Group effect or Group-by-Stimulus interaction was detected (ps>.1), suggesting that performance did not differ across groups. Including age and gender in the model revealed a main effect of age (F(1,54)=8.640, p=.005) but no significant main effect of stimulus or interactions between any variables (all ps>.1).
Conflict-related Activation
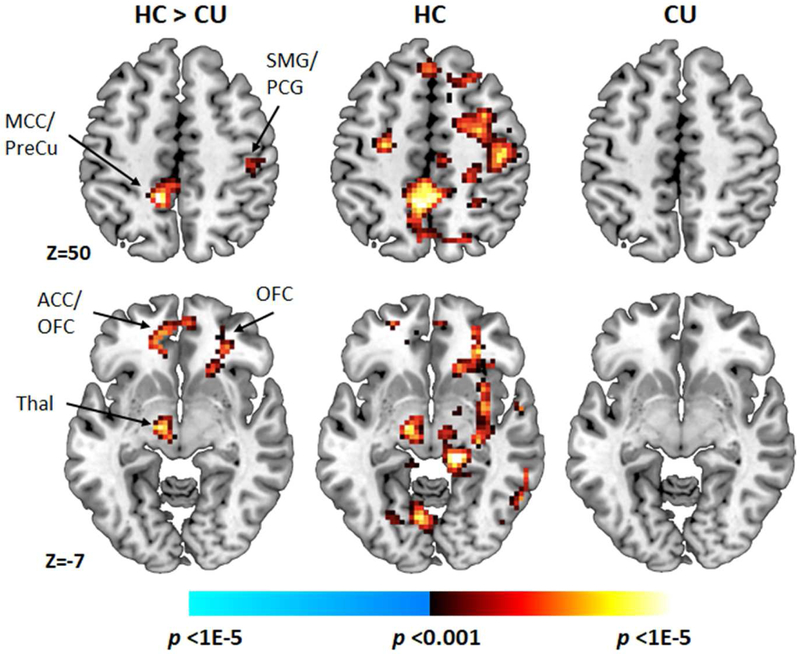
Significant group differences in conflict-related activations were detected bilaterally in lateral PFC (dorsal and ventral regions), vmPFC, middle cingulate, precuneus, and parietal lobe, in left paracentral lobule, pallidum, thalamus, and occipital gyri, as well as in right putamen and precentral gyrus (FWEc; Table 2; Figure 1). Examination of within-group one-sample t-maps revealed that these group differences derived from greater activation of these regions in response to incongruent compared to congruent stimuli in the HC group, but not the CU group (uncorrected). Activation associated with incongruent and congruent stimuli did not differ in the CU group. Effect sizes of these group differences are reported in Table S2 (available online).
Table 2.
Group Differences in Conflict-Related (Incongruent vs. Congruent) Activations, Adjusting for Age and Gender
| Cluster | Areaa | Cluster size | MNI coordinates | t (peak) | pFWEc (cluster) |
|---|---|---|---|---|---|
| #1 | Right orbitofrontal cortex (lateral) Right inferior frontal gyrus (orbitalis) |
79 | 27 35 −10 | 4.92 | .049 |
| #2 | Bilateral middle cingulate cortex Bilateral precuneus |
111 | −12 −46 50 | 4.34 | .016 |
| #3 | Left thalamus | 80 | −12 −13 −7 | 4.49 | .047 |
| #4 | Bilateral orbitofrontal cortex (medial) Left anterior cingulate cortex |
89 | 9 53 −10 | 4.29 | .034 |
| #5 | Right supramarginal gyrus Right postcentral Right rolandric operculum |
97 | 51 −22 26 | 4.28 | .026 |
Note: MNI = Montreal Neurological Institute.
Area labels were based on the Anatomical Automatic Labelling (AAL).
Figure 1. Conflict-Related Neural Activations.
Note: Between-group t-map of conflict-related activations (voxel-wise cluster defining threshold [CDT] of p<.001, FWEc cluster extent correction of p<.05), along with within-group t-maps (CDT of p<.001, uncorrected), adjusting for age and gender. ACC = anterior cingulate cortex; CU = cannabis users; HC = healthy control; MCC = middle cingulate cortex; OFC = orbitofrontal cortex; PCG = postcentral gyrus; PreCu = Precuneus; SMG = supramarginal gyrus; Thal = thalamus.
Conflict-related Functional Connectivity
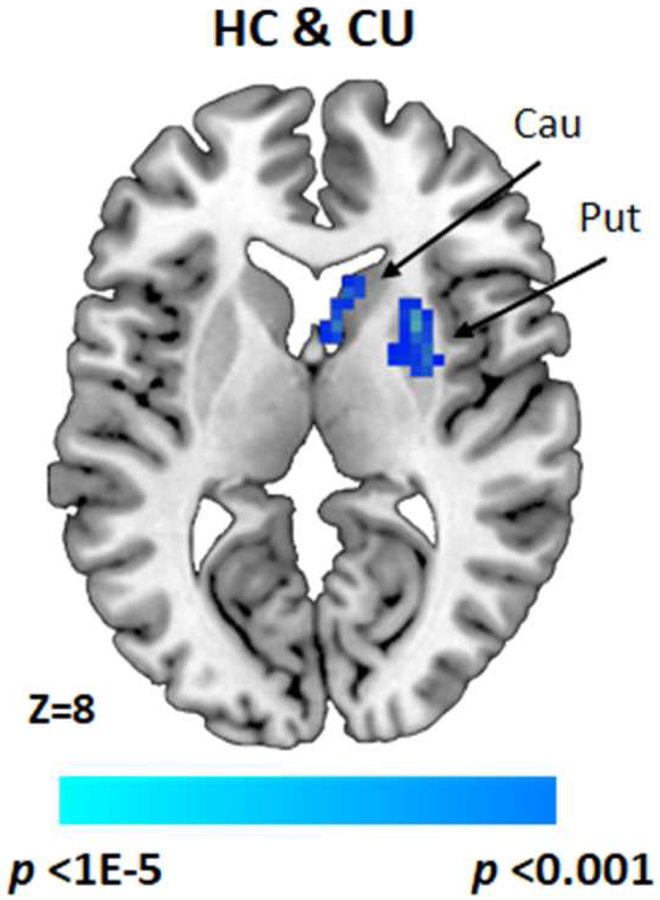
No significant group differences in conflict-related fronto-striatal connectivity were detected. Across both groups, however, significant negative connectivity (i.e., anti-correlations) was detected from vmPFC activation to right putamen (FWEc) and bilateral caudate (uncorrected) (Figure 2; Table 3). Effect sizes for these connectivity findings are reported in Table S2 (available online).
Figure 2. Conflict-Related Functional Connectivity.
Note: T-map showing conflict-related (Incongruent vs. Congruent) ventromedial prefrontal cortex (vmPFC)–putamen (voxel-wise cluster defining threshold [CDT] of p<.001, FWEc cluster extent correction of p<.05) and vmPFC–caudate functional connectivity strength in both HC and CU groups combined (CDT of p<.001, uncorrected), adjusting for age and gender. Cau = Caudate; CU = cannabis users; HC = healthy control; Put = Putamen.
Table 3.
Conflict-Related (Incongruent vs. Congruent) Negative Functional Connectivity of Ventromedial Prefrontal Cortex with Striatum, Across All Participants, Adjusting for Age and Gender.
| Area | Cluster size | MNI coordinates | t (peak) | pFWEc (cluster) |
|---|---|---|---|---|
| Right putamen | 46 | 27 8 8 | −4.17 | .013 |
| Bilateral caudate | 26 | −6 5 2 | −3.91 | .130 |
Note: MNI = Montreal Neurological Institute.
Clinical Correlates
Results for all clinical correlates in the CU group, including p-values adjusted for False Discovery Rate (FDR), are presented in Table S3 (available online). Conflict-related activation in vmPFC correlated positively with the number of days of cannabis use abstinence prior to scanning (r =.447, p=.020, FDR-p=.523; Figure S1A, available online). Conflict-related vmPFC-caudate connectivity also correlated negatively with the age of first regular cannabis use (r=‒ .391, p=.048, FDR-p=.522; Figure S1B, available online). These findings did not survive correction for multiple tests.
Potential Confounding Effects
Our findings of group differences in conflict-related activation did not appreciably change after excluding CU participants with comorbid 1) ADHD (n=4), 2) MDD (n=2), 3) anxiety (n=1), and 4) significant alcohol use (n=1; Figure S2, available online). Findings of group differences also remained significant after controlling for IQ and SES, in addition to age and gender (all ps<.004).
DISCUSSION
Herein, we used an event-related fMRI paradigm to examine the neural correlates of the resolution of cognitive conflict in cannabis-using youth. CU and HC participants performed similarly on the task. Compared to HC participants, CU participants showed reduced activation of fronto-striatal regions in response to conflict. Further, vmPFC activation in the CU group was significantly associated with their cannabis-use abstinence, perhaps reflecting a dose-response effect of cannabis on fronto-striatal function by which the effects of cannabis escalate with recent use. Contrary to our hypothesis, group differences in fronto-striatal functional connectivity were not detected, but vmPFC activation was negatively coupled with striatal activation in both groups, although this finding did not survive correction for multiple tests. Together with findings from prior studies, our results point to disruption in the fronto-striatal circuits that support self-regulatory control. Such disruption likely contributes to impaired control over addiction, thereby leading to the impulsive and compulsive drug seeking/taking that characterize CUD and SUDs more generally. Future studies should examine whether dysfunction in these circuits can be used as a reliable marker of substance use and addiction, as well as targets for circuit-based interventions.
In contrast to HCs, who showed significant conflict-related activation in frontal and parietal cortical regions, striatum, and basal ganglia, CU participants did not differentially activate these regions in response to incongruent compared to congruent stimuli. Thus, they did not engage these fronto-striatal regions to resolve conflict, consistent with previous findings of altered function and structure of fronto-striatal control circuits in chronic cannabis-using individuals.13, 15–23, 25, 26, 35–38, 56–60 This finding is also consistent with reports of reduced dopamine function in the striatum and decreased metabolism in prefrontal regions (particularly vmPFC regions) in addiction.61 For example, previous findings suggest reduced OFC volumes in cannabis-using adolescents37 and adults56, 58 relative to healthy controls, with smaller volumes associated with the initiation of cannabis use in adolescence36. Other data suggest decreased activation in vmPFC and other fronto-striatal regions during decision-making tasks in chronic cannabis-using adults.35, 60 Together, these findings contrast with previous reports of increased activation of vmPFC and other fronto-striatal regions during inhibitory control,57, 59 as well as with reports of increased functional and anatomical connectivity with OFC.38, 58 Such discrepant findings may be due to differences in study designs or differences in the clinical and demographic characteristics of the samples studied.
In the CU group, the duration of cannabis-use abstinence correlated positively with vmPFC activation, such that longer abstinence was associated with more activation of this region. Thus, progressive restoration of fronto-striatal function may occur after drug use has ceased. This exploratory finding was not significant after correcting for multiple tests, but is consistent with at least partial recovery from acute62 and chronic27 effects of cannabis on attentional and information processing abilities after a month or more of abstinence. However, data also suggest persistent deficits in performance and fronto-striatal function during various cognitive tasks following abstinence26, 35, 63 possibly due to an early onset of cannabis use during adolescence. This explanation suggests that adolescents may be particularly vulnerable to the effects of prolonged exposure due to the ongoing development of fronto-striatal circuits and associated cognitive functions. Previous findings in fact suggest greater disturbances in fronto-striatal control processes in chronic cannabis-using adults who became frequent users at early relative to later ages.16, 18, 19, 23, 30 We detected an uncorrected association between age of first regular use and fronto-striatal connectivity. Although in need of replication, these findings suggest that disruption in fronto-striatal functioning may be greater and more persistent if chronic cannabis use is initiated during adolescence, when these circuits are still undergoing maturation. Alternatively, pre-existing fronto-striatal abnormalities may also contribute to the early initiation and maintenance of cannabis use, consistent with findings that reduced OFC volumes at age 12 predict initiation of cannabis use by age 16.36
The present study is limited by its modest sample size, reducing our power to detect effects and precluding thorough examination of potential confounding effects (e.g., age, gender, IQ, SES) and their interactions with cannabis use on our behavioral and brain outcome measures. For example, including age and gender as covariates in our behavioral analyses revealed a main effect of age but no effect of conflict, suggesting that age rather than stimulus type accounted for most of the variance in reaction times across groups. Studies of larger samples are required to properly assess the effects of age and conflict-related processes in healthy and cannabis-using youth. Further, given the low power due to our modest sample size, some of the findings reported herein were not corrected for multiple tests and should thus be interpreted with much caution until replicated in a larger sample. In addition, aspects of cannabis use (i.e., frequency of use, abstinence, age of first regular use and duration of use) were based on self-reports. While informative, such measures may be subject to errors or biases in recall due to current neural functioning, psychopathology, current mood, personality, or other factors64 Future studies could incorporate ecological momentary assessment measures (e.g., using smart phones) of daily cannabis use and other relevant variables over an extended period of time. The cross-sectional study design also precluded examining longitudinal trajectories of fronto-striatal functioning associated with development, long-term chronic use and recovery. In addition, the correlational study design further limits our conclusions pertaining to the specific nature of the relationships observed between cannabis use and fronto-striatal function (e.g., causal or transactional relationships). Thus, future longitudinal studies in larger and more diverse samples should replicate and extend the findings presented herein.
Despite these potential caveats, our findings of altered conflict-related fronto-striatal function in relation to cannabis use in adolescents have important implications for our understanding of adolescent development and the risks/consequences associated with cannabis use. Indeed, due to the various neurobiological changes associated with this sensitive developmental period, an earlier onset of regular use may linearly predict (or result from) alterations in fronto-striatal circuits. The present findings set the stage for future longitudinal studies aimed at understanding the developmental trajectory of fronto-striatal circuits in relation to CUD, as well as to SUD in general, and are a first step towards identifying circuit-based targets for early interventions that reduce addiction behaviors by enhancing self-regulatory capacity. Given that substance use and relapse rates are associated with control processes,65interventions based on neural stimulation, such as transcranial magnetic stimulation (TMS), and behavioral interventions, such as cognitive training, that specifically target fronto-striatal circuits may be helpful as adjunct intervention strategies to complement standard treatment programs for CUD as well as SUDs in general.66
Supplementary Material
Acknowledgments
This work was supported by AACAP/NIDA grant K12DA000357 to Dr. Tau, a NIDA K24DA029647 to Dr. Levin, and a post-doctoral fellowship from Fronds de Recherche du Québec – Santé to Dr. Cyr.
Dr. Cyr served as the statistical expert for this research.
The authors thank the following former research assistants at the New York State Psychiatric Institute for their assistance in participant recruitment and assessment, and data entry: Caitlyn Miller, MA, EdM, MHC-LP, of Union Square Practice, NY; Kelly Schatz, BS, BSN, RN, of the University of Massachusetts Amherst, Baystate Medical Center; Kristen Randolph, BSN, RN, OCN, of the NYU Langone Medical Center; Tania Torres-Sanchez, MD, of Columbia University Medical Center; and Vinodh Chandra, MD, of Georgetown University.
Disclosure: Dr. Levin has received grant or research support from the National Institute on Drug Abuse (NIDA), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), and the Substance Abuse and Mental Health Services Administration (SAMHSA). Dr. Marsh has received grant or research support from the National Institute of Mental Health (NIMH), the Promise Project at Columbia University, Columbia University Office of Research Initiatives, and the Non-Verbal Learning Disability (NVLD) Project. Drs. Cyr and Tau and Ms. Fontaine report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. January 2010;35(1):217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fillmore MT. Drug abuse as a problem of impaired control: current approaches and findings. Behav Cogn Neurosci Rev. September 2003;2(3):179–197. [DOI] [PubMed] [Google Scholar]
- 3.Lubman DI, Yucel M, Pantelis C. Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction. December 2004;99(12):1491–1502. [DOI] [PubMed] [Google Scholar]
- 4.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. June 2003;160(6):1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston LD. Prescription drug use by adolescents: what we are learning and what we still need to know. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. December 2009;45(6):539–540. [DOI] [PubMed] [Google Scholar]
- 6.Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future National Survey Results on Drug Use, 1975–2017: Overview, Key Findings on Adolescent Drug Use. Ann Arbor, MI: University of Michigan, Institute for Social Research; 2018. [Google Scholar]
- 7.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. January 2010;35(1):147–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst M, Korelitz KE. Cerebral maturation in adolescence: behavioral vulnerability. L’Encephale. December 2009;35 Suppl 6:S182–189. [DOI] [PubMed] [Google Scholar]
- 9.Monti PM, Miranda R Jr., Nixon K, et al. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res. February 2005;29(2):207–220. [DOI] [PubMed] [Google Scholar]
- 10.Solowij N, Michie PT. Cannabis and cognitive dysfunction: parallels with endophenotypes of schizophrenia? J Psychiatry Neurosci. January 2007;32(1):30–52. [PMC free article] [PubMed] [Google Scholar]
- 11.Substance Abuse and Mental Health Services Administration OoAS. Results from the2007 National Survey on Drug Use and Health: National Findings (NSDUH Series H–34, DHHS Publication No. SMA 08–4343). Rockville; 2008. [Google Scholar]
- 12.Bhattacharyya S, Atakan Z, Martin-Santos R, et al. Impairment of inhibitory control processing related to acute psychotomimetic effects of cannabis. Eur Neuropsychopharmacol. January 2015;25(1):26–37. [DOI] [PubMed] [Google Scholar]
- 13.Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. May 2009;23(3):266–277. [DOI] [PubMed] [Google Scholar]
- 14.Ranganathan M, D’Souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology. November 2006;188(4):425–444. [DOI] [PubMed] [Google Scholar]
- 15.Battisti RA, Roodenrys S, Johnstone SJ, Pesa N, Hermens DF, Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology. December 2010;212(4):613–624. [DOI] [PubMed] [Google Scholar]
- 16.Ehrenreich H, Rinn T, Kunert HJ, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. March 1999;142(3):295–301. [DOI] [PubMed] [Google Scholar]
- 17.Fontes MA, Bolla KI, Cunha PJ, et al. Cannabis use before age 15 and subsequent executive functioning. The British journal of psychiatry : the journal of mental science. June 2011;198(6):442–447. [DOI] [PubMed] [Google Scholar]
- 18.Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Killgore WD. Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett. March 9 2012;511(2):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychol Addict Behav. September 2012;26(3):496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. November 2004;23(3):914–920. [DOI] [PubMed] [Google Scholar]
- 21.Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. April 2005;23(1):107–118. [DOI] [PubMed] [Google Scholar]
- 22.Harding IH, Solowij N, Harrison BJ, et al. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology. July 2012;37(8):1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagar KA, Dahlgren MK, Gonenc A, Racine MT, Dreman MW, Gruber SA. The impact of initiation: Early onset marijuana smokers demonstrate altered Stroop performance and brain activation. Dev Cogn Neurosci. December 2015;16:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdullaev Y, Posner MI, Nunnally R, Dishion TJ. Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behav Brain Res. December 20 2010;215(1):45–57. [DOI] [PubMed] [Google Scholar]
- 25.Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. October 2009;34(11):2450–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. November 2 2002;59(9):1337–1343. [DOI] [PubMed] [Google Scholar]
- 27.Solowij N Do cognitive impairments recover following cessation of cannabis use? Life Sci. 1995;56(23–24):2119–2126. [DOI] [PubMed] [Google Scholar]
- 28.Pope HG Jr., Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long-term cannabis users. J Clin Pharmacol. November 2002;42(11 Suppl):41S–47S. [DOI] [PubMed] [Google Scholar]
- 29.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. October 1999;2(10):861–863. [DOI] [PubMed] [Google Scholar]
- 30.Filbey FM, McQueeny T, DeWitt SJ, Mishra V. Preliminary findings demonstrating latent effects of early adolescent marijuana use onset on cortical architecture. Dev Cogn Neurosci. December 2015;16:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horga G, Maia TV, Wang P, Wang Z, Marsh R, Peterson BS. Adaptation to conflict via context-driven anticipatory signals in the dorsomedial prefrontal cortex. J Neurosci. November 9 2011;31(45):16208–16216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson BS, Kane MJ, Alexander GM, et al. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res. May 2002;13(3):427–440. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage. July 2004;22(3):1097–1106. [DOI] [PubMed] [Google Scholar]
- 34.Rubia K, Smith AB, Woolley J, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. December 2006;27(12):973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. June 2005;26(2):480–492. [DOI] [PubMed] [Google Scholar]
- 36.Cheetham A, Allen NB, Whittle S, Simmons JG, Yucel M, Lubman DI. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol Psychiatry. April 15 2012;71(8):684–692. [DOI] [PubMed] [Google Scholar]
- 37.Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA. Altered frontal cortical volume and decision making in adolescent cannabis users. Front Psychol. 2010;1:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Larson MP, Rogowska J, Yurgelun-Todd D. Aberrant orbitofrontal connectivity in marijuana smoking adolescents. Dev Cogn Neurosci. December 2015;16:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartman CA, Gelhorn H, Crowley TJ, et al. Item response theory analysis of DSM-IV cannabis abuse and dependence criteria in adolescents. J Am Acad Child Adolesc Psychiatry. February 2008;47(2):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rey JM. Marijuana use disorders and DSM-V. J Am Acad Child Adolesc Psychiatry. February 2008;47(2):121–122. [DOI] [PubMed] [Google Scholar]
- 41.Meyers K Comprehensive Adolescent Severity Inventory (CASI) Administration Manual. Philadelphia, PA: University of Pennsylvania, Treatment Research Institute; 1996. [Google Scholar]
- 42.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. Journal of consulting and clinical psychology. February 2000;68(1):134–144. [DOI] [PubMed] [Google Scholar]
- 43.Sobell LC, Sobell MB. Timeline Follow-Back User’s Guide: A Calendar Method for Assessing Alcohol and Drug Use. Toronto, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- 44.First M, Gil-Ad I, Taler M, Tarasenko I, Novak N, Weizman A. The effects of fluoxetine treatment in a chronic mild stress rat model on depression-related behavior, brain neurotrophins and ERK expression. J Mol Neurosci. October 2011;45(2):246–255. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman J, Birmaher B, Brent D, et al. The Schedule for Affective Disorders and Schizophrenia for School Aged Children: Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 46.DuPaul GJ. Parent and teacher ratings of ADHD symptoms: Psychometric properties in a community-based sample. Journal of Clinical Child Psychology. 1991;20:245–253. [Google Scholar]
- 47.Wechsler D Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 48.Hollingshead AB. Four-factor index of social status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- 49.Marsh R, Horga G, Wang Z, et al. An FMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am J Psychiatry. November 2011;168(11):1210–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cyr M, Fontaine M, Stefan M, et al. A longitudinal functional magnetic resonance imaging study of task control circuits and bulimic symptoms over adolescence. Journal of child psychology and psychiatry, and allied disciplines. November 8 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cyr M, Yang X, Horga G, Marsh R. Abnormal fronto-striatal activation as a marker of threshold and subthreshold Bulimia Nervosa. Hum Brain Mapp. January 10 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satterthwaite TD, Elliott MA, Gerraty RT, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. January 01 2013;64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel AX, Kundu P, Rubinov M, et al. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage. July 15 2014;95:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. February 1 2012;59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. October 1997;6(3):218–229. [DOI] [PubMed] [Google Scholar]
- 56.Battistella G, Fornari E, Annoni JM, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology. August 2014;39(9):2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filbey F, Yezhuvath U. Functional connectivity in inhibitory control networks and severity of cannabis use disorder. Am J Drug Alcohol Abuse. November 2013;39(6):382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Filbey FM, Aslan S, Calhoun VD, et al. Long-term effects of marijuana use on the brain. Proc Natl Acad Sci U S A. November 25 2014;111(47):16913–16918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tapert SF, Schweinsburg AD, Drummond SP, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. October 2007;194(2):173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res. January 30 2011;191(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. September 13 2011;108(37):15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. March 2011;5(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crean RD, Tapert SF, Minassian A, Macdonald K, Crane NA, Mason BJ. Effects of chronic, heavy cannabis use on executive functions. J Addict Med. March 2011;5(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwarz N, Seymour S. Autobiographical memory and the validity of retrospective reports. New York, NY: Springer-Verlag; 1994. [Google Scholar]
- 65.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. August 2005;162(8):1403–1413. [DOI] [PubMed] [Google Scholar]
- 66.Volkow ND, Boyle M. Neuroscience of Addiction: Relevance to Prevention and Treatment. Am J Psychiatry. April 25 2018:appiajp201817101174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.